Abstract
The antioxidant activity of Chinese parsley was evaluated as compared to the synthetic antioxidant ethoxyquin during storage at 45°C for 42 days. The oxidative stabilities of diets were determined according to the changes in headspace oxygen consumption, the formation of oxidation products, and the DPPH radical scavenging activity. The results achieved using different oxidation parameters showed a significant decrease (P < 0.05) in oxidation products and high DPPH radical scavenging activity, suggesting that Chinese parsley is effective in retarding lipid oxidation over a long storage period. On the other hand, the efficiency of ethoxyquin decreased as it was degraded over time. In addition, DPPH radical scavenging activity was strongly correlated (R2 = 0.98, P < 0.01) with the content of caffeic acids, one of the simple polyphenols in Chinese parsley, and thus, caffeic acid may be responsible for the improved oxidative stability. Therefore, results suggested that Chinese parsley, a commonly used plant, can be safely used as a natural antioxidant alternative to synthetic additives for long-term storage in the feed or food industry.
INTRODUCTION
Oxidation of unsaturated fatty acids negatively affects the flavor, odor, color, texture, and nutritional value of foods or feed during storage.Citation[1] Hydroperoxides, the initial products of lipid oxidation, decompose into secondary products, such as esters, aldehydes, ketones, alcohols, hydrocarbons, and furans. These secondary products affect the food quality, also certain oxidation products are potentially toxic even at relatively low concentrations.Citation[2] In order to delay or prevent these undesirable effects, antioxidants are often added to foods to inhibit the initiation and propagation steps terminating the reaction via the competitive binding of reactive oxygen species, destroying or binding free radicals and stabilizing hydroperoxides.[Citation3–5] Ethoxyquin (EQ, 1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline), a synthetic compound, is one of these antioxidants that can scavenge free radicals formed during lipid oxidation.Citation[6 Citation7] Because of its availability and low cost, it is generally allowed in livestock, aquaculture, and pet foods as well as in fishmeal, which is used to prevent proneness to spontaneous fire during shipping through the equatorial seas.Citation[8] However, EQ has unfavorable side-effects in animals; thus, 150 mg kg-1 is the limit allowed in complete animal feed established by the animal food safety laws in Japan and America.
Therefore, there has been a considerable interest in the food industry in finding potentially safe natural antioxidants, especially plant origin, to replace synthetic compounds in food. The food industry has focused the global trend among consumers toward natural antioxidants because of their presumed safety and potential therapeutic or nutritional value. Many isolated plant constituents, including phytochemicals, and also crude extracts of vegetables and fruits, have been recognized to guard against the appearance of free radicals in biological systems as antioxidants. Many plants, especially species from the families Umbelliferae and Apiaceae, have displayed significant antioxidative properties. Among the Umbelliferae species, Chinese parsley (CP, Coriandrum sativum)[Citation9–11] and Western parsley (WP, Petroselinum crispum)[Citation12–14] have been widely studied for their antioxidant activities.
CP is an annual herbaceous plant originally from the Mediterranean and Middle Eastern regions. It is the most widely consumed popular ingredient in the world as a domestic spice, a traditional medicine, and a flavoring agent.Citation[15] It grows 25–60 cm (9–24 in.) in height. It has thin, spindle-shaped roots, an erect stalk, alternate leaves, and small, pinkish-white flowers. The dried fruits are extensively employed as condiments, especially for flavoring sauces, meat products, and bakery and confectionery items.Citation[16] Much information is available regarding its widespread biologic functions and the health benefits owing to its phenolic compounds. CP is considered to have antimicrobialCitation[17] and carminative properties,Citation[18] and many studies have confirmed its antioxidant activity.Citation[15,[Citation19–21]
For evaluating antioxidant potency, assays for radical scavenging ability and assays that test ability to inhibit lipid oxidation are commonly used.Citation[22] Radical scavenging ability tests measure the reduction in either stable radicals or radicals generated by radiolysis, photolysis, or other reactions. Scavenging of the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) is applied extensively with individual and stable radical compound.Citation[23] Oxidation test systems are sometimes accelerated by the action of light or UV radiation but more frequently by elevated temperatures. Any of the substrates, initiators, intermediates, or final products can be used to assess antioxidant activity.Citation[24] In these accelerated systems, many analytical methods, such as headspace oxygen consumption (HOC), peroxide value (PV), thiobarbituric acid value (TBA), acid value (AV), carbonyl value (CaV), iodine value, free fatty acid content, polymer content, viscosity, etc., are used to monitor antioxidant activity.Citation[24]
Our previous studies on biological activities of CP have demonstrated that the lyophilized powder of this plant possesses properties that promote the excretion of heavy metals.Citation[25 Citation26] However, there is little information in the literature regarding the improvement of food storage stability using CP versus synthetic antioxidants. The present study was designed to study the oxidative stability of CP in comparison with EQ in diets under accelerated oxidative stress. HOC measurement, DPPH radical scavenging ability, PV, AV, TBA, and CaV were used to assess the development of rancidity during diet storage.
METHODS AND MATERIALS
Sample CP
CP was purchased from the Japan Agricultural Cooperative Enshu. Immediately after arrival, the leaves and stalks were freeze-dried and crushed into a fine powder (<0.5 mm) using a food processor. Ninety-eight grams of freeze-dried powder were obtained from 1 kg of fresh CP.
Diet Preparation
Diet materials were provided by a diet factory (Tsushi, Mie Prefecture, Japan). No antioxidants or preservatives were added to the raw materials during processing, transport, or storage. The proximate composition of the basal diet on a fresh weight basis was as follows: moisture 8.6, crude protein 57.8, crude fat 11.9, ash 18.5, and crude fiber 2.5 g/100 g diet. Three treatments, the basal diet (control), basal diet plus 20 μg mg−1 of CP, and the basal diet plus 150 μg g−1 of EQ, were submitted to the oxidative stability test. The level of EQ used was the maximum allowed for dietary supplements to livestock, aquaculture, and pet foods.
Oxygen Bomb Test (OBT)
After the diet was prepared, OBT was used as a quick method to evaluate the performance of CP. A glass sample container filled with 35 g of each diet was placed in a 50-ml stainless steel-closed cylinder (a bomb). The bomb was closed tightly and purged with pure oxygen to the pressure of 345 kPa at ambient temperature. Then the bomb was placed in a constant-temperature oven at 60°C for 72 h. The pressure was monitored every 6 h with a pressure transducer connected to the top of the cylinder (SE700T-MV1/4 TC3200-010, Taiatsu Techno Co., Ltd., Tokyo, Japan). The oxygen consumption by the diet was calculated based on the ideal gas law. Tests were carried out in duplicate.
Oxidation Storage Experiment
Samples (70 ml) of each of the preservative treatments were placed into a series of transparent petri dishes. Then, the samples were stored on shelves without covers in an incubator (Model IC-41; Yamato Scientific Co., Ltd., Tokyo, Japan) for 7 weeks at 45 ± 1.8°C, with air blasting and in the dark. Relative humidity was maintained at 22 ± 4% in the chambers. The addition of treatments (25 g) into the petri dishes resulted in a surface to volume ratio of 0.83 cm−1. Three separate petri dishes of each preservative treatment were removed from the incubator weekly to analyze moisture, PV, TBA, AV, and CaV. In addition, EQ concentrations were analyzed.
Peroxide Value
PV and AV were estimated using a titrimetric method following the Feed Safety Law of the Ministry of Agriculture, Forestry and Fisheries of Japan (http://www.famic.go.jp/ffis/feed/sub6.html). PV was calculated using the formula PV = [10(V 1 - V 0)N]/M, where V 1 is the amount of Na2S2O3 used for titration (ml), V 0 is the amount of Na2S2O3 used for the blank (ml), N is the normality of Na2S2O3, and M is the amount of diet (g). PV was expressed as milliequivalents (meq) of active oxygen per kilogram of diet. AV (mg KOH/g) is expressed as the weight of KOH (mg) used to neutralize the lipid (1 g) extracted from the diets. The weight of KOH (mg) was calculated using the following formula: AV = (A - A 0) × F × M/W, where A (ml) is the volume of the titrant used for the diet, A 0 (ml) is the volume of titrant used for the blank, F is the factor of molarity of the alcoholic KOH, M is the chemical formula weight of KOH = 56.106, and W (g) is the weight of the extracted lipid.
Lipid Peroxidation
Thiobarbituric acid reactive substances (TBARS) formation as products of lipid peroxidation was measured using the method of Ohkawa.Citation[27] TBA was calculated as milligrams of malondialdehyde (MDA) per mg of diet.
Carbonyl Value
Diet (0.05 g) was dissolved in 5 ml benzene and then mixed with 3 ml trichloroacetic acid (4.3%) and 5 ml 2,4-dinitrophenylhydrazine (0.05% [w/v benzene]). The mixture was capped and heated for 30 min at 60°C. After it had cooled for 1 h at room temperature, 10 ml of 4% KOH-ethanol solution was added, and the mixture was further diluted to 50 ml with ethanol. The solution was centrifuged at 2000× g for 5 min, followed by the absorbance measurement of the upper layer at 420 nm.
EQ Analyses
After extracting a 0.5-mg sample with acetonitrile (50 mg BHT/L), 20 μL of supernatant was eluted with the mobile phases containing acetonitrile:water (70/30 v/v) at a 0.8 ml/min flow rate through a Wakosil-II 5CHG column, 250 × 4.6 mm i.d. (Wako, Tokyo, Japan). Detection was carried out using a Shimadzu RF-10AxL fluorescence detector (Kyoto, Japan) at an excitation wavelength of 360 nm and an emission wavelength of 435 nm. The LC solution of Shimadzu's workstation software was used to integrate peak areas.
DPPH Radical Scavenging Assay
The antioxidant activities of the extracts were measured on the basis of the scavenging activities of the stable DPPH free radicals.Citation[28] This method is commonly used to investigate the antioxidant activities of natural and synthetic antioxidants. The stable free radical DPPH, which is deep violet in color, reacts with the antioxidants, and converts itself into 1,1-diphenyl-2-picrylhydrazine with discoloration. The stable end-product formed by this reaction would not initiate or propagate further oxidation of lipids. Higher discoloration indicates stronger radical scavenging activity of the sample.
The diet (0.5 g) was extracted a third time in 80% aqueous methanol (4 ml) and centrifuged at 1850× g for 15 min. The three supernatant liquids were combined and then transferred into small sample vials for further analysis and storage. The aqueous extract (20 μl), which was dissolved in 80 μl of Tris-HCl buffer (100 mM, pH 7.4), was mixed with 100 μl of methanol solution of DPPH (500 μM) that had been freshly prepared. The mixture was shaken vigorously and incubated for 20 min in the dark at ambient temperature followed by HPLC measurement at 515 nm. All samples were tested in triplicate, with Trolox (0–1.6 mM) used as the positive control. The percentage of radical scavenging activity was calculated against the DPPH solution without a test compound using the following equation: [(Control area-sample area)/Control area) × 100].
Total Thiols
Thiol groups were assayed by the method of Moron et al.[29] with some modifications. This method is based on the formation of a relatively stable yellow color from the reaction of sulfhydryl groups with DTNB. Briefly, 0.1 ml of methanol extract were mixed with phosphate buffer (pH 8), and 0.9 ml of 10 mM DTNB in methanol. This mixture was incubated for 20 min at room temperature and the absorbance was measured at 415 nm against appropriate blanks. The total thiol content was calculated by using a standard curve constructed from glutathione. The results were expressed as μmol SH/mL of methanol extract.
HPLC Method for DPPH Radical Scavenging Assay and Caffeic Acid
The HPLC analyses of the diet extracts were conducted on a Shimadzu HPLC system (Shimadzu Scientific Instruments Inc., Kyoto, Japan) equipped with a Shimadzu SPD-M20 A UV-Vis detector and TSKgel Octyl-80Ts column (4.6φ × 150 mm, TOSOH, Tokyo, Japan). The mobile phase filtered through a 0.45-μm filter consisted of 70% methano1 in water. The injection volume was 20 μl, and the flow rate was 0.8 ml/min at room temperature. The wavelengths for the detection of caffeic acid and DPPH were set at 280 and 517 nm, respectively. All sample analyses were carried out in triplicate.
Determination of Total Polyphenols Content and Total Flavonoids Content
The concentration of polyphenols in the extracts was determined according to the method described by Negi and Jayaprakasha.Citation[30] The results of the analyses in triplicate are expressed as mg GA/100 mg. The total flavonoid content of the extract was determined according to the method reported by Jia.Citation[31] The results were expressed as μg catechin equivalents per g of diet.
Statistical Analysis
The results are expressed as the mean ± standard deviation of three replicate analyses. Differences between groups were analyzed statistically using student t-tests. A level of P < 0.05 was considered to be significant. A correlation procedure (Pearson's correlation coefficient) was performed to evaluate any relationship between two factors, such as DPPH radical scavenging activity and total polyphenols content.
RESULTS
Headspace Oxygen Consumption
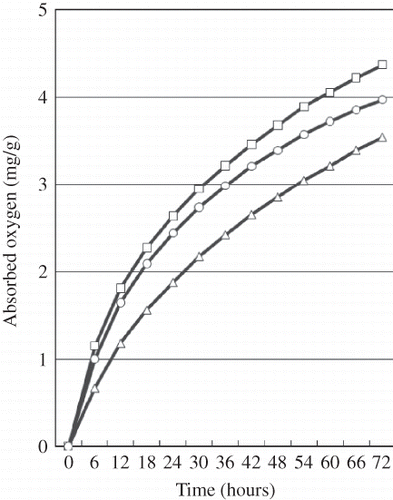
The headspace oxygen consumption of the treatments during 72 h of storage of OBT at 60°C is shown in Oxygen in the headspace of all studied treatments decreased as storage time increased. The HOC under EQ treatment was significantly (P < 0.05) lower than in the control, as expected, but in the case of CP treatment using 2% CP powder that HOC was not only significantly lower than the control but surprisingly lower than expected. That is, the headspace oxygen consumption of the CP treatment was significantly (41.4%) less than that of the control after 6 h of storage (P < 0.05); the headspace oxygen consumption of the EQ treatment was also significantly less (12.9%) than that of the control. At the end of 72 h of storage, the lowest consumption of headspace oxygen (mg/g sample) was found in the CP powder treatment (3.54 ± 0.076), whereas the residual headspace oxygen associated with the EQ treatment and control were 3.97 ± 0.094 and 4.36 ± 0.084, respectively. This result indicates that the presence of CP in a diet reduced oxygen consumption therefore limiting lipid oxidation.
Oxidation Storage Experiment
PV is commonly used to measure hydroperoxides, the primary products in the early stages of lipid oxidation. The formation of hydroperoxides was determined to evaluate the antioxidant activity of CP compared with EQ and the control. The increases in PV of the three treatments are shown in The initial PV of all diets was 1.06 ± 0.104 meq O2/kg diet; it then increased (P < 0.05) gradually during storage and reached levels between 7.9 and 9.4 meq O2 kg/diet at the end of storage. PV increased rapidly (P < 0.05) in the control to 9.84 ± 0.169 meq O2/kg diet after 3 weeks of storage, and after 7 weeks of storage it was also significantly higher (9.35 ± 0.859, P < 0.05) than those of diets containing CP powder (7.92 ± 0.167) or EQ (8.91 ± 0.167). On the other hand, the PV achieved using the CP treatment was lower (P < 0.05) than that of the EQ treatment after 3 weeks of storage at 45°C.
Figure 2 Changes in chemical indicators of lipid oxidation of diet samples during 7 weeks of storage at 45°C. (a) Peroxide value; (b) TBA value; (c) acid value; (d) carbonyl value. Control: open bars; CP powder 2% treatment: point-filled bars; 150 ppm EQ treatment: filled bars. Values are mean ±SD; the different letters indicate significant differences (P < 0.05, n = 3) from the results for the control during the same storage time.
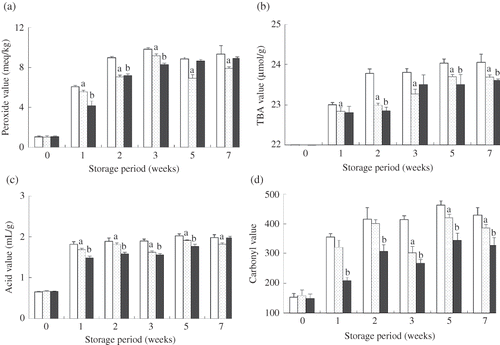
The TBARS assay is one of the most widely used methods to measuring MDA, a secondary oxidation product. The results of the measurement of TBA are shown in A continuous increase in TBA was observed in all samples along with increases in the storage periods. The control exhibited the highest TBA at all the stages of analysis.The initial rate of formation of TBARS was quick and increased in the 2nd week, showing a steady increase in TBA formation up to the 7th week of storage. CP and EQ groups showed a similar behavior, with a significantly slower increase (P < 0.05) in TBARS formation compared to the control up to the 7th week. It is suggested that CP powder at 2% has higher stabilization efficiency in controlling the formation of secondary oxidation products after a long storage period of 42 days under an accelerated set of conditions. The results were in agreement with those obtained for PV.
The AV is a relative measure of secondary lipid oxidation products, the free fatty acid present in diets. The AV variation in diets during storage is shown in AV varied from 0.65 (0-week) to 1.98 (7-week) in the control, to 1.81 using the CP treatment, and to 1.97 using the EQ treatment. The AV of CP was significantly lower (P < 0.05) than that of the control, though it was higher than in EQ up to the 5th week; after this time, it decreased and became less than that of the EQ at the 7th week. In contrast, the AV of EQ continued increasing during all stages of storage period. After the 7-week period, the AV for the control and for the EQ was nearly the same. Based on this parameter, it suggested that CP demonstrates comparable results to that of synthetic antioxidant EQ over the long term.
The effect of the addition of CP and EQ to diets on CaV is shown in The CaV of the control increased suddenly beginning in the 1st week of storage, after which it remained the highest CaV until the end of the 7th week of storage. CP showed a significant difference (P < 0.05) from the control at low levels, similarly to the results of PV, indicating the presence of low amounts of endogenous carbonyl compounds in the diet. However, the difference between CP and EQ for all the periods was statistically significant (P < 0.05), which was unexpected and differed from the results obtained with PV under the experimental conditions employed.
Changes of EQ
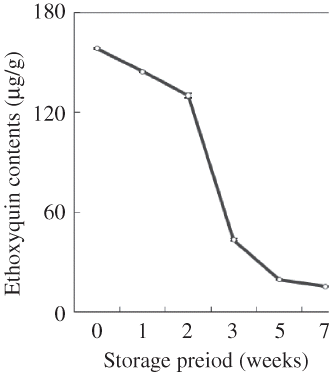
The EQ content of the diets during storage was measured based on the residual amount of EQ. shows the results expressed as the residual EQ percentage. The EQ content diminished with the storage time and eventually reached only 12.3 and 9.7% of the initial content after 5 weeks and 7 weeks of storage, respectively, exhibiting significant low thermal stability (). When the PV contents and the EQ contents in the diets were compared, it emerged that although EQ showed good antioxidant activity with a low PV at the start of storage, EQ degraded gradually with storage time and substantiated the increases of PV, TBA, AV, and CaV.
Free Radical Scavenging and Antioxidant Activities In Vitro
The free radical scavenging activities of diets tested using the DPPH method are depicted in . EQ had the strongest DPPH radical scavenging activity (37.8%) on weight basis followed by CP (30%) and the control (13%) at the beginning of storage; after this, it decreased drastically with time. At week 7, only 7.6% of DPPH radicals were scavenged by EQ, whereas 18.2% were scavenged by CP. Among the three groups, the control showed the lowest radical scavenging activity. On the other hand, regarding thiol group levels, no evident trend was observed along with the storage period. These levels were significantly higher (P < 0.05) in CP groups except during the 2nd week when compared with the control group. Thiol levels in control and EQ groups were not significantly different from each other.
Table 1 Free radical scavenging activity (%) and total thiols (μM) of diets
Correlations of DPPH Radical Scavenging Activity with Polyphenols
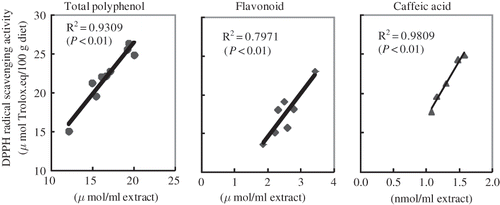
Linear regressions of total polyphenols, total flavonoids, and caffeic acid, which are reported antioxidants of CP with DPPH radical scavenging activity, were analyzed and are presented in The correlation indexes of these regressions were more than 0.7. Especially good correlations for total polyphenols (R 2 = 0.93, P < 0.01) and for caffeic acids (R 2 = 0.98, P < 0.01) were observed in DPPH radical scavenging activity. These values indicate that polyphenols, especially caffeic acids, were a potent source of antioxidant compounds of CP in the present study.
DISCUSSION
Herbs and spices have been used in food as antioxidants to prevent the formation of undesirable oxidation products, and many methods have been developed to evaluate their antioxidant activity.Citation[15 Citation32] In vitro, the primary or secondary oxidation products and free radical scavenging activity, such as DPPH and oxygen radical absorbent capacity, are well known and commonly measured parameters.[Citation33–35] The OBT and conventional oxidation accelerated methods are suitable for assessing the oxidative stability of diverse materials, such as food and feed. However, some studies have suggested that only the one-test system is not relevant in a complex matrix, such as that of diets.Citation[36] The efficacy of antioxidants is greatly affected by many ingredients present in the matrix, such as sugars, water, enzymes, pigments, and salt.Citation[37] Therefore, the most accurate approach to studying antioxidant activity is the analysis of several types of methods. In this study, diets were examined using six methods: OBT, PV, AV, CaV, TBA, and DPPH radical scavenging activity.
Headspace oxygen consumption, which was the result of oxygen reacting to form peroxy radicals, was determined first to investigate the oxidative stability of diets under conditions of accelerated oxidation. The headspace oxygen consumption of the CP treatment was 19 and 12% lower than that of the control and EQ treatments, respectively, which indicated that the CP treatment was the most stable against oxidation. Moreover, EQ did not prevent decreases in headspace oxygen when the diet was still in the initial stages of oxidation. It is presumed that EQ began to decompose under 345 kPa oxygen pressure at 60°C during the initial stage of OBT examination.
Four common chemical indicators of lipid oxidation—PV, TBA, AV, and CaV—were analyzed to determine the contribution of CP in inhibiting the oxidation process. PV increased exponentially after 1 week of storage (), as more hydroperoxides were promoted by the self-catalysis of hydroperoxides formed previously in diets. However, with time, the formation rate of hydroperoxide became lower than the decomposition rate, resulting in a decrease of PV beginning in the fifth week. Hydroperoxide measured by PV comprised the remainder of the formatted amount and the decomposed amount. The diet with EQ showed low PV only until week 1, showing that EQ was effective in inhibiting the formation of hydroperoxides in the diet only during the early stage of storage. In contrast, the PV of the CP treatment was significantly lower than those of both the control (over the entire storage period) and the EQ treatment (after 5 and 7 weeks of storage), showing a protective effect against hydroperoxide formation and significantly longer-lasting oxidative stability of the diet with CP powder over that with EQ.
The other different measurement, TBA, expressed as free MDA, was also used to investigate secondary oxidative development in the diets in this study. In the CP treatment, levels of TBA were relatively low. TBA values are usually high in diets containing fish fat, as it is typically rich in polyunsaturated fatty acids, these eventually derive in MDA. AV increases can be explained from the formation of two types of fatty acids in the fat oxidation. One is the free fatty acid generated by lipid hydrolysis, whereas the other is from hydroperoxide decomposition. The formation of carbonyl compounds (CaV) is attributed to further oxidative degradation of hydroperoxides and polyunsaturated fatty acids. The significantly lower levels of AV and CaV for CP than those for the control after 7 weeks demonstrated that CP has oxidative stability higher than that of the control, and that secondary oxidation products may require longer time. However, EQ showed an obvious and unexpected decrease in the efficiency of preventing the formation of carbonyl compounds throughout the storage period.
The statistical analysis of the data revealed that both the initial products (PV) and the secondary products (AV, CaV, and TBA) of diets including 2% of CP were significantly (P < 0.05) more stable in terms of lipid oxidation compared to the control at a storage temperature of 45°C. The results of this study demonstrate the usefulness of a wide range of methods in simulating antioxidant effectiveness in comparison with a one-test system used to characterize the potential power of antioxidants. With findings similar to ours, Angelo and Jorge have reported CP-delayed lipid oxidation in sunflower oil samples heated to 180°C for 30 h.Citation[38] However, although the reported evidence shows that the EQ content of diets has a marked impact on their oxidative stability,Citation[39] it was observed that its effect was lower than that of CP after a 7-week storage period.
On the other hand, our results suggest that EQ retards lipid peroxidation very well in the initial stages. However, with the passage of time, its efficiency decreases as its degradation occurs such that it becomes ineffective. Thus, the problem is whether the oxidation products of EQ demonstrate residual antioxidant activity and are still sufficient to protect lipids against oxidation in their own right. Also the toxicity of these products has to be considered. Although it has been reported that 2,6-dihydro-2,2,4-trimethyl-6-quinolone and 1,8-bis (1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline) are two oxidation products of EQ,Citation[8] further information is required about these issues, including additional chemical and empirical data. Nevertheless, according to our results, the replacement of EQ with natural antioxidants, such as CP, might be preferable when the oxidative stability of the product is a concern.
In addition, this study shows an average of 18–30% of DPPH radical scavenging activity in diets with 2% CP powder during storage at 45°C in the dark for 7 weeks. The DPPH radical scavenging activity of the control continuously decreased from 13% and was 1% at the end of the test. The decrease in DPPH radical activity with time may be responsible for the degradation of hydrogen- or electron-donating agents, such as phenolic compounds, during autoxidation.Citation[15] It has been reported that antioxidants such as polyphenols and flavonoids have excellent DPPH radical scavenging activity as a result of their hydrogen-donating ability,Citation[40 Citation41] thus delaying the onset of the oxidation process in lipids. Flavonoids are particularly important because they have been found to create antioxidant and free radical scavenging activity in foods, and their antioxidant capacities are much stronger than those of vitamins C and E.Citation[42]
On the other hand, several studies have conclusively shown correlations between total polyphenol (flavonoid) content and antioxidative activity in fruits, vegetables, herbs, and spices.[Citation43–45] This study indicates a direct correlation between the concentration of caffeic acid and antioxidant activity (DPPH radical scavenging activity). The preliminary findings regarding oxidation stability indicate a higher resistance of CP to oxidation in comparison with EQ and the control. Hence, polyphenols, especially caffeic acids (one of simple polyphenols present in CP), seem to be responsible for the radical scavenging activity of CP. It might be also due to their hydrogen-donating ability, according to which they oxidize themselves at the phenolic hydroxyl groups with low oxidation-reduction potential on the benzene ring.Citation[37] As a result, free radicals are removed, and oxidation chain reactions are terminated with low initial levels of hydroperoxides and low levels of secondary products, subsequently leading to better oxidation stability for diets. Thiol-containing molecules, such as glutathione and others such as ascorbate and tocoferols, are components with attributed antioxidant capacity. In this study, DPPH radical scavenging activity and total thiol levels were higher in CP group than in control. As thiol-containing substances are not consumed during storage with CP (possibly due to CP protective), these non-oxidized thiol groups may further contribute to the total antioxidant capacity. Also, the higher level of thiol groups in CP treatment may be due to thiols present originally in the CP itself. These findings are in agreement with those of Al-Ismail et al.Citation[46] who has reported a significant, positive correlation between the antioxidant activity measured by DPPH scavenging activity and the total polyphenol content of the CP extracts. Therefore, CP, a commonly used plant that contains effective phenolic antioxidants to counteract lipid oxidation, can be used safely as a natural antioxidant. This plant is expected to be widely used in the feed or food industry as an alternative to synthetic additives.
ACKNOWLEDGMENT
This research was supported by a special government support program of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- Fridovich , I. 1986 . Biological effects of the superoxide radical . Archives of Biochemistry and Biophysics , 247 : 1 – 11 .
- Min , D.B. and Boff , J.M. 2002 . Chemistry and reaction of singlet oxygen in foods . Comprehensive Reviews in Food Science and Food Safety , 1 : 58 – 72 .
- Shahidi , F. and Wanasundara , P.K. 1992 . Phenolic antioxidants . Critical Reviews in Food Science and Nutrition , 32 : 67 – 103 .
- Hilton , J.W. 1989 . Antioxidants: Function, types and necessity of inclusion in pet foods . The Canadian Veterinary Journal , 30 : 682 – 684 .
- Pappas , A.M. 1991 . Antioxidants—Which ones are best for your petfood products? . Petfood Industry , 33 : 8 – 16 .
- Guo , L. , Xie , M.Y. , Yan , A.P. , Wan , Y.Q. and Wu , Y.M. 2006 . Simultaneous determination of five synthetic antioxidants in edible vegetable oil by GC–MS . Analytical and Bioanalytical Chemistry , 386 : 1881 – 1887 .
- Yamashita , Y. , Katagiri , T. , Pirarat , N. , Futami , K. , Endo , M. and Maita , M. 2009 . The synthetic antioxidant, ethoxyquin, adversely affects immunity in tilapia (Oreochromis niloticus) . Aquaculture Nutrition , 15 : 144 – 151 .
- De Koning , A.J. 2002 . The antioxidant ethoxyquin and its analogues: A review . International Journal of Food Properties , 5 : 451 – 461 .
- Ramadan , M.F. , Kroh , L.W. and Morsel , J.T. 2003 . Radical scavenging activity of Black Cumin (Nigella sativa L.), Coriander (Coriandrum sativum L.), and Niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions . Journal of Agricultural and Food Chemistry , 51 : 6961 – 6969 .
- Melo , E.D. , Mancini , J. and Guerra , N.B. 2005 . Characterization of antioxidant compounds in aqueous coriander extract (Coriandrum sativum L.) . LWT—Food Science and Technology , 38 : 15 – 19 .
- Guerra , N.B. , Melo , E.D. and Mancini , J. 2005 . Antioxidant compounds from coriander (Coriandrum sativum L.) etheric extract . Journal of Food Composition and Analysis , 18 : 193 – 199 .
- Jimenez-Alvarez , D. , Giuffrida , F. , Golay , P.A. , Cotting , C. , Lardeau , A. and Keely Brendan , J. 2008 . Antioxidant activity of oregano, parsley, and olive mill wastewaters in bulk oils and oil-in-water emulsions enriched in fish oil . Journal of Agricultural and Food Chemistry , 56 : 7151 – 7159 .
- Zhang , H. , Chen , F. , Wang , X. and Yao , H. 2006 . Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents . Food Research International , 39 : 833 – 839 .
- Luthria , D.L. , Mukhopadhyay , S. and Kwansa , A.L. 2006 . A systematic approach for extraction of phenolic compounds using parsley (Petroselinum crispum) flakes as a model substrate . Journal of the Science of Food and Agriculture , 86 : 1350 – 1358 .
- Mradu , Gupta . 2010 . Pharmacological properties and traditional therapeutic uses of important Indian spices: A review . International Journal of Food Properties , 13 : 1092 – 1116 .
- Ravi , R. , Prakash , M. and Keshava Bhat , K. 2007 . Aroma characterization of coriander (Coriandrum sativum L.) oil samples . European Food Research and Technology , 225 : 367 – 374 .
- Calucci , L. , Pinzono , C. , Zandomeneghi , M. , Capocchi , A. , Ghiringhelli , S. , Saviozzi , F. , Tozzi , S. and Galleschi , L. 2003 . Effects of gamma-irradiation on the free radical and antioxidant contents in nine aromatic herbs and spices . Journal of Agricultural and Food Chemistry , 51 : 927 – 934 .
- Platel , K. and Srinivasan , K. 2004 . Digestive stimulant action of spices: a myth or reality? . Indian Journal of Medical Research , 119 : 167 – 179 .
- Wangensteen , H. , Samuelsen , A.B. and Malterud , K.E. 2004 . Antioxidant activity in extracts from coriander . Food Chemistry , 88 : 293 – 297 .
- Melo , E.D. , Bion , F.M. , Filho , J.M. and Guerra , N.B. 2003 . In vivo antioxidant effect of aqueous and etheric coriander (Coriandrum sativum L.) extracts . European Journal of Lipid Science and Technology , 105 : 483 – 487 .
- Ramadan , M.F. and Morsel , J.T. 2004 . Oxidative stability of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) crude seed oils upon stripping . European Journal of Lipid Science and Technology , 106 : 35 – 43 .
- Schwarz , K. , Bertelsen , G. , Nissen , L.R. , Gardner , P.T. , Heinonen , M.I. , Hopia , A. , Huynh-Ba , T. , Lambelet , P. , McPhail , D. , Skibsted , L.H. and Tjburg , L. 2001 . Investigation of plant extracts for the protection of processed foods against lipid oxidation . European Food Research and Technology , 212 : 319 – 328 .
- Suh , H.J. , Lee , J.M. , Kim , Z.S. and Chung , S.H. 1999 . Radical scavenging compound in onion skin . Food Research International , 32 : 659 – 664 .
- Antolovich , M. , Prenzler , P.D. , Patsalides , E. , McDonald , S. and Robards , K. 2002 . Methods for testing antioxidant activity . Analyst , 127 : 183 – 198 .
- Jia , H. , Ren , H. , Maita , M. , Satoh , S. , Endo , H. and Hayashi , T. 2006 . Development of functional fish feed with natural ingredients to control heavy metals . Toxicology Mechanisms and Methods , 16 : 411 – 417 .
- Jia , H. , Ren , H. , Endo , H. and Hayashi , T. 2009 . Effect of Chinese parsley Coriandrum sativum on cadmium binding to proteins from the liver and kidney of rainbow trout . Marine and Freshwater Behaviour and Physiology , 42 : 187 – 199 .
- Ohkawa , H. , Oshishi , N. and Yag , K. 1979 . Assay of lipid peroxidation in animal tissue by thiobarbituric acid reaction . Analytical Biochemistry , 95 : 351 – 358 .
- Braca , A. , Tommasi , N.D. , Bari , L.D. , Pizza , C. , Politi , M. and Morelli , I. 2001 . Antioxidant principles from Bauhinia terapotensis . Journal of Natural Products , 64 : 892 – 895 .
- Moron , M.S. , Depierre , J.W. and Mannervik , B. 1979 . Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver . Biochimica et Biophysica , 582 : 67 – 78 .
- Negi , P.S. and Jayaprakasha , G.K. 2003 . Antioxidant and antibacterial activities of Punica granatum peel extracts . Journal of Food Science , 68 : 1473 – 1477 .
- Jia , Z. , Tang , M. and Wu , J. 1998 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxides radicals . Food Chemistry , 64 : 555 – 559 .
- Arumugam , P. , Ramamurthy , P. and Ramesh , A. 2010 . Antioxidant and cytotoxic activities of lipophilic and hydrophilic fractions of Mentha spicata L. (Lamiaceae) . International Journal of Food Properties , 13 : 23 – 31 .
- Decker , E.A. , Warner , K. , Richards , M.P. and Shahidi , F. 2005 . Measuring antioxidant effectiveness in food . Journal of Agricultural and Food Chemistry , 53 : 4303 – 4310 .
- Van Dyck , S.M.O. , Verleyen , T. , Dooghe , W. , Teunckens , A. and Adams , C.A. 2005 . Free radical generation assays: New methodology for accelerated oxidation studies at low temperature in complex food matrices . Journal of Agricultural and Food Chemistry , 53 : 887 – 892 .
- Manda , K.R. , Adams , C. and Ercal , N. 2010 . Biologically important thiols in aqueous extracts of spices and evaluation of their in vitro antioxidant properties . Food Chemistry , 118 : 589 – 593 .
- Thorisson , S. , Gunstone , F.D. and Hardy , R. 1992 . Some oxidation products of ethoxyquin including those found in autoxidising systems . Chemistry and Physics of Lipids , 60 : 263 – 271 .
- Jimenez-Alvarez , D. , Giuffrida , F. , Vanrobaeys , F. , Golay , P.A. , Cotting , C. , Lardeau , A. and Keely Brendan , J. 2008 . High-throughput methods to assess lipophilic and hydrophilic antioxidant capacity of food extracts in vitro . Journal of Agricultural and Food Chemistry , 56 : 3470 – 3477 .
- Angelo , P.M. and Jorge , N. 2008 . Antioxidant evaluation of coriander extract and ascorbyl palmitate in sunflower oil under thermoxidation . Journal of the American Oil Chemists’ Society , 85 : 1045 – 1049 .
- Thorisson , S. , Gunstone , F.D. and Hardy , R. 1992 . The antioxidant properties of ethoxyquin and some of its oxidation products in fish oil and fish meal . Journal of the American Oil Chemists’ Society , 69 : 806 – 809 .
- Jayaprakasha , G.K. , Rao , L.J. and Sakariah , K.K. 2004 . Antioxidant activities of flavidin in different in vitro model systems . Bioorganic Medicinal Chemistry , 12 : 5141 – 5146 .
- Luthria , D.L. 2008 . Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor . Food Chemistry , 107K : 745 – 752 .
- Amic , D. , Davidovic-Amic , D. , Beslo , D. and Trinajstic , N. 2003 . Structure-radical scavenging activity relationship of flavonoids . Croatia Chemica Acta , 76 : 55 – 61 .
- Vinson , J.A. , Hap , Y. , Su , X. and Zubik , L. 1998 . Phenolic antioxidant quantity and quality in foods: Vegetables . Journal of Agricultural and Food Chemistry , 46 : 3630 – 3634 .
- Velioglu , Y.S. , Mazza , G. , Gao , L. and Oomah , B.D. 1998 . Antioxidant activity and total phenolics in selected fruits, vegetables and grain products . Journal of Agricultural and Food Chemistry , 46 : 4113 – 4117 .
- Nuutila , A.M. , Pupponen-Pimia , A.A.R.N.I. and Oksman-Caldentey , M. 2003 . Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity . Food Chemistry , 81 : 485 – 493 .
- Al-Ismail , K. , Herzallah , S.M. and Rustom , A.S. 2007 . Antioxidant activities of some edible wild Mediterranean plants . Italian Journal of Food Science , 19 : 287 – 296 .