Abstract
The presence of sesame oil in extra virgin olive oil has been investigated using Fourier transform infrared spectroscopy and gas chromatography. Frequencies of 1207–1018, 1517–1222, and 3050–2927 cm−1 were chosen for quantification of sesame oil in extra virgin olive oil. Using Fourier transform infrared normal spectra coupled with a partial least square model, the root mean standard error of calibration and root mean standard error of prediction obtained were relatively low, i.e., 0.331 and 1.01% (vol/vol), respectively. Using fatty acid profiles as determined by gas chromatography, the levels of palmitic and oleic acids were decreased linearly with R2 of 0.969 and 0.934, meanwhile the levels of stearic and linoleic acids were increased with R2 of 0.930 and 0.959, respectively, with the increasing levels of sesame oil. From level 10% sesame oil (vol/vol), all these fatty acids are significantly different (p < 0.05).
INTRODUCTION
The authenticity of highly edible oils is of paramount issue, not only for the fats and oils industry, but also for consumers due to the legal conformity, religious reasons, especially halal and kosher issues and economic reasons.[Citation1] It involves misleading the buyer as to the correct nature, composition, and quality. The adulteration of extra virgin olive oil (EVOO) with various cheaper vegetable oils is a common problem that influences the quality of EVOO.[Citation2]
Olive oil is a fatty juice and is straightforwardly consumable after the proper processing of olives and has gained popularity in recent years.[Citation3] Edible olive oils are graded into six categories, namely extra virgin olive oil, virgin olive oil, refined olive oil, common olive oil (a mixture of refined olive oil and virgin olive oil), refined residue oil, and residue olive oil.[Citation4] EVOO is considered as the best olive oil because EVOO is obtained from mechanical extraction and is not treated with artificial processing.[Citation5] Therefore, EVOO can be a target of adulteration with lower price oil, such as sesame oil (SeO).
Several researchers have developed various analytical methods to detect the adulteration resulting from the blending of EVOO with other vegetable oils. Such methods are gas chromatography for determination of fatty acid and sterol contents,[Citation6] liquid chromatography for analysis of the triglyceride compositions,[Citation7] electronic nose for measurement of the aroma and/or volatile compounds,[Citation8] and Fourier transform infrared (FTIR) spectroscopy combined with the powerful chemometrics technique.[Citation9]
FTIR spectroscopy has been widely used in food research and has become a powerful analytical tool in the study of edible fats and oils qualitatively.[Citation10] FTIR is also a promising analytical technique for quantitative analysis of components because the peak absorbances of FTIR spectra are directly proportional to its concentration according to the Beer's law.[Citation11] FTIR spectroscopy combined with chemometrics of linear discriminant analysis to authenticate EVOO and sunflower, corn, soybean, and hazelnut oils from different origins.[Citation12] In addition, Gurdeniz and Ozen[Citation13] have studied the authentication of EVOO from oil adulterants, namely corn, sunflower, rapeseed, and cottonseed oils, using partial least square-discriminant analysis approach. In this study, FTIR spectroscopy in combination with multivariate calibration of partial least square (PLS) and principle component regression (PCR) for analysis of SeO as adulterant in EVOO was used. Furthermore, the changes in fatty acid profiles of EVOO due to the addition of SeO were also analyzed using gas chromatography.
MATERIALS AND METHODS
Materials
Extra virgin olive oil (EVOO), Canola oil (CaO), corn oil (CO), grape seed oil (GSO), soybean oil (SO), sesame oil (SeO), sunflower oil (SFO), and walnut oil (WO) were purchased from the local market in Serdang, Selangor, Malaysia. All solvents and reagents used were of analytical grade. The used EVOO and SeO are the mixtures of three different brand oils having the fatty acid composition as specified in Codex Allimentarius.[Citation14]
Analysis of Fatty Acid (FA) Compositions
In order to determine the FA changes during EVOO adulteration, SeO was mixed with EVOO in the range of 5–60% (vol/vol). These mixtures were kept in controlled room temperature (20°C) before being analyzed. Determination of FA compositions was carried out using GC-FID as a derivative of fatty acid methyl ester (FAME) using an area internal normalization technique as described in Rohman and Che Man.[Citation15] Approximately 100.0 mg of oil samples was accurately weighted in analytical balance (with sensitivity of 0.1 mg), placed in a centrifuge tube, added with 1.2 mL of hexane and transesterified with a solution of 0.25 mL sodium methoxide 2M in anhydrous methanol. The mixture was vigorously mixed for 60 s using a vortex mixer at 2200 rpm. The mixture was subsequently added with 0.5 ml of saturated NaCl and vigorously mixed using a vortex mixer for 15 s in order to separate sodium glycerolate. After that, 1 μL of the clear supernatant was injected into a DB-5 capillary column (0.25 mm internal diameter, 30 m length, and 0.2 μm film thickness; Restex Corp., Bellefonte, PA, USA) and analyzed using a gas chromatograph (Shimadzu GC-2010; Shimadzu Corp., Tokyo, Japan), equipped with a flame ionization detector. The oven temperature was programmed as follows: initial temperature was 100°C (hold for 1 min), then increased into 180°C (8°C/min), increased from 180 to 240°C (10°C/min), and finally held at 240°C for 5 min. The temperatures of detector and injector were maintained at 240°C during the analysis. The flow rate of carrier gas (helium) was 6.8 mL/min. Standard FAMEs (Sigma Chemicals, St. Louis, MO, USA) were used to identify the time retention of FAMEs in the oil samples.
Analysis Using FTIR Spectroscopy
All of oil samples (EVOO, SeO, and the mixture of both oils) were subjected to FTIR spectral measurements. Using a Pasteur pipette, approximately 1.0 ml of the samples was placed on direct contact with single bounce-attenuated total reflectance using Smart ARK accessory. The conditions and parameters used during spectral acquisition are described in . Each FTIR spectra was subsequently subtracted with reference spectra (air) as background. The sample spectra were done in three replicates and displayed as the average spectra.
Table 1 The conditions and parameters of acquisition of FTIR spectra
Statistical Analysis
Fatty acid profiles were subjected to one-way analysis of variance (ANOVA) and followed with Duncan multiple comparison using SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). The significance value (p) less than 0.05 was statistically different. Principal component analysis (PCA) was generated using The Unscrambler (Camo, Woodbridge, NJ, USA). For quantitative analysis of SeO in EVOO using FTIR spectroscopy coupled with PLS and PCR, the selection of frequency regions was performed automatically using software TQ AnalystTM version 6 (Thermo Electron Corporation, Madison, WI, USA) in which the variations between FTIR spectra of pure EVOO and SeO were observed and were chosen for making the calibaration model. The model performance was evaluated by computing the root mean standard error of calibration (RMSEC) and the root mean standard error of prediction (RMSEP) in the calibration and prediction of data set, respectively.[Citation16] PLS and PCR models were performed using the TQ Analyst software.
RESULTS AND DISCUSSION
PCA Classification of EVOO and Selected Vegetable Oils
FA composition of studied vegetable oils is compiled in . FA profile of SeO and EVOO was in accordance with that specified in Codex Allimentarius.[Citation14] In order to classify EVOO and other vegetable oils (CaO, CO, GSO, SO, SeO, SFO, and WO), PCA was used based on their FA profiles. PCA is an unsupervised pattern recognition technique that projects the original data in reduced dimensions defined by the principal components (PCs).[Citation17,Citation18] This technique is useful when there is a correlation present among data.[Citation19] showed the score plot for the projection of PC1 and PC2, which describes 96 and 3% variances, respectively. Therefore, 99% variance can be explained by the first two PCs.
Table 2 FA composition of EVOO and selected vegetable oils
Figure 1 PCA score plot for classification of EVOO and other vegetable oils. See for abbreviations. (Color figure available online.)
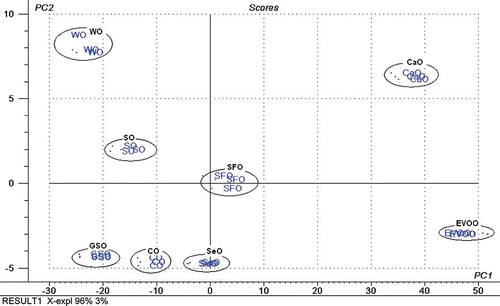
Based on , it is known that among studied vegetable oils, SeO has the close distance with EVOO, meaning that EVOO has the close similarity in FA profile with SeO compared with other studied vegetable oils. For this reason, SeO can be potential oil adulterant in EVOO. In order to guarantee that EVOO and SeO have not been already blended yet with other oils, their FA compositions were used as purity criteria. FA profiles of SeO and RBD-PO were subsequently compared with those compiled in Codex Alimentarius ranges and both oils are in compliance with the Codex standard.[Citation14]
Quantification of SeO in EVOO
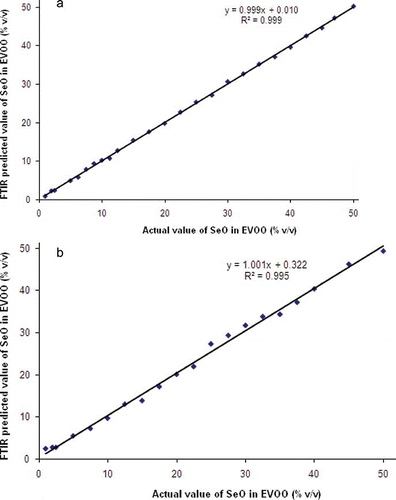
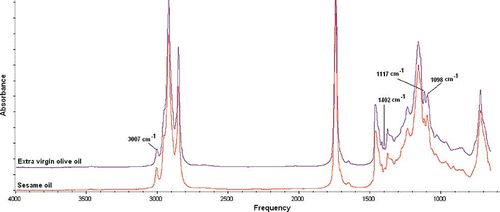
exhibited the FTIR spectra of extra virgin olive oil (EVOO) and sesame oil (SeO) at frequency regions of 4000–650 cm−1. These spectra showed the typical characteristic of absorption bands for common triglycerides.[Citation20] Quantification of SeO as adulterant in the EVOO was performed using PLS and PCR algorithms. For PLS and PCR, the samples of EVOO adulterated with SeO were divided into the calibration and the validation sets. The entire range of FTIR spectra of EVOO and SeO looks very similar with the naked eyes. However, if one examines the spectra closely, there is a minor difference in terms of peak intensities at frequency regions of 3005, 1402, 1117, and 1098 cm−1. The functional groups responsible for IR absorption together with the vibration modes have been reported in our previous article.[Citation9] These frequency differences between EVOO and SeO are exploited for quantitative analysis of SeO in EVOO. Based on the optimization process, the combined frequency regions of 1207–1018, 1517–1222, and 3050–2927 cm−1 were chosen for quantification of SeO in EVOO due to its capability to give the highest values of R 2 and the lowest values of RMSEC, compared with other frequencies.
Figure 2 FTIR spectra of extra virgin olive oil (EVOO) and sesame oil (SeO) scanned at mid infrared region (4000–650 cm−1). (Color figure available online.)
listed the results obtained from PLS and PCR calibration models in terms of R 2, RMSEC, and RMSEP either for normal spectra or its derivatives for analysis of SeO in EVOO. Both PCR and PLS calibrations in the normal spectra revealed the highest of R 2 and the lowest of RMSEC compared with first derivative treatments. PLS with second derivative give the highest values of R 2 and the lowest values of RMSEC; however, the resulted RMSEP is too high, therefore, PLS with second derivative is not chosen for such quantification. The number of factors or PCs used to build PLS and PCR models was based on the minimum predicted residual error of sum of squares values obtained. The number of PCs used for PLS and PCR are 5 and 10 PCs, respectively. Factor analysis with 10 PCs is too high, therefore PLS with lower PCs (5 PCs) was used for analysis of SeO in EVOO rather than PCR with 10 PCs.
Table 3 The performance of multivariate calibrations (PLS and PCR) for quantitative analysis of sesame oil in extra virgin olive oil
The equation obtained for the relationship between actual value (x-axis) and FTIR predicted value (y-axis) of SeO in EVOO in PLS calibration model was shown in The developed model was further used to predict the independent samples, which are different from calibration samples. The scatter plot, expressed as linear regression for the correlation between actual/known concentration and predicted/measured concentration by FTIR spectroscopy, is depicted in These results showed that FTIR spectroscopy in conjunction with multivariate calibration of PLS is well suited for determination of SeO in EVOO.
Analysis of SeO in EVOO Using Gas Chromatography
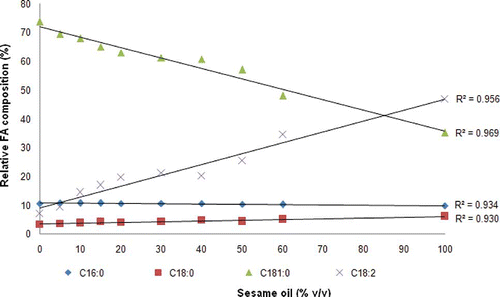
Analysis of FA compositions seems to be a very useful technique for monitoring the authenticity of high price oils like EVOO.[Citation7] The main FA in EVOO is oleic (C18:1) and palmitic (C16:0); meanwhile oleic, linoleic (C18:2), and palmitic acids were present in large amounts in SeO. This difference can be used to analyze SeO adulterated with RBD-PO.
SeO was added to EVOO at concentration ranges of 5–60% (vol/vol), and its FA composition in these mixtures is shown in . The adulteration practice of EVOO with SeO can be investigated by monitoring the level changes of certain fatty acids. The concentrations of palmitic (C16: 0) and oleic (C18:1) acids were lowered linearly with the increasing amount of SeO with R 2 of 0.969 and 0.934, respectively. At a level of 5 and 10% (vol/vol) of SeO in EVOO, these FAs were not significantly decreased (P > 0.05), however, from level 10% (vol/vol) of SeO, there is a significant difference observed. Meanwhile, the concentrations of stearic (C18:0) and linoleic (C18:2) acids were increased with R 2 of 0.930 and 0.959, respectively, with the increasing contents of SeO (). From level 10% (vol/vol) of SeO, stearic, and linoleic acids were significantly increased (p < 0.05).
Table 4 The changes of fatty acid composition
CONCLUSION
The presence of sesame oil in EVOO has been successfully quantified by FTIR spectroscopy using normal spectra with the aid of PLS model. The spectral regions used are the combined frequencies of 1207–1018, 1517–1222, and 3050–2927 cm−1 with the acceptable values of R 2, RMSEC, and RMSEP. The changes in fatty acid profiles of EVOO adulterated with SeO can complement the results obtained from FTIR spectroscopy. During the adulteration of EVOO with SeO, the concentration of palmitic and oleic acids were linearly decreased. Conversely, the concentration of stearic and linoleic acids were increased with the increasing amount of SeO added to EVOO.
ACKNOWLEDGMENTS
Abdul Rohman acknowledges the Directorate of Higher Education, Ministry of National Education, Republic of Indonesia for its financial support during his Ph.D. program in Halal Products Research Institute, UPM, Malaysia. The authors are very grateful to the Ministry of Science, Technology and Innovation (MOSTI), Malaysia, through Science Fund grant No. 05-01-04-SF0285.
Notes
*Y.B. Che Man passed away on 15 July, 2012.
REFERENCES
- Kamm , W. , Dionisi , F. , Hischenhuber , C. and Engel , K-H. 2001 . Authenticity assessment of fats and oils . Food Reviews International , 17 : 249 – 290 .
- Dourtoglou , V.G. , Dourtoglou , Th. , Antonopoulos , A. , Stefanou , E. , Lalas , S. and Poulos , C. 2003 . Detection of olive oil adulteration using principal component analysis on total and region FA content . Journal of the American Oil Chemists’ Society , 80 : 203 – 208 .
- Arvanitoyannis , I.S. and Vlachos , A. 2007 . Implementation of physicochemical and sensory analysis in conjunction with multivariate analysis towards assessing olive oil authentication/adulteration . Critical Reviews in Food Science and Nutrition , 47 : 441 – 498 .
- Boskou , D. 2009 . “ Culinary applications of olive oil-minor constituents and cooking ” . In Olive Oil-Minor Constituents and Health , Edited by: Boskou , D. 1 – 6 . Boca Raton , FL : CRC Press .
- Piravi-Vanak , Z. , Chavami , M. , Ezzatpanah , H. , Arab , J. , Safafar , H. and Ghasemi , J.B. 2010 . Evaluation of authenticity of Iranian olive oil by fatty acid and triacylglycerol profiles . Journal of the American Oil Chemists’ Society , doi: 10.1007/s11746-009-1419-y
- Al-Ismail , K.M , Alsaed , A.K. , Ahmad , R. and Al-Dabbas , M. 2010 . Detection of olive oil adulteration with some plant oils by GLC analysis of sterols using polar column . Food Chemistry , 121 : 1255 – 1259 .
- Christopoulou , E. , Lazaraki , M. , Komaitis , M and Kaselimis , K. 2004 . Effectivenes of determinations of fatty acids and triglycerides for the detection of adulteration of olive oils with vegetable oils . Food Chemistry , 84 : 463 – 474 .
- Mildner-Szkudlarz , S. and Jelen , H.H. 2010 . Detection of olive oil adulteration with rapeseed and sunflower oils using MOS electronic nose and SMPE-MS . Journal of Food Quality , 33 : 21 – 41 .
- Rohman , A. and Che Man , Y.B. 2010 . Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil . Food Research International , 43 : 886 – 892 .
- Che Man , Y.B. , Syahariza , Z.A. and Rohman , A. 2010 . “ Fourier transform infrared (FTIR) spectroscopy: development, techniques, and application in the analyses of fats and oils ” . In Fourier Transform Infrared Spectroscopy , Edited by: Ress , O.J. 1 – 36 . New York : Nova Science Publishers .
- Guillen , M.D. and Cabo , N. 1997 . Infrared spectroscopy in the study of edible oils and fats . Journal of the Science of Food and Agriculture , 75 : 1 – 11 .
- Lerma-Garcia , M.J. , Ramis-Ramos , G. , Herrero-Martinez , J.M. and Simo-Alfonso , E.F. 2010 . Authentication of extra virgin olive oils by Fourier transform infrared spectroscopy . Food Chemistry , 118 : 78 – 83 .
- Gurdeniz , G. and Ozen , B. 2009 . Detection of adulteration of extra-virgin olive oil by chemometric analysis of mid-infrared spectral data . Food Chemistry , 116 : 519 – 525 .
- Codex Alimentarius Commission . 2003 . Amended. Codex Standard for Named Vegetable Oils. Codex Standard 210
- Rohman , A. and Che Man , Y.B. 2009 . Monitoring of virgin coconut oil (VCO) adulteration with palm oil using Fourier transform infrared (FTIR) spectroscopy . Journal of Food Lipids , 16 : 618 – 628 .
- Gallardo-Velázquez , T. , Osorio-Revilla , G. , Cárdenas-Bailón , F. and Beltrán-Orozco , M.C. 2008 . Determination of ternary solutions concentration in liquid-liquid extraction by the use of attenuated total reflectance-Fourier transform infrared spectroscopy and multivariate data analysis . Canadian Journal of Chemical Engineering , 86 : 77 – 83 .
- Cserháti , T. 2009 . Review: Data evaluation in chromatography by principal component analysis . Biomedical Chromatography , 24 : 20 – 28 .
- Shao , Y. , He , Y. , Yidan , B. and Mao , J. 2010 . Near-infrared spectroscopy for classification of oranges and prediction of the sugar content . International Journal of Food Properties , 12 : 644 – 658 .
- He , Y. , Li , X. and Shao , Y. 2007 . Fast discrimination of apple varieties using Vis/NIR spectroscopy . International Journal of Food Properties , 10 : 9 – 18 .
- Safar , M. , Bertrand , D. , Robert , P. , Devaux , M.F. and Genot , C. 1994 . Characterization of edible oils, butters and margarines by Fourier transform infrared spectroscopy with attenuated total reflectance . Journal of the American Oil Chemists Society , 71 : 371 – 377 .