Abstract
This study determined the total phenol, phenolic acid content, the free radical scavenging activity, and ferric reducing antioxidant properties of methanol and aqueous extracts of R. officinalis and O. dictamnus species (Crete, Greece). The methanol extracts showed higher radical scavenging activity (P < 0.05) than the aqueous extracts (mean EC50 value: 0.33 and 0.38 mg/mL, respectively). Rosemary was superior in activity to dittany (mean EC50 value: 0.26 and 1.04 mg/mL, respectively). A number of phenolic acids were also tested for comparison reasons and showed stronger radical scavenging activity than the synthetic antioxidant BHT (mean EC50 value: 0.0821 mg/mL). In the contrary, the aqueous extracts showed significantly higher ferric reducing antioxidant properties (P < 0.05) than the methanol extracts did (mean EC50 value: 0.028 and 0.038 mg/mL, respectively). Rosemary was superior in reducing activity to dittany (mean EC50 value: 0.035 and 0.107 mg/mL, respectively). Caffeic and rosmarinic acids were found to be stronger reducing agents (mean EC50 value: 0.0034 and 0.0021 mg/mL, respectively) than the other phenolic acids as strong as ascorbic acid (mean EC50 value: 0.0033 mg/mL). The aqueous plant extracts contained significantly higher total phenol (P < 0.05) than the methanol extracts did (mean value: 7.55 and 4.07 mg/g dry leaf). Rosemary was a better source of total phenol than dittany (mean value: 5.14 and 3.35 mg/g dry leaf). Rosmarinic and caffeic acids were identified in all the plant extracts and rosmarinic acid was the predominant phenolic acid ranging from 5574.6 to 7542.9 mg/kg dry leaf. The aqueous plant extracts were significantly higher in phenolic acid content than the methanol extracts (P < 0.05) (mean value: 9631.2, 7796.4 mg/kg dry leaf, respectively). Dittany was the highest source of phenolic acid content (P < 0.05) compared to rosemary (mean value: 9134.9 and 8292.7 mg/kg dry leaf, respectively). A positive linear correlation was found between total phenol content, radical scavenging activity and ferric reducing antioxidant properties of dittany extracts (r2 = 0.9998).
INTRODUCTION
Free radical reactions occur naturally in the human body. An over-production of these reactive species due to oxidative stress can cause oxidative damage to biomolecules and the development of chronic diseases, such as aging, coronary heart disease, and cancer.[Citation1] The harmful action of free radicals can be inhibited by antioxidant substances that scavenge them and detoxify the organism. Current research has confirmed that dietary antioxidants play an important role in the prevention of cardiovascular diseases, cancers, and neurodegenerative diseases as well as inflammation.[Citation2–4 Citation Citation−4
Among the various medicinal and culinary herbs, some species, such as plants of the Lamiaceae family, are added to foods for their organoleptic properties and some are consumed as herbal teas. Rosmarinus officinalis L. (Lamiaceae) is an evergreen sclerophyll plant that is well adapted to the limitations of the Mediterranean climate. It is an important spice, which is widely used as a food preservative in a ground form or as an extract,[Citation5] and it has received increasing attention due to its antimicrobial, anti-inflammatory, and antioxidative constituents.[Citation6–8 Citation Citation−8 The main compounds responsible for rosemary's antioxidant properties have been identified as phenolic diterpenes, such as carnosic acid, carnosol, and rosmano.[Citation9] Other compounds, such as rosmarinic acid, caffeic acid, and flavonoids, have also been associated with the antioxidant activities of rosemary.[Citation10,Citation11] Origanum dictamnus L. (dittany), a member of the genus Origanum, is an endemic plant only found in the mountains of the Greek island (Crete) and has been used in folk medicine as an herbal tea and further used in spiced wines. Dittany belongs to the Lamiaceae family as do rosemary, thyme, and oregano, but it has a less penetrating taste than these spices. Dittany extracts showed free radical scavenging activity related to phenolic compounds.[Citation12,Citation13]
Plant secondary metabolites are an enormously variable group of phytochemicals and among the most studied in the Lamiaceae family are phenolic acids, which exist as hydroxycinnamic and hydroxybenzoic acids in free or bound form (esters and glycosides). They are beneficial to human health with various biological effects, such as free radical scavenging, metal chelation, and modulation of enzymatic activity.[Citation14–19 Citation Citation Citation Citation Citation−19
Caffeic acid (CA) (3,4-dihydroxycinnamic acid) and its derivatives, exhibit antioxidant, antimutagenic, anti-inflammatory, hepatoprotective, and antimicrobial properties.[Citation16, Citation20, Citation21] Ferulic acid (FA) (4-hydroxy-3-methoxycinnamic acid), the methoxy derivative of caffeic acid is a ubiquitous phenolic compound in plant tissues and constitutes a bioactive ingredient of many foods. FA was found to protect DNA and lipids against oxidation through reactive oxygen species (ROS).[Citation22–24 Citation Citation−24 Rosmarinic acid (RA) is a diphenolic compound, one of the most important plant secondary metabolites, which occurs particularly in the families of Boraginaceae and Lamiaceae. Originally, RA was identified in rosemary (Rosmarinus officinalis L.); its structure was elucidated as an ester of caffeic acid and 3-(3,4-dihydroxyphenyl) lactic acid.[Citation25–29 Citation Citation Citation Citation−29 RA and its derivatives have been reported to have antioxidant, anti-HIV, anti-inflammatory, and cyclooxygenase inhibitory activity.[Citation16, Citation30, Citation31] Caffeic and rosmarinic acids are found to protect biological systems against various oxidative stresses by inhibition of superoxide anion production in the xanthine/xanthine oxidase system.[Citation18] Hydroxybenzoic acids, such as protocatechuic acid (PA) (3,4-dihydroxybenzoic acid) and gallic acid (GA) (3,4,5-trihydroxybenzoic acid), were also found to be strong antioxidants in emulsion or lipid systems.[Citation32]
Lamiaceae plants were studied as natural antioxidant sources because of their high contents of polyphenols.[Citation17, Citation19, Citation28] Despite the reports on the medicinal or functional properties of these aromatic leaves and their preparations, only a limited number of papers have been published on the determination of phenolic constituents of these materials by using high performance liquid chromatography (HPLC) techniques.[Citation10, Citation19, Citation28, Citation33] In addition, in most of the studies that have been conducted for the presence and the activity of antioxidants, the emphasis has been given to organic solvent extracts obtained from the dried leaves.[Citation12,Citation34] Little is known about the phenolic profiles and antioxidant activity in aqueous extracts or infusions of herbs.[Citation12,Citation35] It is important also to compare the antioxidant activities of plant extracts, which contain more than one phenolic component, with those of individual pure phenolic antioxidants, in order to determine possible synergistic interaction among them.
PRACTICAL APPLICATONS
The studies reported may prove beneficial in the exploitation of natural antioxidant sources for the preservation and/or extension of the shelf life of raw and processed foods.
The aims of this study were: (a) to determine the total phenols (TP) and phenolic acids (PA) in methanol and hot aqueous extracts (infusions) of Rosmarinus officinalis (rosemary) and Origanum dictamnus (dittany), by using UV and HPLC methods; (b) to evaluate the antioxidant properties of the plant extracts and standard phenolic acids by using the free radical scavenging (DPPH) and the ferric reducing antioxidant power (FRAP) assays; and (c) to correlate the antioxidant properties of the plant extracts with the determined phenolic compounds.
MATERIALS AND METHODS
Materials
The air-dried plant material of Origanum dictamnus and Rosmarinus officinalis were purchased from “Creta Land of Plenty” (Rethymno, Crete, Greece). The reagents Folin-Ciocalteau, ferric chloride, DPPH, BHT, rosmarinic acid (purum >95% HPLC), ferulic acid, 3,4-dihydroxybenzoic acid, caffeic acid gallic acid (purum ACS ≥98%), were obtained from Sigma-Aldrich (Steinheim, Germany). Ascorbic acid (99.7%) was obtained from Riedel-de Häen (Seelze, Germany). TPTZ (2,4,6-Tris-(2-pyridyl)-S-triazine) was from Alfa Aesar (Karlsruhe, Germany). Anhydrous sodium carbonate was from Applichem (Darmstadt, Germany). Methanol, acetone, acetic acid, n-hexane (analytical grade), and acetonitrile (HPLC grade) were purchased from Merck (Darmstadt, Germany).
Preparation of Plant Extracts
The solvents (100 mL) were used successively for the extraction of the pulverized leaf material (0.2 mm, 10 g) by stirring for 24 h at room temperature. Hexane was first used to remove the non-polar components from the leaf material followed by acetone and methanol. The mixture was then filtered and the solvent was removed using a rotary evaporator (40°C). For the hot aqueous extracts (infusions), boiling water (100 mL) was poured over the powdered plant material, filtered after 15 min, and lyophilized. The obtained plant extracts were kept in the dark at 4°C until use. Each extraction procedure was carried out three times.
Determination of Antioxidant Activity
2,2′-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay (RSA)
The antioxidant activity of the plant extracts, BHT, gallic, caffeic, ferulic, protocatechuic, and rosmarinic acids was measured in terms of hydrogen donating or radical scavenging activity using DPPH.[Citation36] DPPH is a stable free radical, which accepts a hydrogen radical to become a stable diamagnetic molecule, yellow-colored diphenylpicrylhydrazine. Various concentrations (0.1 mL) of the plant extracts (0.1–5 g/L), BHT (0.02–2 g/L), gallic acid (0.008–0.08 g/L), ferulic acid (0.04–1 g/L), protocatechuic acid (0.008–0.4 g/L), caffeic acid (0.005–0.2 g/L), and rosmarinic acid (0.01–0.1 g/L), were added to 3 mL of 6 × 10−5 M of DPPH in methanol. After a 45-min incubation period at room temperature, the absorbance (abs) was read against a control at 517 nm.
The percentage of the DPPH radical scavenging activity caused by the samples was calculated as follows:
Ac (0) is the absorbance of the control reaction (containing all reagents except for the extract/compound) when t = 0 min and As (t) is the absorbance of the extract/compound at t = 45 min.
The extract/compound effective concentration providing 50% RSA (EC50) was calculated from the plot of % RSA against extract/compound concentration (g/L). All determinations were performed in triplicate.
Ferric reducing antioxidant power assay (FRAP)
This assay measures the change in absorbance at 593 nm owing to the formation of a blue coloured Fe2+-tripyridyltriazine complex compound from a colourless Fe3+-tripyridyltriazine by the action of electron donating antioxidants.[Citation37] An amount of 0.2 ml of various concentrations of the plant extracts (0.01–1.5 g/L), ascorbic acid (0.0005–0.07 g/L), gallic acid (0.003–0.04 g/L), ferulic acid (0.009–0.5 g/L), protocatechuic acid (0.001–0.1 g/L), caffeic acid (0.001–0.04 g/L), and rosmarinic acid (0.001–0.5 g/L) was added to 3 ml of FRAP reagent (10 parts of 300 mM sodium acetate buffer at pH 3.6, 1 part of 10 mM TPTZ (2,4,6-tripyridyl-S-triazine) solution and 1 part of 20 mM ferric chloride) and the reaction mixture was incubated in a water bath at 37°C. The increase in absorbance at 593 nm was measured at 30 min.
The percentage of the ferric reducing/antioxidant power caused by the samples was calculated in the following way:
A 0 is the absorbance of the control reaction (containing all reagents except for the extract/compound) when t = 0 min and A 1 is the absorbance of the extract/compound at t = 30 min.
The extract/compound effective concentration providing 50% FRAP activity (EC50) was calculated from the plot of % FRAP against extract/compound concentration (g/L). All determinations were performed in triplicate.
Determination of Total Phenols by Using Folin-Ciocalteau (FC-TP)
The concentration of total phenols in extracts was measured at 760 nm, based on a colorimetric oxidation/reduction reaction. The oxidizing agent was Folin-Ciocalteau reagent.[Citation38] The sample (5 mL) was transferred to a 50-mL volumetric flask containing 6 mL of H2O to which was subsequently added 2.5 mL of undiluted Folin-Ciocalteau reagent. After 3 min, 5 mL of 20% aqueous Na2CO3 were added and the volume was made up to 50 mL with H2O. The controls contained all the reagents except the extract. After 1 h the absorbance was measured at 760 nm. A standard graph was obtained by repeating the same procedure for all gallic acid (GA) solutions (20–150 ppm). The results were expressed in mg GA per g of dry leaf and were presented as means of triplicates.
Determination of Phenolic Acids by Using HPLC (HPLC-PA)
A high-performance liquid chromatograph consisting of a Thermo Finnigan SCM 1000 solvent degasser (Thermo Fisher Scientific Inc., Boston, MA, USA), a Thermo Finnigann Spectra System P2000 pump model (Thermo Fisher Scientific Inc.), a column oven, and a Fasma 525 UV-Vis detector (Rigas Labs, Thessaloniki, Greece) was used. The column, a Kromasil 100 C18 (250 × 4.6 mm) (MZ-Analysentechnik, Mainz, Germany) was maintained at 28°C. The two solvents used for the isocratic elution of the samples were acetonitrile (v/v) (eluent A) and acetic acid 3.5% in water (eluent B) (23/77, v/v). The flow rate was 1.0 mL/min. Detection wavelength was 280 nm. The sample injection volume was 10 μL. The data were stored and processed with ChromQuest chromatographic software (Scientific Software Inc., San Jose, CA, USA). Working standard solutions of gallic acid (5–50 ppm), caffeic acid (0.5–10 ppm), ferulic acid (0.5–8 ppm), protocatechuic acid (10–100 ppm), and rosmarinic acid (20–200 ppm), were injected into the HPLC, and peak area responses were obtained. A standard graph for each compound was prepared by plotting concentration versus area. Quantification was carried out from integrated peak areas of the plant extracts (1.25–2.5 mg/mL). The results were expressed as mg of phenolic acid per kg of dry leaf and were presented as means of triplicates.
Statistical Analysis
The correlation analyses were performed using linear regression and the Pearson's correlation coefficient (r 2) (SPSS Inc., Chicago, IL, USA). The significance was evaluated by using the analysis of variance (two way-ANOVA) (Minitab Inc., State College, PA, USA). The probability value of P < 0.05 was used as the criteria for significant differences.
RESULTS AND DISCUSSION
Extensive studies have been carried out on the antioxidant activity and phenolic components of many species of the Lamiaceae family and it was demonstrated that this family species had a very strong antioxidant capacity.[Citation19, Citation39–44 Citation Citation Citation Citation Citation−44 The antioxidant activities of the plant extracts depend largely on the composition and concentration of the extracts as well as on the conditions of the test system.[Citation45] The antioxidant activities are influenced by many factors, which cannot be described with one single method. Therefore, it was necessary to perform more than one type of antioxidant activity measurement so as to take into account the various mechanisms of the antioxidant action.
DPPH is a stable nitrogen-centre free radical the colour of which changes from violet to yellow upon reduction by hydrogen or electron donation. Substances that are able to perform this reaction act as radical scavengers. The DPPH scavenging activities—expressed as EC50 values—of dittany and rosemary methanol and aqueous extracts as well as of BHT, hydroxybenzoic (gallic and protocatechuic acids) and hydroxycinnamic acids (caffeic, ferulic, and rosmarinic acids) are shown in . The results showed that the scavenging activities of all the plant extracts against the DPPH radical were concentration dependent. The increase in plant concentration (0.1–1.5 g/L) increased the scavenging activity in a logarithmic way for the methanol (r 2 = 0.9565) and aqueous (r 2 = 0.9678) extracts.
Figure 1 (a) DPPH scavenging activities of: (1) rosemary methanol extract; (2) rosemary aqueous extract; (3) dittany methanol extract; (4) dittany aqueous extract expressed as EC50 values (mg/ml); in comparison with (5) gallic acid; (6) caffeic acid; (7) ferulic acid; (8) protocatechuic acid; (9) rosmarinic acid; and (10) BHT. Data are means ± SD values of triplicate determination. (b) FRAP activities of: (1) rosemary methanol extract; (2) rosemary aqueous extract; (3) dittany methanol extract; (4) dittany aqueous extract expressed as EC50 values (mg/ml); in comparison with (5) gallic acid; (6) caffeic acid; (7) ferulic acid; (8) protocatechuic acid; (9) rosmarinic acid; and (10) ascorbic acid. Data are means ± SD values of triplicate determination.
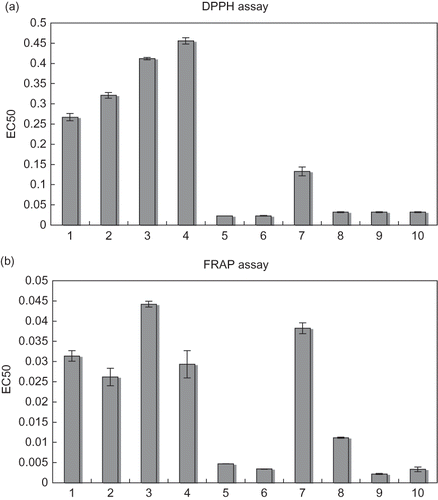
The results from the two-way analysis of variance (solvent, plant) (P < 0.05), showed that the methanol plant extracts showed higher radical scavenging activity (P < 0.05) than the aqueous extracts (mean EC50 value: 0.33 and 0.38 mg/mL, respectively). Rosemary was superior in activity to dittany (mean EC50 value: 0.26 and 1.04 mg/mL, respectively). Similar results in other studies showed that the concentration of extract necessary for a 50% reduction in the concentration of DPPH was approximately 0.2 mg/mL for aqueous and methanol extracts of rosemary leaf [Citation19, Citation35, Citation46] and 0.23 mg/mL for dittany infusions.[Citation47] The aqueous extracts of rosemary showed an increased radical scavenging activity in comparison with those of sage and oregano.[Citation46] The aqueous dittany extracts showed stronger antioxidant activity than the methanol, ethanol, and acetone extracts.[Citation12]
All of the examined phenolic acids—except ferulic acid (mean EC50 value: 0.1325 mg/mL)—showed stronger radical scavenging activity than the synthetic antioxidant BHT (mean EC50 value: 0.0821 mg/mL). Gallic acid and caffeic acid exhibited the same level of radical scavenging activity (mean EC50 value: 0.0229 and 0.0225 mg/mL, resp.) followed by protocatechuic acid and rosmarinic acid (mean EC50 value: 0.0322 and 0.0323 mg/mL, respectively). In similar studies, catechol-type o-diphenols, such as protocatechuic acid and caffeic acid, were found to exhibit high antiradical activity.[Citation16,Citation48] One of the best documented biological activities of ferulic acid (FA) is its antioxidant properties.[Citation49] Rosmarinic and hydroxycinnamic acid compounds have been found to possess strong antioxidant activity and rosmarinic acid much higher than that of a-tocopherol and BHT.[Citation30] Gallic acid was also found to be a strong antioxidant in emulsion or lipid systems.[Citation50] It is almost as effective as the tocopherol analogue Trolox and even more effective than several water soluble antioxidants, such as ascorbic acid.[Citation51]
Fe(III)-reduction is often used as an indicator of electron-donating activity, which is an important mechanism of phenolic antioxidant action.[Citation46,Citation52] The FRAP activities—expressed as EC50 values—of rosemary and dittany methanol and aqueous extracts as well as of ascorbic acid, hydroxybenzoic acids (gallic, protocatechuic), and hydroxycinnamic acids (caffeic, ferulic, and rosmarinic acids) are shown in .
The results showed that the reducing activities of all the plant extracts were concentration dependent. The increase in plant concentration (0.01–0.5 g/L) increased the activity in a logarithmic way for the methanol (r 2 = 0.9874) and aqueous (r 2 = 0.9883) extracts. The results from the two-way analysis of variance (solvent, plant) (P < 0.05) showed that the aqueous plant extracts showed significantly higher reducing capacity (P < 0.05) than the methanol extracts did (mean EC50 value: 0.028 and 0.038 mg/mL, respectively). Rosemary was superior in activity to dittany (mean EC50 value: 0.035 and 0.107 mg/mL, respectively). Rosemary aqueous fraction showed a remarkable potency to donate electron to reactive free radicals converting them into more stable non reactive species, thus, terminating the free radical chain reaction. Caffeic acid and rosmarinic acid were stronger reducing agents (mean EC50 value: 0.0034 and 0.0021 mg/mL, respectively) than gallic acid, protocatechuic acid, and ferulic acid. They showed similar levels of activity with ascorbic acid (mean EC50 value: 0.0033 mg/mL).
The concentration of total phenols (FC-TP) was determined in rosemary and dittany methanol and aqueous extracts. In , the results of the colorimetric analysis are given; they are derived from the absorbance values of the extract solutions compared to the standard solutions of gallic acid equivalents. The total phenol content of the plant extracts ranged from 2.98 to 9.13 mg GA/g dry leaf. The results from the two-way analysis of variance showed that the aqueous plant extracts contained significantly higher TP (P < 0.05) than the methanol extracts did (mean value: 7.55 and 4.07 mg/g dry leaf). Rosemary was a better source of TP than dittany (mean value: 5.14 and 3.35 mg/g dry leaf). In the aqueous rosemary extract, the TP content was found to be 9.13 mg/g dry leaf.
Table 1 FC-total phenols (mg GA/g dry leaf) and HPLC-phenolic acids (mg phenolic acid/kg dry leaf) in plant extracts
There are different reports in the literature on the amount of TP in rosemary. In a previous study, it was reported that the TP concentration in aqueous extracts was 185 mg GA/g of extract.[Citation35] Similar values were reported for rosemary methanol extracts.[Citation8, Citation19] In methanol extracts of herb leaves and stems, a phenolic concentration of 5.07 GA/100 g of herb (dry weight) was found.[Citation53] For fresh rosemary extracts obtained with phosphate buffer[Citation5] and 70% aqueous-methanol[Citation44] was reported concentrations of 2.19 and 7.67 mg GAE/g herb (fresh weight), respectively. The TP content in dittany aqueous-methanol extracts after acid hydrolysis was determined at 13.6 mg GA/g dry sample[Citation33] and in dittany aqueous infusion was found to be 1.09 mg GAE/mL.[Citation47] The aqueous extract of dittany had a significantly higher amount of phenolic compounds (21.7 meq/L) compared to the extracts obtained using methanol (13.8 meq/L), ethanol (7.7 meq/L), or acetone (6.7 meq/L) as solvents.[Citation12]
In the present work, the methanol and hot aqueous extracts (infusions) from dittany and rosemary were examined in order to identify free phenolic acids—hydroxybenzoic and hydroxycinnamic acids—using a simple and rapid isocratic RP-HPLC method. Optimal chromatographic conditions were first determined for a mixture of five hydroxybenzoic and hydroxycinnamic acids (gallic, protocatechuic, caffeic, ferulic, and rosmarinic acid) and the chromatogram is shown in . The most suitable flow rate was found to be 1 mL/min. Based on the UV absorption maximum of all analyzed compounds, the chromatograms were recorded at 280 nm. The optimal separation for the investigated phenolic acids was obtained by the isocratic elution with acetonitrile (eluent A) and acetic acid 3.5% in water (v/v) (eluent B) (23/77, v/v).
Figure 2 HPLC profiles of (a) phenolic acids standards, (b) methanol extract of R. officinalis, and (c) aqueous extract of O. dictamnus. Peak assignment: GA: gallic acid; PRA: protocatechuic acid; CA: caffeic acid; FRA: ferulic acid; and RA: rosmarinic acid.
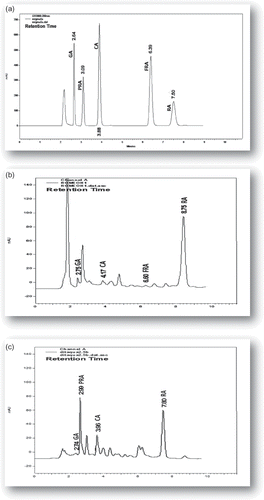
Linear regression analysis was also used to evaluate the calibration curve of each phenolic standard as a function of its concentration. All of the standards showed good linearity (0.9847–0.9998) in a relatively wide concentration range. Repeatability expressed as coefficient of variation (CV%) of multiple independent determinations was ranged from 0.42 to 5.57%. Accuracy of the method was evaluated with a recovery test. Samples of extracts were prepared to contain an analyzed compound of about 0.5–100 μg/ml. An equal volume of a standard solution with the same amount of tested compound was then added. Recoveries ranged from 97.56 to 103.68%.
Dittany and rosemary methanol and aqueous extracts were analyzed under the same chromatographic conditions. and display representative HPLC profiles of the extracts. The separation and identification of the chromatographic peaks in the examined plant extracts were confirmed by comparing the retention times and spiking with the corresponding phenolic standards. shows the content of phenolic acids (HPLC-PA) expressed in mg per kg dry leaf in the methanol and aqueous extracts of dittany and rosemary.
It can be seen from that rosmarinic and caffeic acids were identified in all the plant extracts and the concentrations of caffeic acid ranged from 54 to 599 mg/kg dry leaf, respectively. Rosmarinic acid was the predominant phenolic acid ranging from 5574.6 to 7542.9 mg/kg dry leaf. Ferulic, protocatechuic, and gallic acids were not identified in all the examined extracts and their concentrations ranged up to 4085.1 mg/kg dry leaf. The results from the two-way analysis of variance showed that the aqueous plant extracts were significantly higher in total phenolic acids than the methanol extracts (P < 0.05) (mean value: 9631.2, 7796.4, respectively). Dittany was the highest source of phenolic acids (P < 0.05) compared to rosemary (mean value: 9134.9 and 8292.7, respectively).
In similar studies, HPLC methods were used for the estimation of phenolic compounds, such as caffeic and rosmarinic acids, and their methyl esters in hydrophilic extracts from aromatic herbs of the Lamiaceae family, such as Thymus vulgaris, Mentha piperita, Salvia officinalis, and Rosmarinus officinalis.[Citation10, Citation19, Citation28] The content of rosmarinic and caffeic acids in aqueous-ethanol extracts of Lamiaceae plants, such as rosemary, sage, thyme, balm, spearmint, and lavender, ranged from 2 to 27.4 mg/g dry plant.[Citation10] In rosemary aqueous-methanol extracts, rosmarinic and caffeic acids ranged from 0.2 to 1.70 and 0.4 to 1.65 mg/g dry plant, resp. Ferulic and protocatechuic acids also detected in high amounts (0.75–4.20 mg/g and 1.10–7.25 mg/g dry plant, respectively), but their contribution to the total antioxidant activity of the plant extracts was minimal.[Citation54] Similar chemical profiles were also found by other workers.[Citation5, Citation19, Citation53,55] In dittany infusions, phenolic acids, such as caffeic acid, esters of gallic acid, p-hydroxybenzoic, and vanillic acid, were identified by LC-DAD-MS.[Citation47] In aqueous-methanol extracts after acid hydrolysis gallic and caffeic acids were determined at 0.7 and 23 mg/100 g dry sample, respectively.[Citation33] Caffeic and ferulic acids were also identified among other phenolic acids in oregano methanol extracts.[Citation56] Rosmarinic acid was also the predominant phenolic acid determined in other Lamiaceae plants, such as the methanol extracts of basil,[Citation27] in ethanol, acetone, and methanol extracts of oregano species.[Citation57,Citation58] Rosmarinic acid was the most abundant phenolic compound identified in the aqueous extracts of different species of oregano and thyme even after the removal of the essential oil part.[Citation28,Citation29]
The obtained results showed that there is a positive linear correlation between total phenolic content, free radical scavenging, and ferric reducing antioxidant properties of dittany extracts (r 2 = 0.9998). According to these results, good positive linear correlations between total phenolic content, free radical scavenging, and ferric reducing antioxidant properties of many Lamiaceae plants were found.[Citation5, Citation28, Citation44, Citation46, Citation50, Citation56] Previous studies have reported the relationship between total phenolics and antioxidant activity in tea, fruits, vegetables, and red wine.[Citation20] Other studies showed poor correlation or even could not explain the relationship between total antioxidant activity and phenolic content.[Citation18,Citation39] The relationships between particular antioxidants and antioxidant activity are difficult to explain on the basis of quantitative analysis only. Not only the level of antioxidants but also interactions occur among them, and other constituents might influence the antioxidant activity of herbs.[Citation59]
CONCLUSIONS
The obtained results showed that rosemary exhibited higher free radical scavenging, ferric reducing antioxidant properties, and total phenol content than dittany. Dittany was superior in the content of HPLC determined phenolic acids, especially hydroxybenzoic (gallic, protocatechuic acid) and hydroxycinnamic acids (caffeic, ferulic, and rosmarinic acid), which showed antioxidant activities comparable or even better to that of BHT and ascorbic acid. The positive linear correlation that was found between the total phenols, free radical scavenging, and ferric reducing antioxidant activity of dittany extracts showed that the quantification of phenolic components could be a measure of antioxidant activity for Lamiaceae extracts. The aqueous extracts of rosemary and dittany exhibited higher ferric reducing activity, total phenol, and phenolic acid content than the plant methanol extracts and could be used as safer sources of natural antioxidants free of organic residues.
Further studies on the presence of other bioactive components, should be carried out in order to complete the phenolic identification data and investigate the contribution of minor phenolic compounds to the total antioxidant activity of the plant extracts.
ACKNOWLEDGMENTS
The authors would like to thank Professor S. Raphaelides for providing the technical infrastructure and his valuable contribution and guidance for the manuscript.
REFERENCES
- Halliwell , B. , Gutteridge , J.M.C. and Cross , C.E. 1992 . Free, antioxidants and human disease: Where are now? . Journal of Laboratory and Clinical Medicine , 119 : 598 – 619 .
- Kris-Etherton , P.M. , Hecker , K.D. , Bonanome , A. , Coval , S.M. , Binkoski , A.E. and Hilpert , K. 2002 . Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer . American Journal of Medicine , 113 : 71 – 88 .
- Di Matteo , V. and Esposito , E. 2003 . Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis . Current Drug Targets. CNS and Neurological Disorders , 2 : 95 – 107 .
- Scalbert , A. , Manach , C. , Morand , C. and Remesy , C. 2005 . Dietary of polyphenols and the prevention of diseases . Critical Reviews in Food Science and Nutrition , 45 : 287 – 306 .
- Zheng , W. and Wang , S.Y. 2001 . Antioxidant activity and phenolic compounds in selected herbs . Journal of Agricutlural and Food Chemistry , 49 : 5165 – 5170 .
- Anselmi , C. , Centini , M. , Granata , P. , Sega , A. , Buonocore , A. , Bernini , A. and Facino , R.M. 2004 . Antioxidant activity of ferulic acid and alkyl esters in a heterophasic system: A mechanistic insight . Journal of Agricultural and Food Chemistry , 52 : 6425 – 6432 .
- Romano , C.S. , Abadi , K. , Repetto , V. , Vojnov , A. and Moreno , S. 2009 . Synergistic antioxidant and antibacterial activity of rosemary plus butylated derivatives . Food Chemistry , 115 : 456 – 461 .
- Erkan , N. , Ayranci , G. and Ayranci , E. 2008 . Antioxidant activities of rosemary extract, blackseed essential oil, carnosic acid, rosmarinic acid and sesamol . Food Chemistry , 110 : 76 – 82 .
- Thorsen , M.A. and Hildebrandt , K.S. 2003 . Quantitative determination of phenolic diterpenes in rosemary extracts . Aspects of accurate quantification. Journal of Chromatography A , 995 : 119 – 125 .
- Wang , H. , Provan , G.J. and Helliwell , K. 2004 . Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC . Food Chemistry , 87 : 307 – 331 .
- Lagouri , V. and Nisteropoulou , E. 2009 . Antioxidant properties of O. onites, T. vulgaris and O. basilicum species grown in Greece and their total phenol and rosmarinic acid content . Journal of Food Lipids , 16 : 484 – 498 .
- Moller , J.K.S. , Madsen , H.L. , Aaltonen , T. and Skibsted , L.H. 1999 . Dittany as a source of water extractable antioxidants . Food Chemistry , 64 : 215 – 219 .
- Kouri , G. , Tsimogiannis , D. , Bardouki , H. and Oreopoulou , V. 2007 . Extraction and analysis of antioxidant components from O . dictamnus. Innovative Food Science and Emerging Technologies , 8 : 155 – 162 .
- Rice-Evans , A.C. , Miller , N.J. and Papanga , G. 1996 . Structure-antioxidant activity relationship of flavonoids and phenolic acids . Free Radical Biology and Medicine , 20 : 933 – 956 .
- Kroon , P.A. and Williamson , G. 1999 . Hydroxycinnamates in plants and food: Current and future perspectives . Journal of the Science of Food and Agriculture , 79 : 355 – 361 .
- Lu , Y. and Foo , L. 2001 . Antioxidant activities of polyphenols from sage . Food Chemistry , 75 : 197 – 202 .
- Dapkevicius , A. , Van Beek , T.A. , Lelyveld , G.P. , Van Veldhuizen , A. , Groot , A. , Linssen , J.P.H. and Venskutonis , R. 2002 . Isolation and structure elucidation of radical scavengers from Thymus vulgaris leaves . Journal of Natural Products , 65 : 892 – 896 .
- Parejo , J. 2002 . Comparison between the radical scavenging activty and the antioxidant activity of six distilled and non distilled Mediterranian herbs and aromatic plants . Journal of Agriculture and Food Chemistry , 50 : 6882 – 6890 .
- Kosar , M. , Dorman , H.J.D. and Hiltunen , R. 2005 . Effect of an acid treatment on the phytochemical and antioxidant characteristics of extracts from selected Lamiaceae species . Food Chemistry , 91 : 525 – 533 .
- Lee , K.J. , Choi , J.H. , Hwang , Y.P. , Chung , Y.C. and Jeong , H.G. 2008 . Protective effect of caffeic acid phenethyl ester on tert-butyl hydroperoxide induced oxidative hepatotoxicity and DNA damage . Food and Chemical Toxicoloxy , 46 : 2445 – 2450 .
- Kang , N.J. , Lee , K.W. , Shin , B.J. , Jung , S.K. , Hwang , M.K. , Bode , A.M. , Heo , Y.S. , Lee , H.J. and Dong , Z. 2009 . Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced CoX-2 expression . Carcinogenesis , 30 : 321 – 330 .
- Ogiwara , T. , Satoh , K. , Kadoma , Y. , Murakami , Y. , Unten , S. and Atsumi , T. 2002 . Radical scavenging activity and cytotoxicity of ferulic acid . Anticancer Research , 22 : 2711 – 2717 .
- Abdalla , A.E. and Roosen , J.P. Effect of plant extracts on the oxidative stability of sunflower oil and emulsion . Food Chemistry 1999 , 64 323 – 329 .
- Srinivasan , M. , Sudheer , A.R. , Pillai , K.R. , Kumar , P.R. , Sudhakaran , P.R. and Moon , V.P. 2006 . Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes . Toxicology , 228 : 249 – 258 .
- Harborne , J.B. 1966 . Caffeic acid ester distribution in higher plants . Zeitschrift fur Naturforscung , 21b : 604 – 605 .
- Yang , R. and Shetty , K. 1998 . Stimulation of rosmarinic acid in shoot cultures of oregano (Origanum vulgare) clonal line in response to proline, proline analogue, and praline precursors . Journal of Agricultural and Food Chemistry , 46 : 2888 – 2893 .
- Javanmardi , J. , Khalighi , A. , Kashi , H. , Bais , H.P. and Vivanco , J.M. 2002 . Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran . Journal of Agricultural and Food Chemistry , 50 : 5878 – 5883 .
- Dorman , H.J. , Bachmayer , O. , Kosar , M. and Hiltunen , R. 2004 . Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey . Journal of Agricultural and Food Chemistry , 52 : 762 – 770 .
- Kulisic , T. , Dragovic , V. and Milos , M. 2006 . Antioxidant activity of herbal tea infusions . Food Technology and Biotechnology , 44 : 485 – 492 .
- Chen , J.H. and Ho , C.T. 1997 . Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds . Journal of Agricultural and Food Chemistry , 41 : 2374 – 2378 .
- Kelm , M.A. , Nair , M.G. , Strasburg , G.M. and Dewitt , D.L. 2000 . Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum . Phytomedicine , 7 : 7 – 13 .
- Madsen , H.L. and Bertelesen , G. 1995 . Spices as antioxidants . Trends in Food Science and Technology , 6 : 271 – 277 .
- Proestos , C. , Chorianopoulos , N. , Nychas , G.J.E. and Komaitis , M. 2005 . RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity . Journal of Agricultural and Food Chemistry , 53 : 1190 – 1195 .
- Wettasinghe , M. and Shahidi , F. 1999 . Antioxidant and free-radical scavenging properties of ethanolic extracts of defatted borage seeds . Food Chemistry , 67 : 399 – 414 .
- Dorman , H.J.D. , Peltoketo , A. , Hiltunen , R. and Tikkanen , M.J. 2003 . Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs . Food Chemistry , 83 : 255 – 262 .
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of free radical method to evaluate antioxidant activity . Lebensmittel-Wissenschaft und- Technology , 28 : 25 – 30 .
- Benzie , I.F. and Strain , J.J. 1996 . The ferric reducing ability of plasma as a measure of “antioxidant power” the FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Slinkard , K. and Singleton , V.L. 1997 . Total phenol analysis: automation and comparison with manual methods . American Journal of Enology and Viticulture , 28 : 49 – 55 .
- Hinneburg , I. , Dorman , H.J.D. and Hiltunen , R. 2006 . Antioxidant activities of extracts from selected culinary herbs and spices . Food Chemistry , 97 : 122 – 129 .
- Politeo , O. , Jukic , M. and Milos , M. 2007 . Chemical composition and antioxidant capacity of free volatile aglycones from basil compared with its essential oil . Food Chemistry , 101 : 379 – 385 .
- Bounatirou , S. , Smiti , S. , Miguel , M.G. , Faleiro , L. , Rejeb , M.N. and Naffati , M. 2007 . Chemical composition antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus . Food Chemistry , 105 : 146 – 155 .
- Wojdylo , A. , Oszmiansk , J. and Czemerys , R. 2007 . Antioxidant activity and phenolic compounds in 32 selected herbs . Food Chemistry , 105 : 940 – 949 .
- Mata , A.T. , Proenca , C. , Ferreira , A.R. , Serralheiro , M.L.M. , Nogueira , J.M.F. and Araujo , M.E.M. 2007 . Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices . Food Chemistry , 103 : 778 – 786 .
- Yoo , K.M. , Lee , C.H. , Lee , H. , Moon , B. and Lee , C.Y. 2008 . Relative antioxidant and cytoprotective activities of common herbs . Food Chemistry , 106 : 929 – 936 .
- Wanasundara , U.N. and Shahidi , F. 1998 . Antioxidant and pro-oxidant activity of green tea extracts in marine oils . Food Chemistry , 63 : 335 – 342 .
- Dorman , H.J.D. , Kosar , M. , Kahlos , K. , Holm , Y. and Hiltunen , R. 2003 . Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties and cultivars . Journal of Agricultural and Food Chemistry , 51 : 4563 – 4569 .
- Atoui , K.A. , Mansouri , A. , Boskou , G. and Kefalas , P. 2005 . Tea and herbal infusions: Their antioxidant activity and phenolic profile . Food Chemistry , 89 : 27 – 36 .
- Silva , F.A. , Borges , F. , Guimarães , C. , Lima , J.L. , Matos , C. and Reis , S. 2000 . Phenolic acids and derivatives: Studies on the relationship among structure, radical scavenging activity, and physicochemical parameters . Journal of Agricultural and Food Chemistry , 48 : 2122 – 2126 .
- Graf , E. 1992 . Antioxidant potential of ferulic acid . Free Radical Biology and Medicine , 13 : 435 – 448 .
- Madsen , H.L. , Nielsen , B.R. , Bertelsen , G. and Skibsted , L.H. 1996 . Screening of antioxidative activity of spices. A comparison between assays based on ESR spin trapping and electrochemical measurement of oxygen consumption . Food Chemistry , 57 : 331 – 337 .
- Cholbi , M.R. , Pays , M. and Alcaraz , M.J. 1991 . Inhibitory effects of phenolic compounds on carbon tetrachloride induced microsomal lipid peroxidation . Experientia , 47 : 195 – 199 .
- Yiidirim , A. , Mavi , A. and Kara , A. 2001 . Determination of antioxidant and antimicrobial activities of Runex crispus L . extracts. Journal of Agricultural and Food Chemistry , 52 : 4026 – 4089 .
- Shan , B. , Yizhong , Z.C. , Sun , M. and Corke , H. 2005 . Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents . Journal of Agriculture and Food Chemistry , 53 : 7749 – 7759 .
- Papageorgiou , V. , Gardeli , C. , Mallouchos , A. , Papaioannou , M. and Komaitis , M. 2008 . Variation of the chemical profile and antioxidant behavior of R. officinalis and S. fruticosa grown in Greece . Journal of Agriculture and Food Chemistry , 56 : 7254 – 7256 .
- Zheng , W. and Wang , Y. 2001 . Antioxidant activity and phenolic compounds in selected herbs . Journal of Agriculture and Food Chemistry , 49 : 5165 – 5170 .
- Goze , I. , Alim , A. , Tepe , A.S. , Sokmen , M. , Sergi , K. and Tepe , B. 2009 . Screening of the antioxidant activity of essential oil and various extracts of O .rotundifolium from Turkey . Journal of Medicinal Plants Research , 3 : 246 – 254 .
- Exarchou , V. , Nenadis , N. , Tsimidou , M. , Gerothanasis , I.P. , Troganis , A. and Boskou , D. 2002 . Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage and summer savory . Journal of Agriculture and Food Chemistry , 5 : 5294 – 5299 .
- Pizzale , L. , Bortolomeazzi , R. , Vichi , S. , Uberreger , E. and Conte , L.S. 2002 . Antioxidant activity of sage (S. officinalis and S. fruticosa) and oregano (O. onites and O. indercedens) extracts related to their phenolic compound content . Journal of the Science of Food and Agriculture , 82 : 1645 – 1651 .
- Zaporozhets , O.A. , Krushynska , O.A. , Lipkovska , N.A. and Barvinchenk , V.N. 2004 . A new test for the evaluation of total antioxidant activity of herbal products . Journal of Agriculture and Food Chemistry , 52 : 21 – 25 .