Abstract
The stability and type of vitamin C added to foods is important for enhancing product quality, label claims, and shelf-life. To improve understanding of stability, the effects of deliquescence, storage relative humidity (RH) formulation, and addition of ascorbyl palmitate and dehydroascorbic acid on degradation of vitamin C at 25°C were studied. Individual vitamin C forms (ascorbic acid, sodium, and calcium ascorbate) and mixtures were stored below, near, and above their deliquescence RH for up to 12 weeks. Vitamin C stability was significantly affected by RH and vitamin form (p < 0.0001). Formulation affected deliquescence and chemical stability: mixtures were significantly less stable above RH0mix than below (p < 0.0001). Ascorbyl palmitate-enhanced degradation rates of more soluble vitamin C forms, and the presence of dehydroascorbic acid reduced vitamin blend stability. It is important that vitamin C products be stored below the deliquescence RH to avoid enhanced vitamin losses.
INTRODUCTION
Vitamin C is frequently incorporated into foods and premixes, and its concentration is declared on nutrition fact labels. Additionally, increasing focus on nutritional and functional properties of foods has led to growth of the dietary supplement industry in recent years.Citation[1] These supplements are commonly used to treat chronic conditions, secondary health problems, or for general well-being and disease prevention.Citation[2] Vitamin C is the third most commonly consumed single vitamin, consumed in supplement form by 9.4% of adults.Citation[1] It is, therefore, important to control vitamin C stability in foods and supplements.
Vitamin C is added to foods as ascorbic acid or one of its salt forms (commonly sodium ascorbate or calcium ascorbate), or ascorbyl palmitate (a lipophilic form of vitamin C). It is known that water plays an important role in influencing degradation rates of ascorbic acid, and degradation of solid phase ascorbic acid ensues when moisture is introduced.Citation[3] Shephard, Nichols, and BraithwaiteCitation[4] determined that ascorbic acid degraded in the solid phase in the presence of moisture, resulting in severe brown discoloration. Ascorbic acid degradation was found to increase with increasing moisture content.[Citation5,Citation6] Additionally, moisture present in solid vehicles enhanced the degradation of vitamins A, B1, and C in pharmaceuticals.Citation[7] Recently, the impact of deliquescence on vitamin B1, B6, and sodium ascorbate stability in powder systems was characterized.Citation[8] Deliquescence is a first order phase transformation from solid to solution that occurs at a certain relative humidity (termed RH0) that is specific to that crystalline solid, and has been linked with chemical stability of sensitive food ingredients.[Citation8–12] In addition, a phenomenon known as deliquescence lowering has been witnessed in mixtures of crystalline vitamins.Citation[8] Deliquescence lowering is a reduction in the RH where the solid to solution transition occurs, termed RH0mix, when deliquescent compounds are mixed.Citation[9,Citation11] This is of concern for vitamin C supplements as they commonly contain mixtures of vitamin C forms. Since these vitamin C forms differ in solubility and, therefore, RH0, it is important to understand how each form will interact with water individually and in mixtures.
Previous research has also demonstrated the effect of degradation products on deliquescence lowering and food ingredient stability.Citation[9] Production of a hygroscopic degradation product of vitamin C, such as dehydroascorbic acid (DHAA), could potentially result in greater deliquescence lowering and increased chemical reactivity within the system. However, the degradation mechanism for ascorbic acid depends on several factors and is therefore specific for an individual system.Citation[13] In particular, evidence has suggested that solid phase and solution phase ascorbic acid degradation differ, resulting in different end products.Citation[14]
Since vitamin mixes may be subjected to varying RH conditions during formulation, processing, packaging, storage, and distribution, an understanding of the impact of water-solid interactions on chemical stability within vitamin C powders is important for ensuring delivery of the vitamins in their primary active forms. The objective of this research was to determine the effect of deliquescence and deliquescence lowering, storage RH conditions, and formulation on the chemical stability of different forms of vitamin C: ascorbic acid (A), sodium ascorbate (Na), and calcium ascorbate (Ca). The effects of co-formulation of these water-soluble forms of vitamin C with ascorbyl palmitate (P) (a lipophilic form of vitamin C) and dehydroascorbic acid (DHAA, a degradation product) on moisture uptake and vitamin C stability were also determined.
MATERIALS AND METHODS
Materials
Vitamin C forms were selected based on common usage and varying interactions with water. Sodium ascorbate (≥99%) and dehydroascorbic acid were obtained from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Calcium ascorbate (98–101%) and ascorbyl palmitate were purchased from Spectrum Chemical Mfg. Corp. (Gardena, CA, USA). Ascorbic acid was from Mallinckrodt-Baker (Phillipsburg, NJ). The following salts were used to create saturated salt solutions and control RH in environmental chambers: KCl and K2SO4 (Mallinckrodt-Baker); Mg(NO3)2 (Sigma-Aldrich, Inc.); and NaCl (Sigma-Aldrich, Inc.). Certified HPLC and ACS grade methanol (Mallinckrodt-Baker) and trifluoroacetic acid (TFA) (Sigma-Aldrich, Inc.) were used. Materials for preparing reagents for the microplate reader assay included: orthophosphoric acid (Alfa Aesar, Ward Hill, MA, USA), trichloroacetic acid (TCA), and 2,2 bipyridine (Mallinckrodt-Baker), iron chloride (EMD Chemicals, Gibbstown, NJ, USA), ethanol (Aaper Alcohol & Chemical Co., Inc., Shelbyville, KY, USA), and potassium phosphate monobasic and dibasic (Mallinckrodt Baker, Inc., Paris, KY, USA).
Water Activity Measurements
Samples were prepared by adding 200–500 μL of distilled water to 1–2 g of powder (binary, ternary, and quaternary mixtures were prepared by geometric mixing of equal mass ratios of multiple ingredients). Water activity (aw ) of samples was measured in a chilled mirror dew point instrument (AquaLab 3TE; Decagon, Pullman, WA, USA) at 25°C after a 48 h sample equilibration time. The aw of the saturated solution was used as a prediction of the deliquescence RH (RH0 or RH0mix).Citation[15]
Moisture Sorption Isotherms
Gravimetric sorption analysis was performed using a symmetrical gravimetric analyzer (SGA-100) (VTI Corporation, Hialeah, FL, USA) at 25°C in order to determine the critical relative humidity of deliquescent solids (RH0) and mixtures (RH0mix).Citation[15] For the mixtures, larger quantities of individual components were blended at an equal mass ratio, and a 10–15 mg portion of the sample was used for analysis. Prior to sorption analysis, samples were dried at 60°C in the sorption analyzer. The settings for the sorption analyzer were: equilibrium criterion for the drying step of 0.01% w/w in 2 min, maximum drying time of 30 min, and step equilibrium criterion of 0.001% w/w in 5 min with a maximum step time of 60 min. During the experiment, samples were exposed to increasing RH (from 0 to 94% RH), increasing at 10% intervals from 20–70% RH and at 2% intervals from 70–94% RH. RH0 and RH0mix were determined from the point in the isotherm at which the sample began to rapidly sorb moisture.Citation[15]
Controlled Relative Humidity Storage
Single, binary, ternary, and quaternary mixtures of ascorbic acid (A), sodium ascorbate (Na), calcium ascorbate (Ca), and ascorbyl palmitate (P) were prepared in triplicate and placed in 20-mL glass sample vials. Equal parts by mass of all ingredients were used for each mixture. Additionally, samples of DHAA and binary mixtures of Na and DHAA in ratios of 50:50, 75:25, and 90:10 w/w were prepared via geometric mixing. Controlled RH environmental chambers were prepared using saturated salt solutions: magnesium nitrate (54% RH); sodium chloride (75% RH); potassium chloride (85% RH); and potassium sulfate (98% RH). The RH of each chamber was verified by digital hygrometer (traceable humidity/temperature/dew point meter, Control Co., Friendswood, TX, USA) or water activity (AquaLab 3TE; Decagon Devices, Inc., Pullman, WA, USA). Powder samples were stored in select RH chambers at room temperature (22 ± 5°C) for exposure to RHs above, at/near, and below RH0 or RH0mix for up to 12 weeks. Controls were prepared by analyzing powder mixtures immediately after sample preparation. Following storage treatment, samples were nitrogen flushed and frozen at −20°C until analysis. Physical observations such as formation of a solution and mass gain were recorded as indicators of deliquescence and moisture uptake.
Chemical Stability Determination with HPLC
High performance liquid chromatography (HPLC) was used to determine vitamin C degradation in single and binary formulations without ascorbyl palmitate following the method of Heudi et al.Citation[16] with modifications. The chromatographic system used was an Agilent 1200 Rapid Resolution LC system with photodiode array detector (Agilent Technologies, Milford, MA, USA), and a model 490E programmable multiwavelength detector (Waters Corp, Milford, MA, USA). Separation was achieved using a 3.9 mm × 100 mm (3.5-μm particle size) XTerra RP-C18 column (Agilent Technologies, Foster City, CA, USA). Resolution of vitamin C was achieved using isocratic elution at 0.8 mL per minute with 0.025% trifluoroacetic acid (TFA) (Sigma Co., St. Louis, MO, USA) in polished water (pH 2.6). Detection and identification of the vitamins were performed at 240 nm. A 20-μL injection volume was used in this method. Quantification of compounds of interest was performed using multilevel calibration curves constructed with standards of ascorbic acid.
Chemical Stability Determination by Microplate Reader
An AD/LD 340 Absorbance Detector microplate reader (Beckman Coulter, Inc., Fullerton, CA, USA) was used to quantify vitamin C degradation in a ternary formulation and in formulations containing ascorbyl palmitate and DHAA following the method of Stevens et al.Citation[17] for reduced ascorbate with slight modifications. Samples were dissolved in water and filtered through Whatman #1 filter paper to separate the water soluble vitamin C form from ascorbyl palmitate. Binary mixtures of DHAA and Na were diluted in water without filtering, and all samples were further diluted in TCA. Samples were analyzed on the plate reader at 570 nm.
pH Measurements
An estimation of the pH at the dissolution point of all formulations was determined by preparing saturated solutions of all vitamin C forms, their binary mixtures, a ternary mixture, and a quaternary mixture and measuring the pH on a Beckman model 350 pH/Temp/mV meter (Beckman Coulter, Inc., Fullerton, CA, USA) calibrated from pH 2–10.
Statistical Analysis
A completely randomized three factor factorial design was used for studying the effects of RH, time, and formulation on individual vitamin form stability. An ANOVA model was used for this analysis. Individual differences were tested using Tukey's multiple means comparison procedure. All statistical analysis procedures were conducted using PC SAS software (SAS Institute, Inc, Cary, NC, USA) and α = 0.05.
RESULTS AND DISCUSSION
Deliquescence and Deliquescence Lowering
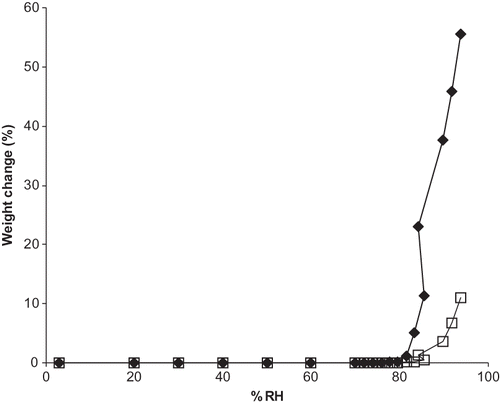
RH0 values for A and Na were in accordance with previously published resultsCitation[8,Citation15] of 98 and 86% RH at 25°C, respectively (, ). RH0 for calcium ascorbate has not been previously reported and was found to be 94% RH. Deliquescence lowering was observed for binary mixtures of vitamin C forms, as well as a ternary and quaternary mixture. RH0mix for binary mixtures ranged from 82% RH for a mixture of A and Na, to 92% RH for a mixture of A and Ca (, ). These values are similar to those expected from Ross equation predictions. The ternary mixture of all water-soluble vitamin C forms (ANaCa) deliquesced at 81% RH, while a quaternary mixture of ANaCaP had RH0mix of 83% RH (). The addition of non-deliquescent ascorbyl palmitate is credited with the higher RH0mix for the quaternary mixture compared to that for the ternary blend. Due to the poor water solubility of P, this vitamin form would not be expected to deliquesce and would, therefore, not contribute to deliquescence lowering. Additionally, P could potentially reduce the deliquescence lowering effects of other ingredients in the mixture by limiting the amount of contact points between them.Citation[11]
Figure 1 Moisture sorption isotherms for single and binary mixtures of vitamin C forms. A. Sorption isotherms for single vitamins forms. Isotherms for each ingredient are shown by: ![]()
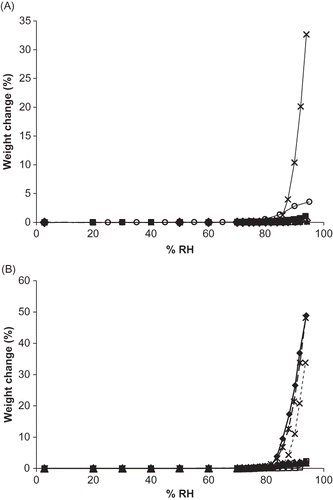
Figure 2 Moisture sorption isotherms for a single, binary, ternary, and quaternary mixture containing sodium ascorbate. Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate, P = ascorbyl palmitate. Isotherms for each mixture are shown by: ![]()
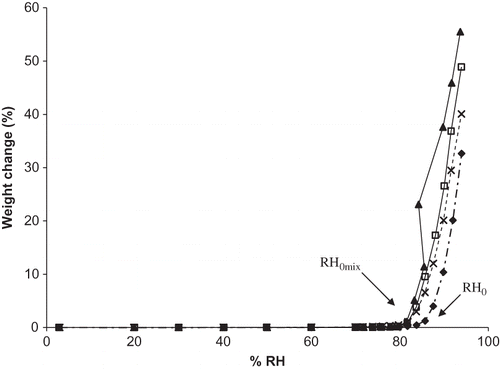
Table 1 Water activity, pH, deliquescence point, and solubility information for ingredients and their mixtures at 25°C
Water activity values for individual forms of vitamin C and their binary mixtures, a ternary mixture, and a quaternary mixture are reported in . The aw of the saturated solution of vitamin forms and their mixtures is a good estimate of RH0 or RH0mix for deliquescent ingredients and their mixtures, respectively.Citation[11] In general, aw values agreed with deliquescence points obtained from moisture sorption isotherm analyses (). Additionally, the RH0mix for multicomponent vitamin C blends was estimated using the Ross equation:
which predicts the aw of a mixture of ingredients from the aw s of the individual components.Citation[18] Calculated χ values, a measure of the deviation of the experimental RH0mix value from the predicted value,Citation[18] are also reported in . Since χ values for mixtures in this study ranged from 0.97 to 1.05, and a value of 1.0 indicates no deviation, there was generally good agreement between predicted RH0mix values and those determined from gravimetric moisture sorption analysis. χ values that deviate from unity indicate the presence of strong solute-solute interactions, as the Ross equation does not take this factor into account; therefore, deviations from predicted RH0mix values can occur as mixture complexity increases.Citation[11,Citation18]
Moisture Sorption Measurements
Of the different forms of vitamin C and DHAA, total moisture sorption at 94% RH (arranged from highest to lowest and reported as percent weight change (% w/w)) was: Na (32.66%), P (3.37%), DHAA (1.06%), A (0.343%), and Ca (0.155%) (). For the water-soluble vitamin C forms, this pattern is in accordance with their molal solubilites with Na having the highest solubility value (3.13 mol/kg), followed by A (1.89 mol/kg),Citation[19] then Ca (1.34 mol/kg)Citation[20] (). Surprisingly, ascorbyl palmitate was second only to sodium ascorbate in terms of moisture sorption during gravimetric moisture sorption analysis, despite its low solubility value (0.014 mol/kg).Citation[21] It was hypothesized that the usually crystalline ascorbyl palmitate contained amorphous regions, resulting in water absorption into the bulk phase. For binary mixtures, moisture sorption isotherm analysis indicated that total moisture sorption at 94% RH followed the order: ANa (48.88%), NaP (48.15%), NaCa (33.81%), ACa (2.29%), CaP (2.00%), and AP (1.92%) (). In general, mixtures containing Na demonstrated greater moisture uptake than binary mixtures without Na, due to its enhanced water solubility compared to the other ingredients.
A comparison of moisture sorption isotherms for a single, binary, ternary, and quaternary mixture all containing Na revealed that a ternary mixture sorbed the greatest amount of moisture (ANaCa), followed by the binary mixture (ANa), then the quaternary mixture (ANaCaP), and finally single Na (). The presence of a greater number of deliquescent ingredients in a blend is expected to enhance weight gain at a given RH above RH0mix due to deliquescence lowering.Citation[11,Citation15] The addition of a non-deliquescent, lipophilic ingredient (P) appears to inhibit some moisture uptake, resulting in less weight gain for the quaternary mixture compared to the binary and ternary mixtures.
Mixtures containing both DHAA and Na in ratios of 50:50, 25:75, and 10:90 DHAA:Na exhibited greater moisture uptake than either DHAA or Na alone (), though the ratio of DHAA:Na did not appear to influence total moisture gain for the mixtures. Compared to all other binary mixtures of Na with other vitamin C forms, DHAA:Na mixtures demonstrated greater total moisture sorption as well as greater deviation from expected moisture sorption (data not shown). The enhancement of moisture uptake for DHAA:Na is somewhat surprising given that DHAA is more hydrophobic than A,Citation[22] but still exhibited ∼0.7% more moisture uptake than ascorbic acid. However, DHAA is very unstable and rapidly degrades further to a variety of end products, especially once in solution.Citation[4,Citation23] Increased moisture sorption accompanied by a reduction in RH0 has been reported for a compound in the presence of degradants or impurities;Citation[24] thus, the production of a complex mixture of soluble degradation products may be responsible for the enhanced moisture sorption observed in DHAA and Na mixtures.
Figure 3 Moisture sorption isotherms for individual DHAA and sodium ascorbate and binary mixtures of the two in ratios of 50:50, 25:75, and 10:90 DHAA:N. Individual ingredients are abbreviated as: N = sodium ascorbate, D = dehydroascorbic acid. Isotherms for individual ingredients and mixtures are shown by: ![]()
Weight Gain and Physical Observations during Controlled RH Storage
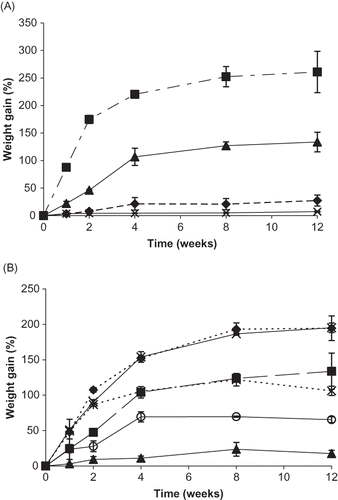
Plots of weight over time for most samples demonstrated initial rapid weight gain before gradually reaching a plateau as equilibrium was established ( and ). The order of moisture uptake for individual vitamin C forms over 12 weeks at 98% RH, from greatest to least, was: Na (260.9% w/w), DHAA, Ca, A, and P (7.09% w/w) (). This trend differs from the order of moisture sorption observed from the moisture sorption balance analysis, where Ca demonstrated the least weight gain and P had greater weight gain than both A and Ca. However, the total moisture uptake recorded during the moisture sorption balance analysis for P, A, and Ca was similar and discrepancies can be attributed to differences in moisture sorption kinetics between controlled RH storage and dynamic moisture sorption measurements.Citation[11] End point moisture uptake for all binary mixtures stored at 98% RH, from greatest to least, followed the order: NaCa (195.2% w/w), ANa, ACa, NaP, CaP, and AP (17.6% w/w) (). Weight gain for the ternary (ANaCa) and quaternary (ANaCaP) mixtures was 202.6 and 108.9% w/w, respectively. It can be noted that the ternary formulation exhibited greater moisture uptake after 12 weeks of storage at 98% RH than all binary mixtures, while mixture ANaCaP only had greater moisture uptake than binary formulations containing ascorbyl palmitate (AP, NaP, CaP). Tracking the amount of moisture present in a system is important for determining solid:solution partitioning of ingredients and possible effects on stability.Citation[25]
Figure 4 Weight gain (%) for individual vitamin C forms and their binary mixtures during storage up to 12 weeks at 98% RH. A. Weight gain (%) for individual vitamin C forms over time. Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate, P = ascorbyl palmitate. Percent weight gain for individual ingredients is shown by: ![]()
After 12 weeks of storage at 98% RH (above RH0 for Na and Ca and at RH0 for A), Na, Ca, and DHAA samples formed brown liquids with some precipitate present; A appeared as slightly yellow, damp, caked crystals with some drops of visible moisture; and P formed a caked white powder. During storage at 54% RH, below RH0, browning of Na and Ca was observed after 12 weeks but the powders appeared dry with moisture uptakes of 2 ± 0.1% and 0.5 ± 1% for Na and Ca, respectively. After 12 weeks at 54% RH, A yellowed slightly and caked, DHAA appeared similar to the starting material (a brown powder), and P formed a white slightly caked powder. Brown discoloration and the presence of liquid was observed in mixtures ANa, ACa, NaCa, NaP, CaP, ANaCa, and ANaCaP at 98% RH. Sample AP formed a caked white powder with some yellow liquid present. Below RH0mix (54% RH), browning was observed (except in AP) but no liquid formation was observed in any sample. It has been established that ascorbic acid degradation can result in browning,Citation[4] and physical observations of enhanced browning and visual moisture present corresponded to enhanced degradation.
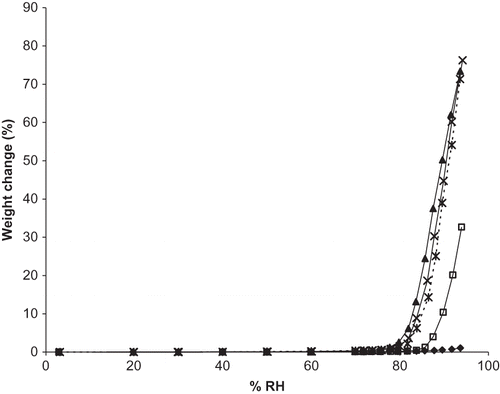
Interestingly, above the RH0 of sodium ascorbate (86% RH) the experimental isotherm for formulation ANaCa had enhanced moisture sorption compared to that predicted by the moisture uptake of the individual mixture components (). Below RH0mix (at 76, 78, and 80% RH) the difference between the experimental and predicted moisture gain was <0.07%. At and slightly above RH0mix for ANaCa (at 81 and 83% RH), the discrepancy between experimental and predicted moisture sorption was 0.1 and 5%, respectively. Once RH0 of Na (the most water-soluble mixture component) was exceeded, large differences in experimental and predicted moisture gain were observed. At 89, 92, and 94% RH the differences were 34, 39, and 44%, respectively. Sodium ascorbate appears to be dominating the moisture sorption behavior of the mixture, likely due to its increased capacity to interact with water. This trend holds for the other vitamin mixtures and was also observed for blends of sodium ascorbate with sucrose, citric acid, and green tea powder.Citation[25]
Figure 5 Experimental and predicted moisture sorption isotherms for a ternary mixture of vitamin C forms (ANC). Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate. Sorption isotherms are shown by: ![]()
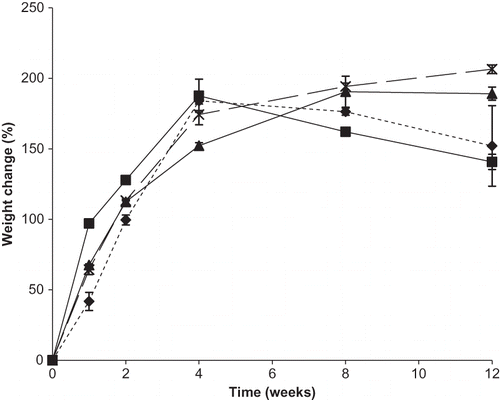
Unlike other samples, weight loss was observed for individual DHAA and a 50:50 ratio of DHAA:Na after 4 weeks (). Weight loss could be due to further decomposition of DHAA breakdown products, with release of CO2.Citation[14,Citation26] Compared to dynamic moisture sorption measurements, increasing amounts of Na in binary DHAA:Na mixtures resulted in increased moisture uptake. Weight gain for binary DHAA and Na mixtures after 12 weeks of storage at 98% RH ranged from 141% w/w for a 50:50 ratio to 207% w/w for a 10:90 ratio of DHAA to Na. This is likely due to the increased solubility of Na compared to DHAA.
Figure 6 Weight gain (%) for individual DHAA and binary mixtures of DHAA and sodium ascorbate in ratios of 50:50, 25:75, and 10:90 DHAA:N over time during storage at 98% RH. Individual ingredients are abbreviated as: N = sodium ascorbate, D = dehydroascorbic acid. Percent weight gain for mixtures is shown by: ![]()
Chemical Stability of Different Forms of Vitamin C
RH and formulation significantly affected vitamin stability (p < 0.0001). Chemical stability at 98% RH was not significantly different for Ca and Na, but A was significantly more stable than either of these salt forms (p < 0.0001) (). Degradation at 98% RH varied from 100.0 ± 0.2% for Ca to 9.6 ± 4.8% for A (). Additionally, A degradation was not influenced as greatly by RH as Ca or Na degradation, as demonstrated by its stability across storage RH conditions (), although it is important to note that A was not stored above its RH0 (98% RH). The more acidic conditions in a saturated solution of A compared to the pH of Na or Ca solutions would provide another stabilizing factor, since ascorbate is reportedly more stable at pH values below its pKa1 of 4.2.[Citation27–29] Ascorbic acid degradation following 12 weeks of storage at RH0 (98% RH) was not significantly different from degradation below RH0 (54% RH) (p = 1.000). Both Ca and Na were greatly influenced by RH as significantly greater degradation occurred for both of these vitamin C forms above RH0 compared to below (p < 0.0001) (). Generally Na and Ca were stable when stored below RH0, with degradation of only 2 ± 2.5% and 1 ± 1.6% for Na and Ca, respectively. Thus, storing ingredients below RH0 could maintain chemical stability.
Figure 7 Vitamin C stability for individual vitamin forms and their binary mixtures during storage up to 12 weeks at 98% RH. A. Percent vitamin C remaining over time for different vitamin forms. Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate. Percent vitamin C remaining for individual vitamins is shown by: ![]()
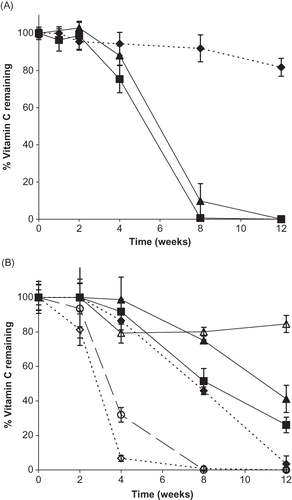
Table 2 Endpoint vitamin C degradation after 12 weeks of storage at select RHs for individual vitamin C forms and in vitamin C ingredient mixtures. Results are reported as % degradation with standard deviations and different letters indicate significant differencesa. Each formulation was stored at three RHs selected to be above, near, and below the deliquescence point of the blend
Chemical Stability of Mixtures of Various Forms of Vitamin C
RH had the largest effect on vitamin C stability in mixtures, as this factor had the largest mean square error value in the ANOVA table generated from the effects of RH, formulation, time, and their interactions on vitamin stability. All formulations except AP were significantly more stable below RH0mix than above () (p < 0.0001), and AP was stable across all RH storage conditions, which were at or below the RH0 of A. Of the mixtures, samples of vitamin salt forms (Ca and Na) with ascorbyl palmitate and a quaternary mixture of all vitamin C forms were the least stable after 12 weeks of storage at 98% RH (). A mixture of A with P was the most stable, with only 15.4 ± 5.0% degradation after 12 weeks of storage at 98% RH (). Of the binary mixtures that did not contain P, stability (from greatest to least stable) followed the order: NaCa, ACa, and ANa with a degradation after 12 weeks of storage at 98% RH of approximately 59, 75, and 88%, respectively. This is in accordance with the distance of storage RH from RH0mix for mixtures ACa and ANa. Storing samples at RHs further above their RH0mix will result in greater moisture sorption, contributing to greater molecular mobility and chemical reactivity.[Citation30–32] Both samples were stored at 98% RH; however, the RH0mix for ACa is 87% RH and this sample demonstrated a weight gain of 133.8%, while the RH0mix is 84% RH for ANa and 194.6% moisture uptake was observed. Therefore, it would be expected that a mixture of ANa would exhibit greater degradation than mixture ACa. Plotting percent vitamin C remaining over time for each binary mixture shows similar trends for each sample (). All demonstrated sharp decreases in stability in the first 2 weeks, followed by more gradual degradation. Thus, short exposure to high RHs could have significant effects on product stability.
Impact of Ascorbyl Palmitate on Degradation of Water Soluble Vitamin C Forms
Co-formulation with P did not assert any protective effects on the endpoint degradation of more soluble vitamin C forms; however, it did influence their degradation rates ( and ). Compared to degradation of individual Na and Ca, mixtures of these vitamin forms with P showed significantly greater degradation after 4 weeks of storage (p < 0.0001 and p = 0.0003 for Na and Ca, respectively), before complete degradation was observed after 8 and 12 weeks at 98% RH ( and ). A comparison of binary mixtures of all vitamin C forms indicates that degradation rates are increased when mixed with P compared to mixing that same vitamin form with other more soluble vitamin C ingredients. This can be explained in part by comparing the theoretical amounts of dissolved water-soluble vitamin C in the different formulations. The binary mixture NaP sorbed 420.9 ± 16.2 mg of moisture after storage for 4 weeks at 98% RH, while mixture NaCa sorbed 618.7 ± 28.2 mg moisture under these conditions. Assuming independent dissolution of the vitamin forms and using the molal solubility of sodium ascorbate,Citation[19] dissolution maxima of 261.0 mg and 383.7 mg in formulations NaP and NaCa, respectively, would be expected. This corresponds to complete dissolution of the sodium ascorbate present in these samples. As ascorbate degradation commonly follows first-order kinetics,Citation[3,Citation33,Citation34] the excess water could decrease the ascorbate concentration in the blend of NaCa compared to NaP, potentially reducing degradation rates.
Impact of Dehydroascorbic Acid on Degradation of Vitamin C
DHAA was mixed with sodium ascorbate at various ratios of DHAA to Na: 50:50, 25:75, and 10:90. Compared to Na stored individually, addition of DHAA at all ratios did not significantly affect Na degradation (p = 1.00) when stored at 98% RH for 12 weeks: complete degradation of ascorbate occurred in all samples (, ). However, by examining degradation below RH0 or RH0mix (54% RH) the impact of the presence of a degradation product, DHAA, on sodium ascorbate degradation can be elucidated. Addition of DHAA to Na at a ratio of 50:50 DHAA:Na resulted in significantly greater Na degradation compared to individual Na after 12 weeks of storage (p = 0.0051) (). Ratios of 25:75 and 10:90 DHAA:Na did not exhibit significantly different Na degradation than Na alone (p = 1.00), and very little Na degradation occurred in those samples following the 12-week period (). This is important as it demonstrates that the presence of DHAA in vitamin C powders in sufficient quantities can decrease vitamin C stability at lower RHs.
Figure 8 Percent of reduced ascorbate remaining over time for binary mixtures of dehydroascorbic acid and sodium ascorbate in ratios of 50:50, 25:75, or 10:90 DHAA:N stored at 98% RH. Individual ingredients are abbreviated as: N = sodium ascorbate, D = dehydroascorbic acid. Percent reduced ascorbate remaining in mixtures is shown by: ![]()
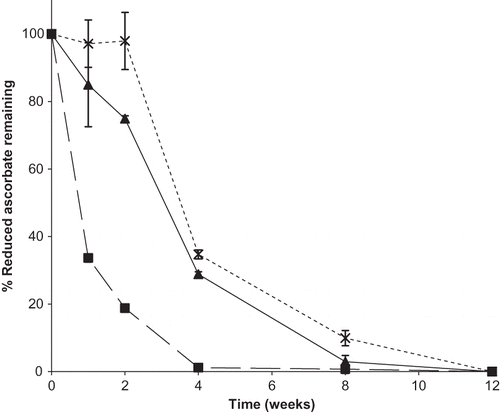
In addition to the ratio of DHAA and Na in mixtures, storage RH affected Na stability in these blends. For binary mixtures containing a 50:50 or 10:90 ratio of DHAA:Na, increasing RH resulted in significantly decreased ascorbate stability (p < 0.0001) (). At a ratio of 25:75 DHAA:Na, mixtures were significantly more stable after a 12-week storage period at 54 and 75% RH compared to 98% RH (p < 0.0001), but stability did not differ between 54 and 75% RH storage (p = 1.00) (). Therefore, both storage RH and the amount of a degradation product, DHAA, present in vitamin C powders will influence the stability of the available ascorbate.
Previous research has demonstrated the effect of formation of degradation products on deliquescence lowering and subsequent chemical instability of the parent compound.Citation[32] Salameh and TaylorCitation[35] reported the occurrence of sucrose hydrolysis to fructose and glucose upon deliquescence of sucrose:citric acid mixtures. Production of hygroscopic degradation products further decreased RH0mix and enhanced moisture sorption, resulting in decreased chemical stability.Citation[35] A similar mode of action is presumed for vitamin C degradation as DHAA is produced in the initial stages. DHAA is the first degradation product of ascorbic acid in solution, followed by irreversible hydrolytic or oxidative degradation to 2,3-diketogulonic acid and subsequent decomposition to a variety of furan, ketoacid, and carboxylic acid products.Citation[4,Citation36–39] Since sufficient water and solute dissolution is required to drive hydrolytic degradation of vitamin C, the enhanced moisture sorption promoted by the presence of a second deliquescent compound in the mixture reduces RH0mix, which results in greater chemical instability.
Relationship between Deliquescence, Moisture Sorption, and Vitamin C Stability
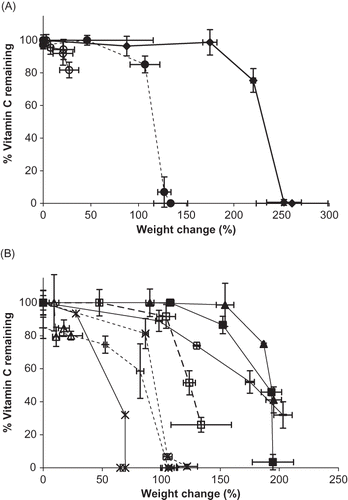
As moisture sorption increased, vitamin C stability decreased ( and ). A broad range of total moisture sorbed was observed, dependent on the hygroscopicity of the individual vitamin form or blend. The deliquescence event drastically increases moisture sorption,Citation[11] and is, therefore, of major concern for vitamin C stability. Formulation also influences deliquescence behavior,Citation[8,Citation11,Citation12,Citation25] with reduced deliquescence points for blends of vitamin C compared to individual vitamins. Generally, the vitamin C forms and mixtures with lower deliquescence points exhibited greater moisture uptake, and a lag period is observed for all samples, during which appreciable vitamin C degradation does not commence until sufficient moisture sorption has occurred. Differences in stability were evident between formulations, though total moisture sorption did not necessarily dictate stability. While Na sorbed the most moisture of the individual vitamin C forms, similar losses in vitamin quantity were observed for Ca with a lesser amount of moisture uptake (). Of the blends, the most stable formulation with increasing moisture sorption was AP, while the least stable formulation (most sensitive to moisture sorption) was CaP (). The inability to fully describe vitamin C stability, both individually and in mixtures based on total moisture sorption data, may be a result of solution-formation properties unique to each formulation, such as moisture uptake rate, reactant concentration, pH, or formation of degradation products.
Figure 9 Percent weight gain versus % vitamin C remaining for vitamin C forms and their blends stored at 98% RH and 25°C up to 12 weeks. A. Percent weight gain versus % vitamin C remaining for individual vitamin C forms. Individual ingredients are abbreviated as: A = ascorbic acid, N = sodium ascorbate, C = calcium ascorbate. Trends for individual vitamins are shown by: ![]()
CONCLUSION
Deliquescence is known to contribute to enhanced chemical instability for vitamin C. Various forms of vitamin C are available and are included in dietary supplements and food and beverage premixes. These forms differ in solubility and, therefore, in RH0. This study demonstrated that stability differences do exist for different forms of vitamin C when stored above their deliquescence point and that the difference in storage RH vs. RH0 also affects stability. Additionally, ascorbyl palmitate, a lipophilic form of vitamin C, did not protect more soluble vitamin C forms from degradation in the presence of moisture. The presence of a vitamin C degradation product (DHAA) contributed to decreased stability. This work highlights the importance of careful consideration of ingredient solubility, moisture-mediated reactions, and storage RH when selecting a specific vitamin form for mixture formulation.
ACKNOWLEDGMENTS
This research was supported in part by USDA-NRICGP Grant #07-35503-18405 and by a grant from the Lilly Endowment, Inc. to the School of Pharmacy and Pharmaceutical Sciences at Purdue University. Sarah Verkamp is also thanked for her contribution to this work.
REFERENCES
- Marigny Research Group . 2006 . Nutritional Supplements in the U.S. , New Orleans, LA, , USA : MarketResearch.com, Inc. .
- Muth , M.K. , Nardinelli , C. and Beach , R.H. 2001 . The marketplace for dietary supplements: Recent studies . Drug Information Journal , 35 ( 3 ) : 973 – 983 .
- Lee , S.H. and Labuza , T.P . 1975 . Destruction of ascorbic-acid as a function of water activity . Journal of Food Science , 40 ( 2 ) : 370 – 373 .
- Shephard , A.B. , Nichols , S.C. and Braithwaite , A. 1999 . Moisture induced solid phase degradation of L-ascorbic acid—Part 1. A kinetic study using tristimulus colorimetry and a quantitative HPLC assay . Talanta , 48 ( 3 ) : 585 – 593 .
- Kirk , J. , Dennison , D. , Kokoczka , P. and Heldman , D. 1977 . Degradation of ascorbic-acid in a dehydrated food system . Journal of Food Science , 42 ( 5 ) : 1274 – 1279 .
- Sablani , S.S. , Al-Belushi , K. , Al-Marhubi , I. and Al-Belushi , R. 2007 . Evaluating stability of vitamin C in fortified formula using water activity and glass transition . International Journal of Food Properties , 10 ( 1 ) : 61 – 71 .
- Wai , K.N. , Banker , G.S. and Dekay , H.G. 1962 . Stability of vitamins A, B1, and C in selected vehicle matrices . Journal of Pharmaceutical Sciences , 51 ( 11 ) : 1076
- Hiatt , A.N. , Ferruzzi , M.G. , Taylor , L.S. and Mauer , L.J. 2008 . Impact of deliquescence on the chemical stability of vitamins B-1, B-6, and C in powder blends . Journal of Agricultural and Food Chemistry , 56 ( 15 ) : 6471 – 6479 .
- Salameh , A.K. , Mauer , L.J. and Taylor , L.S. 2006 . Deliquescence lowering in food ingredient mixtures . Journal of Food Science , 71 ( 1 ) : E10 – E16 .
- Ortiz , J. , Ferruzzi , M.G. , Taylor , L.S. and Mauer , L.J. 2008 . Interaction of environmental moisture with powdered green tea formulations: Effect on catechin chemical stability . Journal of Agricultural and Food Chemistry , 56 ( 16 ) : 4691 – 4697 .
- Mauer , L.J. and Taylor , L.S. 2010 . Water-solids interactions: Deliquescence . Annual Review of Food Science and Technology , 1 : 41 – 63 .
- Hiatt , A.N. , Taylor , L.S. and Mauer , L.J. 2010 . Effects of co-formulation of amorphous maltodextrin and deliquescent sodium ascorbate on moisture sorption and stability . International Journal of Food Properties , 14 ( 4 ) : 726 – 740 .
- Fennema , O.R. 1996 . Food Chemistry , 3rd , New York : Marcel Dekker .
- Shephard , A.B. , Nichols , S.C. and Braithwaite , A. 1999 . Moisture induced solid phase degradation of L-ascorbic acid—Part 2. Separation and characterization of the major degradation products . Talanta , 48 ( 3 ) : 595 – 606 .
- Salameh , A.K. and Taylor , L.S. 2005 . Deliquescence in binary mixtures . Pharmaceutical Research , 22 ( 2 ) : 318 – 324 .
- Heudi , O. , Kilinc , T. and Fontannaz , P. 2005 . Separation of water-soluble vitamins by reversed-phase high performance liquid chromatography with ultra-violet detection: Application to polyvitaminated premixes . Journal of Chromatography A , 1070 ( 1–2 ) : 49 – 56 .
- Stevens , R. , Buret , M. , Garchery , C. , Carretero , Y. and Causse , M. 2006 . Technique for rapid, small-scale analysis of vitamin C levels in fruit and application to a tomato mutant collection . Journal of Agricultural and Food Chemistry , 54 ( 17 ) : 6159 – 6165 .
- Ross , K.D. 1975 . Estimation of water activity in intermediate moisture foods . Food Technology , 29 ( 3 ) : 26
- Windholz , M. 1983 . The Merck Index an Encyclopedia of Chemicals, Drugs, and Biologicals , 10th , Merck, NJ : Rahway .
- Mishelevich , A. and Apelblat , A. 2008 . Solubilities of magnesium-L-ascorbate, calcium-L-ascorbate, magnesium-L-glutamate, magnesium-D-gluconate, calcium-D-gluconate, calcium-D-heptagluconate, L-aspartic acid, and 3-nitrobenzoic acid in water . Journal of Chemical Thermodynamics , 40 ( 5 ) : 897 – 900 .
- Swern , D. 1949 . Solubility and specific rotation of L-ascorbyl palmitate and L-ascorbyl laurate . Journal of the American Chemical Society , 71 ( 9 ) : 3256
- Rose , R.C. 1987 . Solubility properties of reduced and oxidized ascorbate as determinants of membrane permeation . Biochimica et Biophysica Acta , 924 ( 1 ) : 254 – 256 .
- Deutsch , J.C. and SanthoshKumar , C.R. 1996 . Dehydroascorbic acid undergoes hydrolysis on solubilization which can be reversed with mercaptoethanol . Journal of Chromatography A , 724 ( 1–2 ) : 271 – 278 .
- Guerrieri , P. , Salameh , A.K. and Taylor , L.S. 2007 . Effect of small levels of impurities on the water vapor sorption behavior of ranitidine HCl . Pharmaceutical Research , 24 ( 1 ) : 147 – 156 .
- Ortiz , J. , Kestur , U. , Taylor , L.S. and Mauer , L.J. 2009 . Interaction of environmental moisture with powder green tea formulations: relationship between catechin stability and moisture-induced phase transformations . Journal of Agricultural and Food Chemistry , 57 ( 11 ) : 4691 – 4697 .
- Monsalve , A. , Powers , J.R. and Leung , H.K. 1990 . Browning of dehydroascorbic acid and chlorogenic acid as a function of water activity . Journal of Food Science , 55 ( 5 ) : 1425 – 1428 .
- Lee , Y.C. , Kirk , J.R. , Bedford , C.L. and Heldman , D.R. 1977 . Kinetics and computer-simulation of ascorbic-acid stability of tomato juice as functions of temperature, pH and metal catalyst . Journal of Food Science , 42 ( 3 ) : 640
- Weast , R.C. 1988 . CRC Handbook of Chemistry and Physics, 1st Student , CRC Press, FL : Boca Raton .
- Yuan , J.P. and Chen , F. 1998 . Separation and identification of furanic compounds in fruit juices and drinks by high-performance liquid chromatography photodiode array detection . Journal of Agricultural and Food Chemistry , 46 ( 4 ) : 1286 – 1291 .
- Adams , J.R. and Merz , A.R. 1929 . Hygroscopicity of fertilizer materials and mixtures . Industrial and Engineering Chemistry , 21 : 305 – 307 .
- Liu , Y. and Laskin , A. 2009 . Hygroscopic properties of CH3SO3Na, CH3SO3NH4, (CH3SO3)(2)Mg, and (CH3SO3)(2)Ca particles studied by micro-FTIR spectroscopy . Journal of Physical Chemistry A , 113 ( 8 ) : 1531 – 1538 .
- Mauer , L.J. and Taylor , L.S. 2010 . Deliquescence of pharmaceutical systems . Pharmaceutical Development and Technology , 15 ( 6 ) : 582 – 594 .
- Kirk , J. , Dennison , D. , Kokoczka , P. and Heldman , D. 1977 . Degradation of ascorbic-acid in a dehydrated food system . Journal of Food Science , 42 ( 5 ) : 1274 – 1279 .
- Singh , R.K. , Lund , D.B. and Buelow , F.H. 1983 . Storage stability of intermediate moisture apples—Kinetics of quality change . Journal of Food Science , 48 ( 3 ) : 939 – 944 .
- Salameh , A.K. and Taylor , L.S. 2006 . Role of deliquescence lowering in enhancing chemical reactivity in physical mixtures . Journal of Physical Chemistry B , 110 ( 20 ) : 10190 – 10196 .
- Tatum , J.H. , Shaw , P.E. and Berry , R.E. 1969 . Degradation products from ascorbic acid . Journal of Agricultural and Food Chemistry , 17 ( 1 ) : 38
- Niemela , K. 1987 . Oxidative and nonoxidative alkali-catalyzed degradation of L-ascorbic-acid . Journal of Chromatography , 399 : 235 – 243 .
- Gibbons , E. , Allwood , M.C. , Neal , T. and Hardy , G. 2001 . Degradation of dehydroascorbic acid in parenteral nutrition mixtures . Journal of Pharmaceutical and Biomedical Analysis , 25 ( 3–4 ) : 605 – 611 .
- Kurata , T. and Sakurai , Y. 1967 . Degradation of L-ascorbic acid and mechanism of nonenzymic browning reaction. 3. Oxidative degradation of L-ascorbic acid (degradation of dehydro-L-ascorbic acid) . Agricultural and Biological Chemistry , 31 ( 2 ) : 177