Abstract
This study was conducted to search for novel natural bioactive compounds (peptides and phenolic compounds) with hypoglycemic, antioxidant, and anti-diabetic potential from barley protein fractions and isolates. Protein isolate and globulin, prolamin, glutein-1, and glutein-2 fractions of barley flour were extracted from barley flour. Free and bound phenolic compounds were extracted from protein isolate and protein fractions. Protein isolate and protein fractions from barley were subjected to pancreatic hydrolysis to obtain peptides. Peptide and both free and bound phenolic compounds were subjected to determine antioxidant and their potential to inhibit the key enzymes relevant to diabetes and hypertension using in vitro models. The extracted phenolic from prolamin fraction and protein isolate revealed the highest content of total phenolics (2.0–2.4 mg/g), antioxidant activity (65–73%), angiotensin converting enzyme-inhibitory activity (73–87%), and α-amylase inhibitory activity (57–77%) as compared to that of other protein fractions. Hydrolysates of prolamin fraction exhibited the highest antioxidant activity (77.7%) and angiotensin converting enzyme-inhibitory activity (61.3%) as compared to all other protein fractions and protein isolate. Poor correlations were obtained between total phenolic content, antioxididant activity, ACE-inhibitory activity, and α-amylase inhibitory activity of phenolic compounds from protein fractions and isolate. On the other hand, positive correlations were obtained between antioxididant activities, angiotensin converting enzyme-inhibitory activity, and the degree of hydrolysis of peptides from hydrolyzed protein fractions and protein isolate. Our findings indicated that prolamin fraction and protein isolate were recommended to be used as hypoglycemic, antioxidant, and anti-diabetic ingredients as potential candidates in functional, nutraceutical, and pharmaceutical industries.
INTRODUCTION
Grain barley has been considered to be an important food cereal in many parts of Asia, arid and the semi-arid areas. Barley (Hordeum vulgare L.) has many applications in the food industry, such as sponge cake, bread, brewing industry, and animal feeding.[Citation1,Citation2] Recently, blending of barley with human diets has been studied extensively due to the presence of β-glucan and phenolic compounds that reduce cholesterol and blood glucose levels.[Citation3,Citation4] Over the past years, increasing interest in barley food ingredients for human consumption has been observed due to the presence of bioactive compounds that are important to our health.[Citation5] Hydrolyses of food proteins is important for food, pharmaceutical, and nutraceutical industries. It plays a major role in the improvement of function, antioxidant properties, and nutritional properties of foods.[Citation6] Several studies have reported that bioactive peptides are generated from foods or from enzymatic digestion of food proteins, such as fish,[Citation7] milk,[Citation8] chickpea, and mushroom.[Citation9] Phenolic compounds are bioactive compounds that possess antioxidant properties that protect health against oxidation by products.[Citation10] The antioxidant compounds enhance the health benefits in humans. An example is the reduction of chronic diseases due to a decrease in the oxidative damage, which is caused by reactive oxygen species (ROS) to DNA, lipids, and proteins.[Citation11]
α-Amylase has been considered as the major enzyme that can control postprandial hyperglycemia.[Citation12,Citation13] The α-amylase was found in both salivary and pancreatic secretions and is responsible for hydrolyzing oligosaccharides to maltose.[Citation14] Diabetes diseases are frequently associated with hypertension, which may eventually cause cardiovascular disease.[Citation15] Angiogenesis converting enzyme (ACE) is responsible for the reduction of the hypertension by decreasing the excessive activation of the Renin Angiotensin System (RAS).[15,16] Several ACE inhibitors have been used in treating chronic diseases, such as cardiovascular disease. Therefore, ACE inhibitors are considered currently to be crucial in treating patients who suffer from high blood pressure.[Citation16,Citation17] The decrease in the absorption of glucose caused by the inhibition of the digestive enzymes would thus lead to the degradation of starch and oligosaccharides and inhibiting the increase in postprandial blood glucose and blood pressure.
The main objective of this study was to search for novel natural bioactive compounds (peptides and phenolic compounds) with hypo-glycemic, anti-oxidant, and anti-diabetic potential from barley protein fractions. The specific objectives of this study were to (1) evaluate the effect of barley extracted phenolic compounds from the isolated partial homogenous protein fractions on antioxidant activity, ACE inhibitory activity, and α-amylase inhibitory activity; and (2) evaluate the effect of barley peptide obtained from hydrolyzed isolated partial homogenous protein fractions through gastrointestinal enzymes on ACE inhibitory activity, degree of hydrolysis, and antioxidant activity.
MATERIALS AND METHODS
Plant Materials
Barley grains (ACSAD 178) were obtained from the Agriculture Research Center for Technology Transfer (Ar Ramtha, Jordan). The grain seeds were ground and sieved to remove the bran then stored in a plastic container at 4°C for further analysis.
Preparation of Protein Isolate from Barley
Proteins were extracted from 10 g of full-fat barley mixed with dilute NaOH (2 N, 100 ml). The mixture was adjusted to pH 11.0 and stirred at 23°C/60 min in a water bath, and then centrifuged (10,000× g, 15 min). The extract was filtered through cheese cloth. The supernatant was adjusted to pH 4.6 using diluted HCl (0.1 N). The precipitate protein were separated by centrifugation at (10,000× g, 15 min) and then freeze dried for further analysis.
Preparation of Partial Homogenous Protein Fractions from Barley
Extraction of barley proteins was carried out according to a modified procedure of Kwon et al.[Citation18] A sequential solvent extraction procedure with the following four solvents was used to extract proteins from full-fat barley, including 0.5 M NaCl, 0.1 M NaOH, 50% glacial acetic acid, and 70% ethanol. The extraction conditions were as follows: meal to solvent ratio of 1:10 (w/v) for 2 h at room temperature. Insoluble residue was removed by centrifugation at (10,000× g for 30 min). The resulting supernatant was collected and the residue was re-suspended in 0.5 M NaCl and with the same extraction steps were followed with 0.01 M NaOH, 50% acetic acid, and finally with 70% ethanol. The fractionated proteins from NaCl, NaOH, acetic acid, and ethanol were designated as globulin, glutein-1, glutein-2, and prolamin fractions, respectively. Each fraction was then lyophilized, and protein powders were stored at 4°C for further analysis.
Extraction of Free Phenolic Compounds from Barley and Proteins
A sample (1 g) of barley, protein isolate, and partial homogeneous protein fractions were extracted with 25 ml of methanol for 1 h at 24°C in a water bath and centrifuged (10,000× g, 10 min). The supernatant was filtered in a cheese cloth then flushed under a stream of nitrogen and stored at –18°C for further analysis. The phenolic compounds obtained by this method were designated as free phenolic compounds at room temperature (FP-23°C). The residue remaining from methanol extraction at room temperature was extracted with 25 ml methanol for 1 h at 60°C in a water bath and centrifuged (10,000× g, 10 min). The supernatant was filtered in a cheese cloth then flushed under a stream of nitrogen and stored at –18°C for further analysis. The phenolic compounds obtained by this technique were designated as free phenolic compounds using heat treatment (FP-60°C). The residue was kept for further extraction.
Extraction of Bound Phenolic Compounds from Barley and Proteins
The residue remaining from methanol/heat extraction described in the above section was hydrolyzed with diluted alkaline solution (25 ml, pH 12.0, 0.1 N NaOH) for 12 h at 24°C in a water bath with shaking in order to extract bound phenolic compounds, then the extract was centrifuged (10,000× g, 10 min). The supernatant was filtered using a cheese cloth. The bound phenolic compounds of lyophilized supernatant were extracted with 25 ml methanol for 1 h at 24°C in a water bath and centrifuged (10,000× g, 10 min). The phenolic compounds obtained by this technique were designated as bound phenolic compounds using base hydrolysis (BP-base). The supernatant was filtered in a cheese cloth and stored at –18°C for further analysis. The residue remaining after extraction was hydrolyzed with dilute acid solution (25 ml, pH 2.0, 0.1 N HCl) for 12 h at 24°C in a water bath with shaking in order to extract bound phenolic compounds. The bound phenolic compounds of lyophilized supernatant were extracted with 25 ml methanol for 1 h at 24°C in a water bath and centrifuged (10,000× g, 10 min). The supernatant was filtered in a cheese cloth and stored at –18°C for further analysis The phenolic compounds obtained by this technique were designated as bound phenolic compounds using acid hydrolysis (BP-acid).
Determination of Total Phenolic Contents
The Folin-Ciocalteu spectrophotometric method was used to determine the total phenolic compound content of each extract with some modifications; a standard curve was prepared using a stock solution (50 mg/50 ml) from gallic acid.[Citation19] Ten ml of the extract containing phenolic compounds were first diluted to the 50-ml mark of distilled water after adjusting the volume of methanol. A volume of 1 ml was taken for analysis, then 7.5 ml of distilled water was added. After that, 0.5 ml of Folin-Ciocalteu reagent was added and mixed well in the test tube, then after 4 min, 1 ml of 5% sodium carbonate (Na2CO3) was added. The contents were mixed, and after 1 h, the intensity of the green colour was measured at 725 nm in comparison to the standards. The total phenolic contents were expressed as milligrams of gallic acid equivalents per gram of dry matter (mg of GAE/g). The analysis was repeated three times for each extract.
Determination of the Antioxidant Activity
Antioxidant activity of phenolic extract was determined according to the method described by Lee et al.[Citation20] and Emmons et al.[Citation21] with some modifications. Five milligrams of β-carotene was dissolved in 50 ml of chloroform, and emulsion was prepared as follows: 3 ml β-carotene solution, 50 μl linoleic acid, and 400 mg Tween 20 were added. Chloroform was removed under a stream of nitrogen, and 100 ml of distilled water was added, then the mixture was shaken and saturated with oxygen. Three ml of the β-carotene/linoleic acid emulsion were mixed with 100 μl of sample solution and incubated in a water bath at 50°C for 60 min. Oxidation of the emulsion was monitored by measuring absorbance at 470 nm over 60 min. The control samples contained 100 μl of solvent instead of the sample and water, which were used as blank. The antioxidant activity (AA%) is expressed using EquationEq. (1):
where AA% is the antioxidant activity, DRc is the degradation rate of the control, DRc = (In (A/B)/60), DRs is the degradation rate in the presence of the sample and DRs = (In (A/B)/60), A is initial absorbance at time 0 min, and B is the absorbance after 60 min.
Hydrolysis of Proteins from Barley and Proteins
Protein isolates (1% in distilled water, pH 2.0, 0.1 N HCl) were hydrolyzed according to the method described by Megias et al.[Citation22] and Chen et al.[Citation23] with some modifications, using gastrointestinal enzymes (pepsin and trypsin; enzyme/substrate ratio 1:50 W/W). The mixture of pepsin enzyme and substrate was acidified to pH 2.0 using 0.1 N HCl, heated to 37°C and incubated and held for 1, 3, and 6 h followed by digestion with trypsin (1:1 W/W) for another 1, 3, and 6 h at pH 7.5 and 37°C. The mixture was centrifuged (10,000× g, 10 min), the supernatant was filtered with cheese cloth, cooled immediately, and then lyophilized.
Degree of Hydrolysis for Barley and Proteins
The degree of hydrolysis (DH) of hydrolyzed samples was measured according to the method described by Church et al.[Citation24] using the o-phthaldialdehyde (OPA) method. The OPA reagent was prepared daily by mixing 25 ml of 100 mM sodium tetraborate, 2.5 ml of 20% SDS (w/w), and 40 mg of OPA, which dissolved in 1 ml of methanol and 100 μl of β-mercaptoethanol followed diluting to a final volume of 50 ml of distilled water. A 50 μl of hydrolysates aliquot was added directly to 2 ml of OPA reagent in a cuvette and the solution was mixed and incubated for 2 min at room temperature. The DH was determined by measuring the absorbance at 340 nm. The percentage of DH was calculated by using EquationEq. (2):
where MW is the average of amino acids molecular weight (120), Δ340 nm is the absorbance at 340 nm, δ is the dilution factor (1/41), ϵ is the constant value (6000 m−1 cm−1), and ρ is the mg ml−1 (0.1%).
Measurement Activity of Digestive Enzymes for Carbohydrate (α-Amylase Inhibition)
The activity of α-amylase was determined according to the method described by McCue et al.[Citation25] with modifications. Starch solution [0.5% (w/v)] was prepared by mixing 0.125 g of potato starch in 25 ml of pH 6.9 in phosphate buffer at 65°C for 20 min. The α-amylase was prepared by mixing 0.030 g of the enzyme in 100 ml of distilled water. Phenolic extracts were dried and dissolved in phosphate buffer (1 mg/ml) to (12.5 μg/ml). Sodium potassium tartrate tetrahydrate (12.0 g) was mixed in 8.0 ml of 2 M NaOH. The sodium potassium tartrate solution was mixed with 96 nM of 2% 3, 5-dinitrosalicylic acid 1:1 (v/v). Forty μl of phenolic extracts and control were mixed with 400 μl starch solution and was left to react with 200 μl α-amylase solution in alkaline buffer at room temperature. The maltose concentration was measured after 3 min of reaction at 540 nm of absorbance. The activity of α-amylase was determined using EquationEq. (3):
Angiotensin Converting Enzyme (ACE) Inhibitory Activity
ACE inhibition was determined in vitro using the method previously described by Cushman and Cheung.[Citation26] Hip-His-Leu was prepared by dissolving in 50 mM HEPES-HCl buffer containing 300 mM NaCl (pH 8.3). Two hundred μl of Hip-His-Leu solution (0.3% w/v) was mixed with 80 μl of phenolic extracts and hydrolyzed proteins. ACE enzymes (0.33 U) was dissolved in 1 ml of distilled water. Fifty microliters of ACE was mixed to initiate the reaction followed by incubation for 15 min at 37°C. The reaction was stopped by the addition 250 μl of HCl (1 M). The hibburic acid librated from the reaction was removed from the solution using 2 ml of ethyl acetate. One ml of ethyl acetate layer was separated and evaporated by heating in boiling water for 15 min followed by the addition of 3 ml of distilled water. The absorbance of the sample was measured at 228 nm. For each tested hydrolyzed proteins and phenolic extracts, a blank was prepared by adding 250 μl HCl of 1 M before incubation. The reaction on ACE during the absorbance of inhibitors was carried out by replacing the protein extract with distilled water; a blank was also prepared. The amount of hippuric acid librated from the reaction in the absence of an inhibitor was defined as 100% ACE activity. The ACE activity was calculated as EquationEqs. (5) and Equation(6):
where 2 is the conversion factor since hippuric acid detected is 1/2 of the total amount produced in the assay (2 ml of ethylacetate is added, and 2 ml is removed for measurement) and 3 is the total volume of hippuric acid solution. The 9.8 is mill molar extinction coefficient of hippuric acid at 228 nm and 15 is the time (min) of the assay per the unit definition. The 0.91 is the extinication efficiency of ethyl acetate and 0.05 is the volume (ml) of used enzyme.
Statistical Analysis
Means were the average of three replicates. Data were analyzed using the general linear model (GLM) with the SAS Version 8.2 software package (SAS 2002 Institute Inc., Cary, NC, USA). Means were separated by LSD analysis at a least significant difference P value (0.05).
RESULTS AND DISCUSSION
illustrates the total content of free and bound phenolic compounds for extracts from barley flour, partial homogeneous protein fractions, and protein isolate. The total extracted free phenolic content ranged from 0.32 to 0.52 mg/g from barley flour, while the total extracted bound phenolic content ranged from 0.93 to 0.72 mg/g from barley protein isolate. For barley flour and their proteins, the total free phenolic extracted with no prior hydrolysis represents 33 to 46% of the total phenolic compounds. The remaining 67 to 84% was considered as BP extracted with hydrolysis. This suggests relatively high content of bound phenolic compound in barley and their proteins. The contents of FP-23°C extracts were varied significantly for all samples except for globulin and glutein-2 fractions that were not varied significantly (0.14 mg/g). The highest content of FP-23°C extract was obtained in prolamin fraction (0.20 mg/g) followed by barley flour, protein isolate, glutein-1 and glutein-2, and globulin fractions with values of 0.17, 0.16, 0.15, 0.14, and 0.14 mg/g of dry weight, respectively. While for FP-60°C extracted from barley flour and its proteins, the contents were varied significantly except for protein isolate and glutein-2 fraction. shows that the highest content of BP-base was extracted from protein isolate and barley flour with values of 0.72 and 0.69 mg/g, respectively, whereas the lowest content of BP-base was extracted from globulin and glutein-2 fractions with values of 0.21 and 0.29 mg/g. The content of BP-base was not varied significantly for barley flour and protein isolate. For all protein fractions, the contents of BP-base were varied significantly. The BP-acid extracted from barley flour and barley proteins were varied significantly. The highest content BP-acid was extracted from protein isolate and prolamin fraction with values of 1.00 and 1.36 mg/g, respectively, followed by barley flour, globulin, glutein-2, and glutein-1 fractions with values of 0.91, 0.47, 0.72, and 0.81 mg/g, respectively. The contents of phenolic compounds in barley cultivars were in the range between 0.18 to 42 mg/g for free phenolic compounds and from 2.0 to 3.4 mg/g for bound phenolic compounds.[Citation27] Nordkvist et al.[Citation28] reported that the insoluble bound phenolic acids in barley ranged from 0.8 to 0.88, 0.34 to 0.6, and 0.03 to 0.09 mg/g for husk, aleuronr layer, and endosperm, respectively. Liu and Yao[Citation29] found that the content of free phenolic compounds was 1.32–1.96 mg/g. The total free phenolic content varied from 1.03 to 1.87 mg/g in barley.[Citation30] To our knowledge, no information has been reported about the interaction and the distribution of free and bound phenolic compounds in protein isolates and protein fractions.
Table 1 Content of total free and bound phenolic compounds extracted from barley flour, partial homogeneous protein fractions, and protein isolate expressed as (mg/g DW)
shows the antioxidant activity of free and bound phenolic compounds for barley flour, partial homogeneous protein fractions, and protein isolates based on methanol extraction, methanol extraction/heat, alkaline hydrolysis, and acid hydrolysis carried out in sequence. Bound phenolic compounds that were extracted using alkaline hydrolysis and acid hydrolysis showed higher antioxidant activity from barley flours and all proteins fractions as compared to free phenolic compounds. The antioxidant activity of FP-23°C extracts has changed significantly for all samples studied. The change in the activity is drastic and lies within the range (∼46 to 56%) except for globulin and glutein 2 fractions for which the activity change was not significant and its value was fixed at ∼46%. The highest contents of FP-23°C were obtained in prolamin fraction (55.6%) and barley flour (52.7%). For FP-60°C extracted from barley flour and barley proteins, the contents were changed drastically within the range (52 to 70%) except for globulin and glutein 2 fractions (∼47%). The highest contents of BP-60°C were extracted from protein isolate (70.0%), barley flour (63.0%), and prolamin fraction (0.69%). The antioxidant activity of extracted BP-base has varied significantly for barley flour, protein isolate, and protein fractions. The maximum antioxidant activities of BP-base were in protein isolate (70.2%), barley flour (68.4%), and prolamin (66.8%), whereas the lowest antioxidant activity of BP-base was extracted from glutein 1 fraction (27.5%). The antioxidant activity of extracted BP-acid has varied significantly for all samples except for glutein 1 fraction and barley flour for which the activity was obtained to be ∼59%. The highest contents of BP-acid extracts were extracted from protein isolate, prolamin fraction, and barley flour and found to be 72.8, 65.4, and 60.4%, respectively. Zhao et al.[30] reported that the aqueous methanol extracts had antioxidant activity that lies within the range (50–65%) in barley flour. In colored barley, Kim et al.[Citation31] found that the antioxidant activities were varied from 46.4 to 86.3% in colored barley and from 66.5 to 70% in unhulled barley.
Table 2 Antioxidant activities (%) of free and bound phenolic compounds extracted from barley flour, partial homogeneous protein fractions, and protein isolate
shows the effects of free and bound phenolic compounds extracts from barley flour, partial homogeneous protein fractions, and protein isolates on ACE inhibitory activity. The ACE inhibitory activity of FP-23°C extract from either globulin and glutein 1 fractions or glutein 2 and prolamin fractions were found to vary significantly. The highest value of ACE inhibitory activity of FP-23°C extracted from prolamin fraction was 79.3%. The corresponding values of ACE activities for glutein 2, barley flour, isolate, gluelin 1, and globulin fractions are 71.7, 71.0, 65.0, 52.0, and 41.0%, respectively. The values of ACE inhibitory activity of FP-60°C extracted from barley flour, partial homogeneous protein fractions, and protein isolate were found to vary drastically. The highest value for ACE inhibitory activity of FP-60°C extracted from prolamin fraction was 82%, while the lowest value for ACE inhibitory activity of FP-60°C extracted from globulin fraction was 56.3%. Our results revealed that the maximum ACE inhibitory activity for BF-base extracted from prolamin fraction had values of 87.0 and 80.7%, respectively. These results indicate that the value of ACE inhibitory activity was not significant for BP-base extracted from globulin and glutein 1 fractions, whereas its value for BF-base extracted from glutein 2 and protein isolate were not significant and exhibits values in the range of ∼72–73%. We also found that for BP-acid extracted from globulin and glutein 2, the ACE inhibitory activities were not large. On the other hand, the BP-acid extracted from prolamin and protein isolate exhibits the highest ACE inhibitory activity that varies significantly within the range ∼78–81%. Thus, our results suggest that the extracted free and bound phenolic compounds from prolamin fractions show a higher ACE inhibitory activity as compared to other protein fractions.
Table 3 ACE inhibitory activity (%) of free and bound phenolic compounds extracted from barley flour, partial homogeneous protein fractions, and protein isolate
demonstrates the effects of free and bound phenolic compounds extracts from barley flour, partial homogeneous protein fractions, and protein isolate on inhibitory activity of α-amylase. Our results have demonstrated that α-amylase inhibitory activity for FP-23°C, FP-60°C, and BP-base extracts from all protein fractions exhibits significant values; however, for BP-acid extracted from globulin and glutein 2 fractions, it adopts values that do not vary significantly. The highest value of α-amylase inhibitory activity was obtained from prolamin fraction. It exhibits a value of 66.0% for FP-23°C, 68.0% for FP-60°C, 72.0% for BP-base, and 76.7% for BP-acid. The lowest values for α-amylase inhibitory activity were found in glutein 1 fraction. It adopts a value of 36.0% for FP-23°C, 39.3% for FP-60°C, 32.3% for BP-base, and 43.7% for BP-acid. In general, bound phenolic compounds exhibit higher values of α-amylase inhibitory activity as compared to free phenolic compounds in all protein fractions and isolates from barley flour. These results suggest that the extracted free and bound phenolic compounds from prolamin fractions adopt higher values of α-amylase inhibitory activity as compared to those extracted from other protein fractions.
Table 4 α-Amylase inhibitory activity (%) of free and bound phenolic compounds extracted from barley flour, partial homogeneous protein extracts, and protein isolate
indicates the effect of peptide obtained from hydrolyzed protein from barley on ACE inhibitory activity and antioxidant activity. The ACE inhibitory activity of peptide obtained from hydrolyzed protein from either globulin and glutein 2 fractions or glutein 2 and protein isolate fractions were found not to vary significantly. The highest value of ACE inhibitory activity of peptide was obtained in prolamine (61.5%), while the lowest value for ACE inhibitory activity of peptide was obtained from glutein fraction (33.0%). The antioxidant activity of peptide obtained from hydrolyzed protein was found to vary significantly for all protein fractions and protein isolates. The highest content of antioxidant activity of peptide was obtained from hydrolyzed prolamin fraction that adopts a value of 77.7%. The corresponding values for peptides extracted from globulin, protein isolate, barley flour, glutein 2, and glutein 1 were 54.3, 51.3, 47.0, 47.0, and 38.3%, respectively.
Table 5 ACE inhibitory activity (%) and antioxidant activity (%) of barley peptides hydrolyzed with combined pepsin and trypsin enzymes at 6 h extracted from barley flour, partial homogeneous protein fractions, and protein isolate
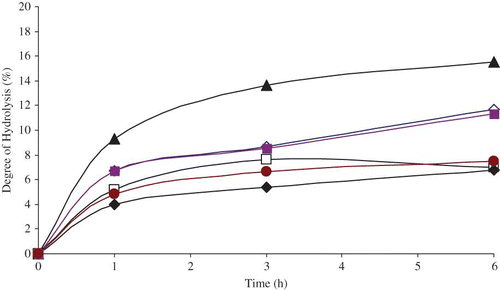
– show the effect of time on the degree of hydrolysis with selected pancreatic enzymes. With all samples, as the incubation time increases for pepsin, trypsin, and combined pepsin-trypsin enzymes, the extent of degree of hydrolysis increases. The optimum degree of hydrolysis was obtained for an incubation time of 6 h. For hydrolysis with pepsin enzyme, the highest degree of hydrolysis of 15.5% was obtained for protein isolate. Values of 12.5 and 12.4% were obtained for prolamin fraction and barley flour, respectively, for an incubation time of 6 h. The maximum degree of hydrolysis for trypsin exhibits values of 14.3, 11.8, and 12.8% at an incubation time of 6 h in prolamin, globulin, and protein isolate, respectively. For the combined pepsin-trypsin, the corresponding values obtained were 15.6, 11.7, and 11.3%, respectively. The lowest degrees of hydrolysis were obtained in glutein 2 fractions with values 7.3, 2.4, and 6.8% for pepsin, trypsin, and combined pepsin-trypsin, respectively, at an incubation time of 6 h.
Figure 1 Effect of incubation time of pepsin enzyme on degree of hydrolysis (DH) for barley flour and protein fractions: ◊ Globulin-1-fraction, □ Glutein-fraction, ♦ Glutein-fraction, Prolamin-fraction, Protein isolate, Barley flour. (Color figure available online.)
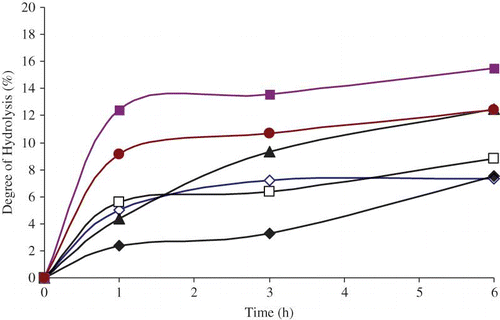
Figure 2 Effect of incubation time for trypsin enzyme on degree of hydrolysis (DH) for barley flour and protein fractions: ◊ Globulin-1-fraction, □ Glutein-fraction, ♦ Glutein-fraction, Prolamin-fraction, Protein isolate, Barley flour. (Color figure available online.)
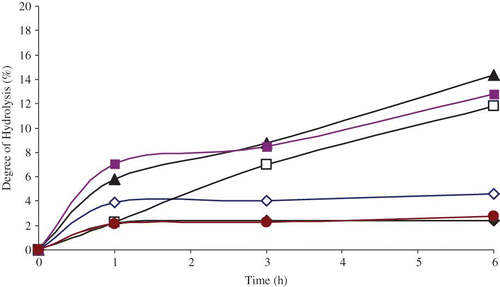
Figure 3 Effect of incubation time for combined pepsin and trypsin enzymes on degree of hydrolysis (DH) for barley flour and protein fractions: ◊ Globulin-1-Fraction, □ Glutein-Fraction, ♦ Glutein-Fraction, Prolamin-Fraction, Protein isolate, Barley flour. (Color figure available online.)
Correlation coefficients between total phenolic content, antioxidant activity, and ACE inhibitory activity and α-amylase inhibitory activity of phenolic compounds of barley are shown in . In general, total phenolics, ACE inhibitory activity, α-amylase inhibitory activity, and the antioxidant activity showed a positive correlation in all groups (). The ACE inhibitory of phenolic compounds was highly correlated with antioxidant activity and α-amylase inhibitory activity of phenolic compounds with correlation coefficients of 0.647 and 0.627, respectively. On the other hand, the total content of phenolic compounds had the lower positive correlation with antioxidant activity, ACE inhibitory, and α-amylase inhibitory of phenolic compounds () with correlation values of 0.524, 0.367, and 0.509, respectively. shows the correlation coefficients among degree of hydrolysis, antioxidant activity, and ACE inhibitory activity of barley peptides. The ACE inhibitory activity of peptide was highly correlated with antioxidant activity (0.919) and degree of hydrolysis (0.850). The degree of hydrolysis of peptides was positively correlated with antioxidant activity (0.940). These results suggested that the antioxidant activity, ACE inhibitory, and α-amylase inhibitory of these samples could be associated not only with the extracted phenolic compounds but with other compounds or it could be related to the content of individual phenolic acid.[Citation32–34] Several studies reported that the total phenolic content was positively correlated with antioxidant activity in wine.[Citation35–37] Cheplick et al.[Citation38] reported that antioxidant activity has weak correlation with α-amylase inhibitory activity in raspberry. Hassimotto et al.[Citation39] reported no correlation between total phenolic content and antioxidant activity. Minussi et al.[Citation37] found that the content of phenolic compounds was highly correlated with α-amylase inhibitory activity. Cheplick et al.[38] reported that the α-amylase inhibitory activity was not correlated with antioxidant activity. It has been found that ACE inhibitory activity was highly correlated with antioxidant activity, α-amylase inhibitory activity, and total phenolic contents.[Citation40] Kim et al.[31] found that the content of total phenolic compounds has a weak positive correlation with the antioxidant activity in barley. No correlation was observed between total phenolic content and the antioxidant activity in wheat extract.[40] Many studies found a positive correlation between phenolic content and antioxidant activity in olive oils, fruits, vegetables, grain and olive oils.[41,42]
Table 6 Correlation coefficients (r) between content, α-amylase inhibitory activity, antioxidant activity, and ACE inhibitory activity of phenolic compounds of barley proteins
Table 7 Correlation coefficients (r) between degree of hydrolysis, antioxidant activity and ACE inhibitory activity of barley peptides hydrolyzed with combined pepsin and trypsin enzymes at 6 h
CONCLUSIONS
In summary, our results suggested that protein fractions derived from barley grains could be used as hypoglycemic, antioxidant, and anti-diabetic potential candidates in several applications. The total phenolic content of bound phenolic compound was extremely (p < 0.05) higher than the content of free phenolic compounds for all protein fractions and isolate. However, protein hydrolysates, which were prepared by hydrolysis of prolamin fraction using pancreatic hydrolysis with combined pepsin and trypsin enzymes to obtain peptides, yielded the highest antioxidant activity and ACE-inhibitory activity as compared to all other protein fractions and protein isolate. The most striking result we found was that the extracted bound phenolic from prolamin fraction and protein isolate showed the highest content of total phenolics, antioxidant activity, ACE-inhibitory activity, and α-amylase inhibitory activity as compared to those extracted from all other protein fractions. As a result of this detailed study, hydrolyzed peptide and both free and bound phenolic compounds from barley prolamin fraction as well as other barley protein fractions and protein isolate could be utilized as natural potential active ingredients for the natural hypertension, antioxidant, and anti-diabetic products in the modern food industry.
REFERENCES
- Gupta1 , M. , Bawa , A.S. and Semwal , A.D. 2009 . Effect of barley flour incorporationon the instrumental texture of sponge cake . International Journal of Food Properties , 12 : 243 – 251 .
- Ereifej , K.I. , Al-Mahasneh , M.A. and Rababah , T.M. 2006 . Effect of barley flour on quality of Balady bread . International Journal of Food Properties , 9 : 39 – 49 .
- Newman , R.K. , Lewis , S.E. , Newman , C.W. , Boik , R.J. and Ramage , R.T. 1989 . Hypochoesterolemic effect of barley foods on healthy men . Nutritional Reports International , 39 : 749 – 760 .
- Cavallero , A. , Empilli , S. , Brighenti , F. and Stanca , A.M. 2002 . High-β-glucan barley fractions in bread making and their effects on human glycemic response . Journal of Cereal Science , 36 : 59 – 66 .
- Manach , C. , Scalbert , A. , Morand , C. , Remesy , C. and Jimenez , L. 2004 . Polyphenols: Food sources and bioavailability . American Journal of Clinical Nutrition , 79 : 727 – 747 .
- Wang , W. and Gonzalez de Mejia , E. 2005 . A new frontier in soy bioactive peptides that may prevent age-related chronic diseases . Comprehensive Reviews in Food Science and Food Safety , 4 : 63 – 68 .
- Kohama , Y. , Matsumoto , S. , Oka , H. , Teramoto , T. , Okabe , M. and Mimura , T. 1988 . Isolation of angiotensin I-converting enzyme inhibitor from tuna muscle . Biochemical and Biophysical Research Communication , 156 : 332 – 337 .
- Pihlanto-Leppala , A. 2001 . Bioactive peptides derived from bovine whey proteins: Opioid and ACE-inhibitory peptides . Trends food Science and Technology , 11 : 347 – 356 .
- Ma , M.S. , Bae , I.Y. , Lee , H.G. and Yang , C.B. 2006 . Purification and identification of angiotensin I-converting enzyme inhibitory peptide from buckwheat (Fagopyrum esculentum Moench) . Food Chemistry , 96 : 36 – 42 .
- Gutteridge , J.M.C. and Halliwell , B. 1994 . Antioxidants in Nutrition, Health and Disease , 111 – 123 . New York : Oxford University Press .
- Shahidi , F. and Naczk , M. 2004 . Phenolic in Food and Nutraceutical , 1 – 558 . Boca Raton , Florida, USA : CRC Press .
- Ali , H. , Houghton , P.J. and Soumyanath , A. 2006 . α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus . Journal of Ethnopharmacology , 107 : 449 – 455 .
- Lee , Y.A. , Cho , E.J. , Tanaka , T. and Yokozawa , T. 2007 . Inhibitory activities of proanthocyanidinis from persimmon against oxidative stress and digestive enzyme related to diabetes . Journal Nutritional Science Vitaminology , 53 : 287 – 292 .
- Ramasubbu , N. , Ragunath , C. , Mishra , P.J. , Thomas , L.M. , Gyemant , G. and Kandra , L. 2007 . Human salivary alpha-amylase Trp58 situated at subsite-2 is critical for enzyme activity . European Journal Biochemistry , 271 : 2517 – 2529 .
- Segura , J. and Ruilope , L.M. 2006 . Antihypertensive therapy in patients with metabolic syndrome . Current Opinion Nephrology Hypertension , 15 : 493 – 497 .
- Shlipak , M.G. , Browner , W.S. , Noguchi , H. , Massie , B. , Frances , C.D. and McClellan , M. 2001 . Comparison of the effects of angiotensin converting-enzyme inhibitors and beta blockers on survival in elderly patients with reduced left ventricular function after myocardial infarction . American Journal of Medicine , 110 : 425 – 433 .
- Remuzzi , G. and Ruggenenti , P. 2006 . Overview of randomised trials of ACE inhibitors . Lancet , 368 : 555 – 556 .
- Kwon , K. , Park , K.H. and Rhee , K.C. 1996 . Fractionation and characterization of proteins from coconut (Cocos nucifera L.) . Journal of Agriculture and Food Chemistry , 44 : 1741 – 1747 .
- Hoff , J.E. and Singleton , K.I. 1977 . A method for determination of tannins in foods by means of immobilized protein . Journal of Food Science , 42 : 1566 – 1569 .
- Lee , Y. , Howard , L.R. and Villalón , B. 1995 . Flavonoids and antioxidant activity of fresh pepper (Capsicum annuum) cultivars . Journal of Food Science , 60 : 473 – 476 .
- Emmons , C.L. , Peterson , D.M. and Paul , G.L. 1999 . Antioxidant capacity of oat (Avena sativa L.) extracts. 2. In vitro antioxidant activity and contents of phenol and tocol antioxidants . Journal of Agriculture and Food Chemistry , 47 : 4894 – 4898 .
- Megias , C. , del Mar , Y , Pedroche , J. , Lquari , H. , Giron-Calle , J. , Alaiz , M. , Millan , F. and Vioque , J. 2004 . Purification of an ACE inhibitory peptide after hydrolysis of sunflower (Helianthus annuus L.) protein isolates . Journal of Agricultural and Food Chemistry , 52 : 1928 – 1932 .
- Chen , H.-M. , Muramoto , K. and Yamauchi , F. 1995 . Structural analysis of antioxidative peptides from soybean b-conglycinin . Journal of Agricultural and Food Chemistry , 43 : 574 – 578 .
- Church , F.C. , Swaisgood , H.E. , Porter , D.H. and Catignani , G.L. 1983 . Spectrophotometric assay using o-Phthaldialdehyde for determination of proteosis in milk and isolated milk proteins . Journal of Dairy Science , 66 : 1219 – 1227 .
- McCue , P. , Kwon , Y.I. and Shetty , K. 2005 . Anti-amylase, anti-glucosidase and anti-angiotensin I-converting enzyme potential of selected foods . Journal of Food Biotechnology , 29 : 278 – 294 .
- Cushman , D.W. and Cheung , H.S. 1971 . Spectrometric assay and properties of the angiotensin-converting-I-enzyme of rabbit lung . Biochemical Pharmacology , 20 : 1637 – 1648 .
- Madhujith , T. and Shahidi , F. 2009 . Antioxidative potential of barley as affected by alkaline hydrolysis and release of insoluble-bound phenolics . Food Chemistry , 117 : 615 – 620 .
- Nordkvist , E. , Salomonssona , A. and Aman , P. 1984 . Distribution of insoluble bound phenolic acids in barley grain . Journal of the Science of Food and Agriculture , 35 : 657 – 661 .
- Liu , Q. and Yao , H.Y. 2007 . Antioxidant activities of barley seeds extracts . Food Chemistry , 102 : 732 – 737 .
- Zhao , H. , Fan , W. , Dong , J. , Lu , J. , Chen , J. , Shan , L. , Lin , Y. and Kong , W. 2008 . Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties . Food Chemistry , 107 : 296 – 304 .
- Kim , M.J. , Hyun , J.N. , Kim , J.A. , Park , J.C. , Kim , M.Y. , Kim , J.G. , Lee , S.J. , Chun , S.C. and Chung , I.M. . 2007 . Relationship between phenolic components, anthocyanins content and antioxidant activity in colored barley germplasm . Journal of Agriculture and Food Chemistry , 55 : 4802 – 4809 .
- Kwon , Y.I. , Vattem , D.V. and Shetty , K. 2006 . Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension . Asia Pacific Journal of Clinical Nutrition , 15 : 107 – 118 .
- Lee , J. , Bae , I.Y. , Lee , H.G. and Yang , C. 2006 . Tyr-Pro-Lys, an angiotensin-I-converting enzyme inhibitory peptide derived from broccoli (Brassica oleracea Italica) . Food Chemistry , 99 : 143 – 148 .
- Gouda , K.G.M. , Gowda , L.R. , Rao , A.G.A. and Prakash , V. 2006 . Angiotensin-I-converting enzyme inhibitory peptide derived from glycinin, the 11S globulin of soybean (Glycine max) . Journal of Agriculture and Food Chemistry , 54 : 4568 – 4573 .
- Fogliano , V. , Verde , V. , Randazzo , G. and Ritieni , A. 1999 . Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines . Journal of Agriculture and Food Chemistry , 47 ( 3 ) : 1035 – 1040 .
- Henn , T. and Stehle , P. 1998 . Total phenolics and antioxidant activity of commercial wines, teas and fruit juices . Ernahrungs-Umschau , 45 ( 9 ) : 308 – 313 .
- Minussi , M. , Rossi , L. , Bologna , L. , Cordi , D. and Rotilio , G.M. 2003 . Phenolic compounds and total antioxidant potential of commercial wines . Food Chemistry , 82 : 409 – 416 .
- Cheplick , S. , Kwon , Y. , Bhowmik , P. and Shetty , K. 2007 . Clonal variation in raspberry fruit phenolics and relevance for diabetes and hypertension management . Journal of Food Biochemistry , 31 : 656 – 679 .
- Hassimotto , N.M.A. , Genovese , M.I. and Lajolo , F.M. 2005 . Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps . Journal of Agriculture and Food Chemistry , 53 : 2928 – 2935 .
- Blair , R. and Reichert , R.D. 1984 . Carbohydrate and phenol constituents in a comprehensive range of rapeseed and canola fractions: Nutritional significant for animals . Journal of the Science of Food and Agriculture , 35 : 29 – 35 .
- Yu , L. , Haley , S. , Perret , J. , Harris , M. , Wilson , J. and Qian , M. 2002 . Free radical scavenging properties of wheat extracts . Journal of Agriculture and Food Chemistry , 50 : 1619 – 1624 .
- Gorinstein , S. , Martin-Belloso , O. , Katrich , E. , Lojek , A. , Ciz , M. , Gligelmo-Miguel , N. , Haruenkit , R. , Park , Y. , Jung , S. and Trakhtenberg , S. 2003 . Comparison of the contents of the main biochemical compounds and the antioxidant activity of some Spanish olive oils as determined by four different radical scavenging tests . Journal of Nutritional Biochemistry , 14 : 154 – 159 .