Abstract
Starch from the corms of local aroid cultivars (cv.) Colocasia esculenta (cv. Mukhia Kochu), Xanthosoma caracu (cv. Boga Dohi Kochu), Xanthosama sagittifolium (cv. Kola Dohi Kochu), and Amorphophallus paeonifolius (cv. Ool Kochu) were isolated and their morphology, composition, structure, and physicochemical properties were studied. The yield of starch was in the range of 10.23–14.81% (A. paeonifolius–C. esculenta) of fresh weight corm (peeled off). The scanning electron microscopy of isolated starch of Colocasia esculenta revealed its round polygonal shape with a diameter of 0.71–1.25 μm. The shape of the starch of Xanthosoma caracu and Xanthosama sagittifolium were found to be polygonal with a diameter of 1.25–2.21 and 2.1–2.84 μm, respectively. There was considerable variation among the four aroids with respect to total amylose content, amylose leaching, phosphorus and carbon content, susceptibility towards enzymes, and acid hydrolysis and crystalline nature. The smallest sized starch of Colocasia esculenta, with a yield of 13.81 ± 3.86% (fresh weight), showed great potential to be a source for baby food, fine printing paper, plastic sheets, carrier material for cosmetics, laundry stiffening agent, and other applications.
INTRODUCTION
Starch is considered as one of the strategic materials of the future. Its consumption is increasing and also the utilization of starch in industries is on the rise. The highest utilization of the biopolymer is in the paper, textile, and chemical industries where it serves as the raw material for the production of many items. Edible aroids are a subsidiary food in tropical and subtropical countries.[Citation1] It is important to determine the composition of food commodities for dietary purposes. Several authors evaluated the chemical composition of corms and cormels of the aroid species X. sagittifolium and C. esculenta.[Citation1] Starches from C. esculenta and X. sagittifolium were reported by Perez et al.[Citation2] Rasper et al.[Citation3] indicated that the compositions of food commodities are dependent on variety, location, season, method of processing and storage. In North East India, the starchy crop potato (Solanum tuberosum) is grown in the winter; taro (Colocasia and Xanthosoma spp.) and Amorphophallus spp (grown widely) are dominant in the rainy summer (March to August). Only a limited number of reports are available on systematic assessment of C. esculenta.[Citation4] Colocasia species originated from the North Eastern region of the country and dispersed to other parts of India and world.[Citation5] The corms of Colocasia esculenta (cv. Mukhia Kochu) are fused together, each having an independent petiole. The single corm is cone shaped; the fused corm does not influence the basic shape resulting in a broad upper side and cone-shaped distal part. Environment influences size and distribution of starch granules.[Citation6] The starch type depends on the environmental condition in which the plant grows. In Hordeum sp., the stress induced by high temperatures tends to reduce the number of A-type starch granules, but proportionately the effect is less in the case of B-type granule and it is hypothesized that small granules are formed in vesicles, bobbed off from out-growth of A-type granules containing amyloplast.[Citation7] The hypothesis was supported by the presence of B-type granules as revealed through transmission electron microscopy and confocal laser scanning microscopy. Raeker et al.[Citation8] reported the presence of significant difference in amylose content among aroid cultivars. The negative correlation between amylose content and starch granule size explains, at least in part, cultivar dependency of granule size. Starch, being a low-cost and renewable resource, could be used as fillers in the development of biodegradable polymer products.[Citation9] Starch with smaller granules shows a slightly higher viscosity in the starch-filled poly-hydroxyl-ester-ether composites.[Citation10] Plastic film incorporated with starch produces porous structures enhancing accessibility of plastic molecules to oxygen and microorganisms.[Citation11,Citation12] There are several reports on Colocasia and Xanthosoma starch granules, but information on Amorphophallus paeonifolius is limited.[Citation13] Gunaratne and Hoover[Citation14] provided information on starch physicochemical properties of X. sagittifolium. Sefa-Dehen and Sackey[Citation15] reported physical properties of starch in Colocasia and Xanthosoma species. Starch morphology and granule size are genetically controlled.[Citation16] It is also known that genetic variation and environmental condition influence structure and properties of starch. Therefore, an effort has been made to determine the granule size, crystalinity, composition, and physicochemical properties of starch present in different aroid species grown in NE India.
Xanthosoma species selected for the study were X. caracu (cv. Boga Dohi Kochu) and X. sagittifolium (cv. Kola Dohi Kochu). Both species differ in stem color, X. caracu light green and X. sagittifolium black color. Looking into edibility and medicinal use, the species Amorphophallus paeonifolius was selected. C. esculenta was selected as being the most widely cultivated species with a large number of cultivars.
MATERIALS AND METHODS
Corms from C. esculenta, X. caracu, X. sagittifolium, and A. paeonifolius were grown under constant field conditions at Tezpur University, Tezpur 784028, Dist. Sonitpur, Assam, India.
Chemicals
α-Amylase was purchased from Himedia Laboratories (Mumbai, India), potassium metabisulfite, sodium-chloride, iodine, potassium iodide, and hydrochloric acid were purchased from Merck India Limited (Bangalore, India).
Starch Granule Isolation
Mature corms were collected from uprooted plants. After uprooting, corms were washed thoroughly to remove the dirt and outer cover. The corms were then cut into squares of about 1–3 cm3, weighting 100 g, and soaked in a double volume of potassium-metabisulfite at the concentration of 50 mg.l−1. Starch isolation was done following the method of Jayakody et al.[Citation17] with minor modifications. The blended corm paste was suspended in a double volume of extraction solution. The suspension was filtered through a double-layered cheese cloth. The process was repeated twice. The filtrate was centrifuged at 1000 × g for 30 min at room temperature. The process was repeated four more times with double-distilled water. The upper brown layer contaminating proteins and polysaccharides was removed. The lower whitish part containing starch was isolated and washed with distilled water three more times to obtain pigment free white starch.
Proximate Composition Analysis
Proximate composition, such as moisture, ash, and lipids, was performed using the AACC method.[Citation18] The free lipids were extracted using chloroform:methanol (2:1, v/v) at 25°C. The bound lipid was estimated using the chloroform/methanol treated lipid extraction using n-propanol:water (3:1, v/v) for 7 h at 50°C. The total lipid was estimated by hydrolyzing starch in 24% HCL and then extracting thrice with an equal volume of chloroform:methanol (2:1, v/v) at 25°C. Total phosphorus was estimated by the method described by Morrison.[Citation19]
Scanning Electron Microscopic Examination of Starch Granules
To determine the starch granule size, scanning electron microscopy (SEM) of the starch was done using JOEL model no. JSM-6390LV (Oxford Instrumentation Ltd., Tokyo, Japan). For the analysis, the dried starch powder was sprinkled on the carbon tape and then coated with 30 nm platinum coat using JOEL auto fine coater (model no. JFC-1600; Oxford Instrumentation Ltd.). The SEM was operated at 15–20 KV (15 KV for A. paeonifolius, X. caracu, and X. sagittifolium; and 20 KV for C. esculenta) and under 1 Pascal pressure with the spot size fixed at 34.
Elemental Composition Analysis Using EDX of Starch Granules
Atomic compositions of the starches with respect to C, O, and P were determined using Energy Dispersive X-ray diffraction (EDX, micro-analysis). Samples were visualized under the scanning electron microscope (2,000×–4,000× magnifications) using X-ray source 0–20 KV for starch surface analysis with EDX software.
Amylose Content Estimation
Apparent and total amylose content was determined using the method described by Gunaratne and Hoover.[Citation14] Starch (20 mg, dry basis) was dissolved in 90% dimethylsulfoxide (8 ml) (DMSO) in screw cap reaction vials. The contents were vigorously mixed for 30 min and then heated in a water bath (shaking intermediately) at 85°C for 20 min. The vials were then cooled to ambient temperature and the contents were diluted with water to 25 ml in a volumetric flask. One milliliter of diluted solution was mixed with water (40 ml) and 5 ml of I2/KI solution (0.0025 M KI) and then adjusted to a final volume of 50 ml. After allowing the content to stand at ambient temperature, the absorption was measured at 600 nm. The total amylose content of the sample was determined by the above procedure, but with a defatting procedure before it with hot n-propanol:water (3:1, v/v) for 7 h. In order to correct overestimation of apparent and total amylase content, amylase content was calculated from a standard graph prepared using potato amylase and amylopectin over the range of 0–100% amylose and 100–0% amylopectin.
X-Ray Diffraction Study of the Starch Granules
The aroid starches were analyzed by a powder X-ray diffraction method for quantitative phase identification. The X-ray powder diffraction patterns were obtained using Miniflex goniometer (Rigaku Corporation, Tokyo, Japan) with scanning mode 2θ; scanning type being continuous; X-ray 30 KV/15 mA; divergence slit being variable; scattering slit 4.2°; receiving slit 0.3 mm; step 0.02 and using Kb filter from 10–40°. The starch was equilibrated at 100% relative humidity for 24 h at 25°C prior to analysis. The equation of the degree of crystallinity is as follows:
Starch Granule Damage Estimation
The method followed for estimating starch damage was based on the principle that amylose leached more rapidly from damage starch granule when extracted by sodium sulphate as compared to sound starch granules. Starch (1 g) was extracted with 25 ml of extraction solution (sulphosalicylic acid 2 g/1000 ml 1.41 M sodium sulphate) for 15 min at 50°C with thorough shaking at 5 min intervals. Celite (0.25 gm) was added to the suspension followed by brief stirring. The mixture was allowed to stand for 1–2 min and was later filtered through Whatman no. 1 filter paper. A 10-ml aliquot from the above suspension was mixed with 10 ml of diluting solution (gelatin 50 g and 2.5 ml of H2O2 in 500 ml boiled distilled water) and 0.5 ml of iodine reagent (0.55 g I2 and 1.10 g KI in 25ml distilled water) was added. This mixture was kept in a water bath at 30°C for 15 min. The absorbance was measured at 560 nm against reagent blank. Fifty units of iodine absorbance value corresponded to 9% starch damage was used as standard for calculations.[Citation20]
Functional Group Analysis Using FT-IR Spectroscopy
The FT-IR spectra of the purified starch were assessed in the wave length range of 4000–400 cm−1. A pure grade commercial starch sample (Merck India Ltd., Mumbai, India) was used as the control.
Gelatinization Parameter Analysis Using DSC
Gelatinization parameter was measured using DSC equipped with thermal analysis data recording software. Water (11 μl) was added with a microsyringe to starch (3 mg) in the DSC pan, which were then sealed, reweighed, and allowed to stand for 2 h at room temperature in order to attain an even distribution of water. The scanning temperature range and heating rates were 20–120°C/min, respectively. An empty aluminium pan thermogram was taken as reference in all measurements. The transition temperature reported is the onset (To ), peak (Tp ), and conclusion (Tc ). The enthalpy of gelatinization (ΔH) was estimated by integrating the area between the thermogram and baseline under the peak and expressed in joul per unit weight of dry starch (J/gm).
Water Binding Capacity Analysis
The procedure described by Sugimoto et al.[Citation21] was used with slight modification. A starch (5 gm) was added to 75 ml distilled water in 100 ml centrifuge bottle. The bottle was stoppered and agitated on a magnetic stirrer for 1 h, then centrifuged for 10 min at 2200 g. The water was decanted and the bottle was allowed to further drain for 10 min and weighed. The amount of water held by starch was determined. The binding capacity was calculated from the formulae:
Amylose Leaching Colorimetric Measurement
Amylose leaching was assessed by heating the isolated starch (2 mg ml−1 deionized water) at 50, 60, 70, and 80°C for half an hour.[Citation17] The tubes were centrifuged at 2000 × g for 10 min. The supernatant (4 ml) was used to determine amylose content by the method of Gunaratne and Hoover.[Citation14]
Acid Hydrolysis of Starch Granules
Starch was hydrolyzed in triplicate with 2.2 M HCl (1 g starch/40 ml, 2.2 M HCl) at 35°C in a water bath (GE Health Care, Piscataway, USA) for a period of 15 days. The starch slurry was vortexed daily to re-suspend the deposited starch granules. Aliquots taken for analysis during the interval were neutralized with 2.2 M NaOH and centrifuged (2000 × g, 10 min). The amount of total reducing sugar (glucose equivalent) in the supernatant was determined by Somogyi-Nelson method.[Citation22] The extent of hydrolysis was calculated by using the formula given by Jayakody et al.:[Citation17]
Enzymatic Hydrolysis of Starch Granules
Enzymatic digestibility of starch was assessed using an amorphous suspension of α-amylase in 2.9 M sodium chloride containing 3 mM calcium chloride (Himedia, Mumbai, India), in which the concentration of α-amylase was 32 mg protein ml−1 and specific activity 1,122 U.mg−1 protein. Starch granules (20 mg dry weight) were suspended in 10 ml 0.02 M phosphate buffer (pH 6.9) containing 0.006 M NaCl. α-Amylase 5.5 μl was added, the mixture gently mixed, and digested at 37°C in a water bath (New Brunswick Scientific, Enfield, CT, USA) for 72 h. The reaction mixture was vortexed regularly at an interval of 12 h to suspend the deposited granules. The digestion reaction was terminated by adding 5 ml of absolute ethanol. The hydrolysate was recovered by centrifugation at 2000 × g for 5 min. Aliquots of the supernatant were analyzed for reducing sugar (maltose) content following the method of Somogyi-Nelson:[Citation22]
Statistical Analysis
All the experiments were repeated three times in triplicate. Statistical analysis was done using the origin 6.1-Scientific Graphing and Data Analysis Software (OriginLab Corporation, Northampton, MA, USA) as well as Microsoft Excel 2007(Microsoft Corporation, Redmond, WA, USA).
RESULTS AND DISCUSSION
Chemical Composition of Aroid Starch Granules
Total moisture, ash, amylose, carbon, oxygen and phosphorus content in all four species were analyzed and the data obtained are presented in . Total moisture, ash, amylose, carbon, oxygen and phosphorus content in all four species were analyzed and the data obtained are presented in . As observed in the present investigation the starch granule size of A. paeonifolius (5–12 μm) () was found to be smaller as reported by Hoover (3–30 μm).[Citation1] In the case of C. esculenta, the observed amylose content of 22.4 ± 4.5% () against 21.4% reported by Hoover[Citation1] both of which were lower as compared to the observation done by Perez et al. (30.62 ± 0.16 %).[2] The total lipid content of C. esculenta was reported as 0.39% against observed value of 0.09 ± 0.02% (), which was found to be very low. The moisture content of C. esculenta and X. sagittifolium starch were observed to be 11.2 ± 0.74 and 12.2 ± 1.2% (), respectively, against the earlier reported value of 14.01 ± 0.05 and 13.43 ± 0.01%.[Citation2] The ash content of C. esculenta and X. sagittifolium starch were found to be 1.3 ± 0.6 and 1.2 ± 0.8%, respectively, against the earlier reported value of 0.31 ± 0.01 and 0.20 ± 0.04%.[Citation2]
Table 1 Chemical composition of aroid corms on dry weight basis; shape and size of starch granules
Starch Granule Morphology
In protein-starch separation, the small starch granules are entrapped in proteins and fine fiber sediments generated during centrifugation.[Citation11,Citation23,Citation24] These problems are encountered in case of C. esculenta and X. caracu. After the centrifugation, a dark brown layer is observed on top of the white starch. The upper layer is scraped off, but it causes loss of some small granules.[Citation23] The desiccated free flowing starch after purification was studied by FT-IR analysis using the commercial starch as the control. The size of the isolated starch granules was measured using scanning electron microscope (JOEL) at 2,000–4,000× magnification (at 15 kV and 20 kV) are shown in and data obtained are presented in . In the case of X. sagittifolium, the starch granule size ranges from 2.1–2.84 μm and was very small as compared to 2.0–12.5 μm as reported by Pérez et al.[Citation2] In case of C. esculenta, Perez et al.[Citation2] found the granule size of 0.5–5.0 μm, which was very high as compared to that of 0.71–1.25 μm as observed in the present investigation.
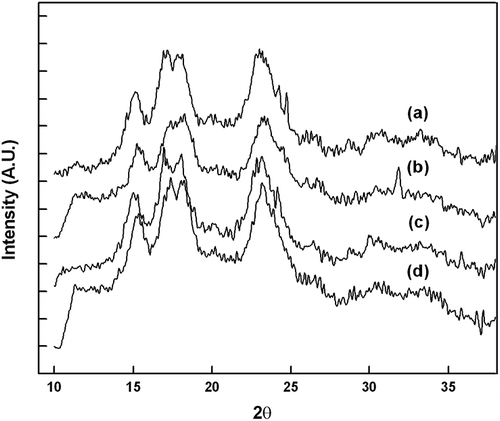
X-Ray Pattern and Crystallinity of Starch Granules
Starch is broadly divided into two types A and B; both are based on parallel standard double helixes. The A-type starch helixes are more closely packed as compared to B-type. The type of starch (A or B) can be determined by X-ray diffraction studies. The starch granules of all four aroids were subjected to XRD and the pattern obtained is presented in . A-type starch (mostly cereals) exhibits reflection at 15.3, 17.0, 18.0, 20.0, and 23.4° 2θ angles. They also differ (B > A) in the content of intra-helical water.[Citation25] The double helices of A and B-type starch are packed in a pseudo hexagonal array. The lattice associated with B type starch has a large void (channel), which can accommodate 36 water molecules. However, in A-type starch, the lattice contains a helix in the center rather than a column of water. In both A and B-type starch, there is a spacing of double helix that corresponds to 1.1 nm distance between the axes of the two double helices.[Citation18] Aroid species mostly exhibit A-type starch pattern except an extra peak at 31.9° (2θ) in the case of C. esculenta. The crystallinity of the sarch granules were found in the order of A. paeonifolius > X. caracu > X. sagittifolium > C. esculenta.
Functional Group Detected Using FT-IR Spectroscopy
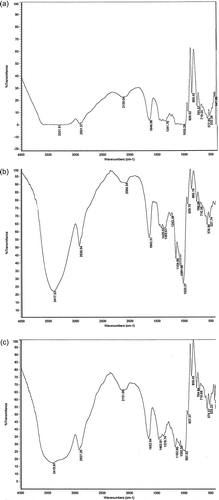
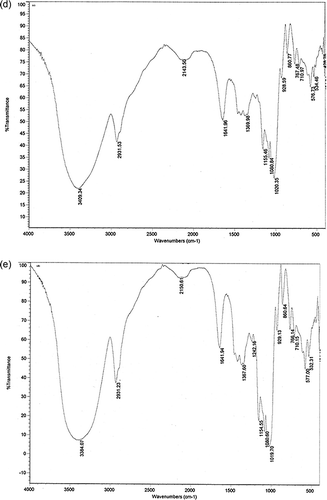
The starch of all four aroids was assessed using FT-IR (Nicolet) to determine the presence of functional groups leading to structure elucidation. Data obtained are graphically presented in . The wide band observed at 3331.91 cm−1 could be attributed to O–H bond stretching of the starch and its width was ascribed to the formation of inter and intramolecular hydrogen bonds, which were observed maximum in A. paeonifolius. Similar type of observation was also reported by Dragunski and Pawlicka.[Citation26] The characteristic peak between 1019 and 1156 cm−1 attributed to C–O bond stretch in C–O–C of bonding[Citation27] and the peaks near 1081 and 1154 cm−1 could be attributed to C–O stretch in C–O–H bonding. The peak near 1154 was not very prominent in A. paeonifolius. The peak near 2930 cm−1 might be attributed to the asymmetric stretching of C–H, while the band near 1644 cm−1 was ascribed to the adsorbed water and the bands near 1420 and 1368 cm−1 to the angular deformation of C–H; the later one was prominent in A. paeonifolius. The IR spectra not only revealed the purity of the starch isolated but also the functional groups present in the starch of aroid species.
Figure 3 FT-IR patterns of the purified aroid starches along with commercial starch from Merck (India) showing their purity: (a) A. aeonifolius, (b) X. caracu, (c) C. esculenta, (d) X. sagittifolium, (e) Starch soluble (Merck). (Continued)
As seen in the , the gelatinization parameters of the aroid satches vary greatly from species to species. The order of ΔH was found to be A. paeonifolius > C. esculenta > X. sagittifolium > X. caracu > starch soluble. The order of TP was found to be C. esculenta > A. paeonifolius > X. caracu > X. sagittifolium > starch soluble. The ΔH generated due to the thermal decomposition of the starch granules which suggests the complexity of the starch copolymer.
Table 2 DSC analysis data of the aroid and commercial starch
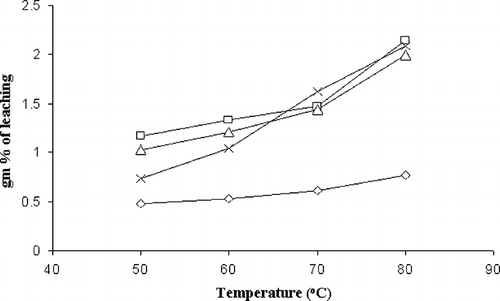
Amylose Leaching Recorded Using Colorimetric Method
The small granule-starch tend to leach more amylose out of the intact granules at temperature 50°C and above than larger granules.[Citation6] The amount of amylose leached was quantified through iodine test and data thus obtained for all four aroids are presented graphically in .
Acid Hydrolysis Results
Starch granules from the aroid species were hydrolyzed for 15 days with 2.2 M HCl at 35°C and the reducing sugar was estimated as per the standard method and are presented graphically in . The amorphous region of the starch was degraded during initial days, which were followed by slow degradation of the crystalline region. The difference in the extent of acid hydrolysis of starch might be attributed to granule size, interaction in relation to amorphous and crystalline regions, composition of starch in respect of phosphate content and amylose/amylopectin ratio.[Citation3] In B-type starch, α-1,6 branch is located mainly in the amorphous region making it very susceptible to acid hydrolysis; whereas, in A-type starch, α-1,6 branch is located in the crystalline region making it resistant to hydrolysis by H3O+. Starch hydrolysis could be explained on the basis of granule surface area and composition. Low amylose content and large granule size starch in A. paeonifolius as compared to other three aroid species might be responsible for the reduced hydrolysis during the initial and later periods. Several workers reported wide variation (9–25 days) in regard to the time period required for the degradation of the crystalline region by H3O+ depending on the source of starch.[Citation1,Citation28] Data suggested that during the hydrolysis, only amorphous regions were degraded by H3O+. But, the aroids did not possess sharp difference in their crystallinity. Difference in the rate and extent of acid hydrolysis
Figure 5 Acid hydrolysis of the aroid starches with respect to time (days): A. paeonifolius, ◊; X. sagittifolium, ×; X. caracu, □; C. esculenta, Δ.
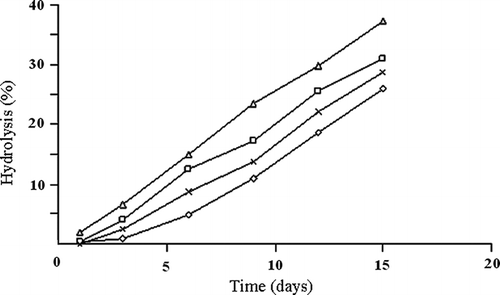
among starches has been attributed to granule size; interaction between starch chains;[Citation29] amylopectin chain length distribution;[Citation30] and phosphorus content.[Citation1] But, most of these factors were negated by the size of starch granule, which was prominently revealed by the result. Difference in hydrolysis among the aroid starches could be attributed to the interplay of granule size, phosphorus content, and total amylose content. The difference in the extent of hydrolysis among the aroids suggested the combined effect of these three factors. In X. caracu and X. sagittifolium, the difference was negated by the granule size; and the interaction of amylose-amylopectin might be responsible for the higher rate of hydrolysis of X. caracu. The extent of hydrolysis of aroid starch () was much lower than those reported in potato, cassava, yam and sweet potato,[Citation28] in which starch hydrolysis exceeded 70% after 12 days.[Citation14]
Enzymatic Hydrolysis Results
The susceptibility of aroid starch to α-amylase was analyzed and data obtained are presented in . The extent of hydrolysis follows the order C. esculenta > X. caracu > X. sagittifolium > A. paeonifolius. The difference in in vitro digestibility of starch among the species could be attributed to the interplay of many factors such as starch source, granule size, amylose/amylopectin ratio, extent of molecular association between starch chains, degree of crystallinity and unit cell structure.[Citation17] α-Amylase could simultaneously solubilize both amorphous and crystalline regions of the starch granules.
Starch damage was found to be in the order of A. paeonifolius > X. sagittifolium > C. esculenta > X. caracu. The damage of A. paeonifolius was found to be about double as compared to the others in the present investigation. Normally, amylose leaching increases due to starch damage. The starch damage 4.54% might be very small factor as compared to 5–12 μm granule size, which plays the prevalent role in the amylose leaching property in the present investigation. The one way ANOVA analysis suggested the starches of the aroids are different at of significance level, α = 1.0 with a p value of 0.99.
CONCLUSION
The results showed major difference in composition and physicochemical properties among aroid starch. However, variations were caused due to the interplay of factors like granule size, crystallinity, and phosphorus content. The small granule sized starch could be used in foods with minimum modifications. There is an increasing interest in starch manufactured from novel materials for use in special food products like baby food. Starch having small and narrow granule size, as found in the C. esculenta could be used in fine printing paper and plastic sheets, as a binder with orally active ingredients, and as a carrier material in cosmetics. Micro-granular starches are suitable for one-layer-thick honeycomb coatings and thus, C. esculenta starch could be used in the cosmetic, paper, textile and photographic industries. A well-established use of small granule starch, mainly rice starch, is as a cold-water laundry-stiffening agent. The small granule size affords superior penetration of starch into the fabric. The stiffness of textiles and fabrics so treated is less affected by humidity than those treated with other starches.[Citation6] Study was going on for the synthesis of edible films for food coating. The thrust of starch research can be estimated from the present research going on in the field of acid thinning, resistant starch, starch extrudates, starch-gum, starch blend and thermal treatment to starch.[Citation30–35 The starches analyzed here were not reported earlier from this biodiversity rich and origin land of C. esculenta.
REFERENCES
- Hoover , R. 2001 . Composition, molecular structure, and physicochemical properties of tuber and root starches: A review . Carbohydrate Polymers , 45 ( 3 ) : 253 – 267 .
- Pérez , E. , Schultz , F.S. and de Delahaye , E.P. 2005 . Characterization of some properties of starches isolated from Xanthosoma sagittifolium (tannia) and Colocassia esculenta (taro) . Carbohydrate Polymers , 60 : 139 – 145 .
- Rasper , V. and Coursey , D.G. 1996 . Properties of starches of some West African yams . Journal of the Science of Food and Agriculture , 18 : 240 – 244 .
- Lakhanpaul , S. , Velayudhan , K.C. and Bhat , K.V. 2003 . Analysis of genetic diversity in Indian taro [Colocasia esculenta (L.) Schott] using random amplified polymorphic DNA (RAPD) markers . Genetic Resources and Crop Evolution , 50 : 603 – 609 .
- Kuruvilla , K.M. and Singh , A. 1981 . Karyotypic and electrophoretic studies on taro and its origin . Euphytica , 30 ( 2 ) : 405 – 413 .
- Lindeboom , N. , Chang , P.R. and Tyler , R.T. 2004 . Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: A review . Starch/Stärke , 56 ( 3–4 ) : 89 – 99 .
- Langeveld , S.M.J. , Wijk , R.V. , Stuurman , N. , Kijne , J.W. and dePater , S. 2000 . B-type granule containing protusions and interconnections between amyloplasts in developing wheat endosperm revealed by transmission electron and GFP expression . Journal of Experimental Botany , 51 : 1357 – 1361 .
- Raeker , M.O. , Gaines , C.S. , Finney , P.L. and Donelson , T. 1998 . Granule size distribution and chemical composition of starches from 12 soft wheat cultivars . Cereal Chemistry , 75 : 721 – 728 .
- Ratto , J.A. , Stenhouse , P.J. , Auerbach , M. , Mitchell , J. and Farrell , R. 1999 . Processing, performance and biodegradability of a thermoplastic aliphatic polyester/starch system . Polymer , 40 ( 24 ) : 6777 – 6788 .
- Zhou , G. , Willett , J.L. , Carriere , C.J. and Wu , Y.V. 2000 . Effect of starch granule size on viscosity of starch-filled poly (hydroxy ester ether) composites . Journal of Polymers and the Environment , 8 ( 3 ) : 145 – 150 .
- Lim , S.T. , Lee , J.H. , Shin , D.H. and Lim , H.S. 1999 . Comparison of protein extraction solutions for rice starch isolation and effects on residual protein content on starch pasting properties . Starch/Stärke , 51 : 120 – 125 .
- Ahamed , N.T. , Singhal , R.S. , Kulkarni , P.R. , Kale , D.D. and Pal , M. 1996 . Studies on chenopodium quinoa and amaranthus paniculatas starch as biodegradable fillers in LDPE films . Carbohydrate Polymer , 31 : 157 – 160 .
- Rani , V.S. , John , J.K. , Moorthy , S.N. and Raja , K.C.M. 1998 . Effect of pretreatment of fresh Amorphophallus paeoniifolius on physicochemical properties of starch . Starch/Stärke , 50 ( 2–3 ) : 72 – 77 .
- Gunaratne , A. and Hoover , R. 2002 . Effect of heat-moisture treatment on the structure and physicochemical properties of tuber and root starches . Carbohydrate Polymers , 49 : 425 – 437 .
- Sefa-Dehen , S. and Sackey , E.K. 2002 . Starch structure and some properties of cocoyam (Xanthosoma sagittifoliun and Colocasia esculenta) starch and raphides . Food Chemistry , 79 : 435 – 444 .
- Jane , J.L. , Kasemsuwan , T. , Leas , S. , Ia , A. , Zobel , H. , Il , D. and Robyt , J.F. 1994 . Anthology of starch granule morphology by scanning electron microscopy . Starch/Stärke , 46 : 121 – 129 .
- Jayakody , L. , Hoover , R. , Liu , Q. and Donner , E. 2007 . Studies on tuber starches. II. Molecular structure, composition and physicochemical properties of yam (Dioscorea sp.) starches grown in Sri Lanka . Carbohydrate Polymers , 69 ( 1 ) : 148 – 163 .
- American Association of Cereal Chemists . 1984 . “ Method 76-30A ” . In Official Methods of the AACC , 8th , St. Paul , MN : The Association .
- Morrison , W.R. 1964 . A fast simple and reliable method for the micro determination of phosphorus in biological materials . Analytical Biochemistry , 7 : 218 – 224 .
- Medcalf , D.G. and Gilles , K.A. 1965 . Determination of starch damage by rate of iodine absorption . Cereal Chemistry , 42 : 546 – 557 .
- Sugimoto , Y. , Nishihara , K. and Fuwa , H. 1986 . Some properties of taro and yam starch . Journal of the Japanese Society of Starch Science , 33 : 169 – 176 .
- Somogyi , M. 1952 . Notes on sugar determination . Journal of Biological Chemistry , 195 : 19 – 23 .
- Andersson , A.A.M. , Andersson , R. and Aman , P. 2001 . Starch and byproducts from a laboratory-scale barley starch isolation procedure . Cereal Chemistry , 78 : 507 – 513 .
- Xie , X.J. and Seib , P.A. 2002 . Laboratory wet-milling of grain sorghum with abbreviated steeping to give two products . Starch/ Stärke , 54 : 169 – 178 .
- Imberty , A. 1988 . A revisit to the three dimenational structure of B-type starch . Biopolymers , 27 : 1205 – 1221 .
- Dragunski , D.C. and Pawlicka , A. 2001 . Preparation and characterization of starch grafted with toluene poly (propylene oxide) diisocyanate . Materials Research , 4 ( 2 ) : 77 – 81 .
- Fang , J.M. , Fowler , P.A. , Tomkinson , J. and Hill , C.A.S. 2002 . The preparation and characterization of a series of chemically modified potato starches . Carbohydrate Polymers , 47 : 245 – 252 .
- McPherson , A.E. and Jane , J.L. 1999 . Comparison of waxy potato with other root and tuber starches . Carbohydrate Polymers , 40 : 57 – 70 .
- Hoover , R. and Manuel , H. 1995 . A comparative study of the physicochemical properties of starches from two lentil cultivars . Food Chemistry , 53 : 275 – 284 .
- Srichuwong , S. , Sunarti , T.C. , Mishima , T. , Isono , N. and Hisamatsu , M. 2005 . Starches from different botanical sources. II. Contribution of starch structure to swelling and pasting properties . Carbohydrate Polymers , 62 : 25 – 34 .
- Singh , H. , Sodhi , N.S. and Singh , N. 2009 . Structure and functional properties of acid thinned sorghum starch . International Journal of Food Properties , 12 : 713 – 725 .
- Brumovsky , L.A. , Brumovsky , J.O. , Fretes , M.R. and Peralta , J.M. 2009 . Quantification of resistant starch in several starch sources treated thermally . International Journal of Food Properties , 12 : 451 – 460 .
- Yuliani , S. , Torley , P.J. and Bhandari , B. 2009 . Physical and processing characteristics of extrudates made from starch and d-limonene mixtures . International Journal of Food Properties , 12 : 482 – 495 .
- Ylmaz , M.T. , Sert , D. and Karakaya , M. 2009 . Rheological and sensory properties of spray dried Pekmez mixtures with wheat starch-gum . International Journal of Food Properties , 12 : 691 – 704 .
- Gupta , M. , Bawa , A.S. and Semwal , A.D. 2009 . Morphological, thermal, pasting, and rheological properties of barley starch and their blends . International Journal of Food Properties , 12 : 587 – 604 .
- Gupta , M. , Gill , B.S. and Bawa , A.S. 2008 . Gelatinization and X-ray crystallography of buckwheat starch: Effect of microwave and annealing treatments . International Journal of Food Properties , 11 : 173 – 185 .