Abstract
The seeds of Zanthoxylum armatum DC, on hydrodistillation, yielded 1.2% of the essential oil. The oleoresins were extracted by a Soxhlet extractor using ethanol, ethyl acetate, and isopropyl alcohol. Gas chromatography-mass spectrometry analysis of the essential oil resulted in the identification of 38 components, of which linalool (62%) and limonene (18.1%) were the major components. The major components of oleoresins were linoleic acid, palmitoleic acid, and linalool. The antioxidant potential of essential oil and oleoresins were evaluated by 2,2′-diphenyl picrylhydrazyl radical scavenging, Fe2+ chelating, ferric thiocyanate method, and various lipid peroxidation assays. The essential oil showed maximum antioxidant potential, whereas oleoresins showed moderate antioxidant activity.
*This article is part 73 in a series on essential oils.
INTRODUCTION
At present, there is increasing consumer demand for herbal and natural products, particularly in medicines, cosmetics, and food additives. Due to possible toxicologicalCitation[1, Citation2] effects of synthetic food additives on human health, the general trend towards reducing the use of synthetic food additives have caused great interest in nutritional research. In the literature, there are many reports on antimicrobial and antioxidantCitation[3– Citation5] properties of essential oils and extracts of plants. The antioxidant capacity can be explored in the food industry by using aromatic plants as a source of antioxidants to prevent the rancidity and oxidation of lipids.Citation[6] In fact, in recent years, researchers have focused on medicinal plants for extracting natural antioxidantsCitation[7, Citation8] that can replace synthetic ones, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate, etc.
Zanthoxylum armatum DC (Rutaceae) is an erect shrub or a small tree commonly growing in hot valleys of the Himalayas. It is commonly known by the name ‘Tomer’ or ‘Timur’ and is extensively used in the Indian system of medicine as a carminative, stomachic, and to relieve toothache. Its fruits and seeds are also extensively used in fever, dyspepsia, and to kill roundworms. Kalia et al.Citation[9] and Mehta et al.Citation[10] have reported that the essential oil of its fruits exhibited good antibacterial, antifungal, and antihelmintic activities. The larvicidal activity of essential oil and petroleum extract against mosquito vectors has also been reported.Citation[11, Citation12] Continuing with the investigations,Citation[13– Citation16] the chemistry and antioxidant properties of the essential oil and oleoresins extracted from the seeds of Zanthoxylum armatum are reported here.
MATERIALS AND METHODS
Chemicals
BHA, BHT, glacial acetic acid, potassium ferricyanide, ferrous chloride, and ferric chloride were purchased from S D Fine Chemicals Ltd. (Mumbai, India). NaOH, ferrozine, chloroform, p-anisidine, methanol, ethanol, and isopropyl alcohol were supplied by Merck (Mumbai, India). Analytical grade diphenyl picrylhydrazyl (DPPH) and linoleic acid (Fluka Chemicals, Mumbai, India) and thiobarbituric acid (TBA) and potassium iodide (Qualigens Fine Chemicals, Mumbai, India) were purchased and used.
Extraction of Essential Oil and Oleoresins
The seeds of tomer were purchased from the local market of Gorakhpur, India in April, 2008, and voucher specimens were deposited at the Herbarium of the Science Faculty of DDU Gorakhpur University, Gorakhpur, India. The seeds were washed, sun dried, and pulverized into a fine powder. Spice powder in the amount of 150 g was subjected to hydrodistillation in a Clevenger's type apparatus for 5 h according to a European Pharmacopoeian procedure.Citation[17] The light yellow colored oil obtained (yield 1.2%) was dried over a minimum amount of anhydrous sodium sulfate and stored at 4 ± 1°C. Oleoresins were obtained by extraction of spice powder with a suitable solvent (here, ethanol, ethyl acetate, and isopropyl alcohol were used). For this, 30 g of ground spice was loaded on the Soxhlet's apparatus and extracted with the solvent for 5–6 h. After complete extraction, the solvents were distilled off to obtain viscous oleoresins, which were stored at 4°C.
Chemical Analysis
Chemical composition of volatile oil and oleoresins of tomer were analyzed by gas chromatography-mass spectrometry (GC-MS) technique using a Hewlett-Packard gas chromatograph (Model 6890, Hewlett-Packard, Agilent Technologies, Buenos Aires, Argentina) coupled with a quadruple mass spectrometer (Model HP 5973, Hewlett-Packard, Agilent Technologies, Buenos Aires, Argentina) and a Perkin Elmer Elite-5MS capillary column (5% phenylmethylsiloxane; length 30 m × inner diameter 0.25 mm × film thickness 0.25 μm, Argentina). The injector, interphase, ion source, and selective mass detector temperatures were maintained at 250, 280, 230, and 150°C, respectively. Helium (He) was used as a carrier gas at a flow rate of 1.0 mL/min for essential oil and at 1.5 mL/min for the oleoresins. For the oil, the oven temperature was programmed at: 60°C for 1 min; then increased from 60 to 185°C at the rate of 1.5°C/min and held at 185°C for 1 min; then again increased from 185 to 275°C at the rate of 9°C/min and held at 275°C for 2 min. The oven temperature for oleoresins was programmed as follows: 70°C (zero min), increased from 70° to 280°C at the rate of 5°C/min and held at 280°C for 20 min.
Antioxidant Properties
A number of different methods are necessary to adequately assess the in vitro antioxidant properties of a particular substance. Hence, several standard methods have been used to evaluate the antioxidant activity of the essential oil and oleoresins and compared them with a few synthetic antioxidants viz. BHA and BHT.
Oxidation of Mustard Oil
Sample preparation
The unrefined crude mustard oil samples (50 g each) containing 200 ppm (w/v) concentration of essential oil and oleoresins of tomer were prepared. Synthetic antioxidants, such as BHA and BHT, were also added to mustard oil at the same concentration. Mustard oil without any additive was taken as a control sample. All the samples were exposed to accelerated oxidation by incubating at 70°C in darkness.
Peroxide value
Total peroxide and hydroperoxide oxygen content of the mustard oil samples was determined by measuring peroxide value (PV) at regular intervals according to the procedure prescribed by Horwitz.Citation[18] An oil sample (5 g) was dissolved in 30 mL of glacial acetic acid-chloroform (3:2) solution and mixed with 0.5 mL of saturated KI solution. After 1 min, 30 mL of distilled water was added followed by titration with 0.01N Na2S2O3 using starch as an indicator. Titration was continued, shaking the flask vigorously until the blue color just disappeared. The peroxide value (meq of peroxide/kg of oil) was calculated as:
Anisidine value
The anisidine value of mustard oil samples was estimated by the method described earlier.Citation[19] An oil sample (5 g) was dissolved in isooctane to make up the volume of 50 mL. Further, a 5 mL aliquot of this solution was mixed with 1 mL of 0.25% anisidine reagent and left for 10 min in the dark at room temperature. Absorbance of the solution was then recorded spectrophotometrically (Aimil Spectrochem NV201, Aimil Ltd., New Delhi, India) at 350 nm. A blank test, without anisidine reagent, was also performed. The anisidine value was calculated as:
TBA value
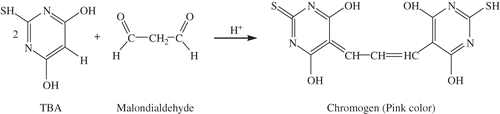
The TBA value of different samples was determined according to the method previously reported by Pokorny and Dieffenbacher.Citation[20] About 100 mg of the oil sample was dissolved in 25 mL of 1-butanol. A 25 mL aliquot of the above solution was mixed thoroughly with 5.0 mL of TBA reagent (200 mg TBA in 100 mL 1-BuOH) and incubated at 95°C. After 2 h, the reaction mixture was cooled to room temperature under running water and absorbance was measured at 530 nm. At the same time, a reagent blank test (without TBA reagent) was also done. The TBA value (meq of malondialdehyde/g) was calculated as:
Individual Complementary Assays
Total antioxidant activity (ferric thiocyanate [FTC] method)
The total antioxidant activity was determined according to the FTC methodCitation[21] with some modifications. The reaction medium is a mixture of 2.5 mL solution of tomer oil/oleoresins (1 mg/100 mL in absolute alcohol), 2.5 mL of 2.51% linoleic acid emulsion, and 5 mL of 0.05 M-phosphate buffer (pH 7.0). The mixed solution (10 mL) was incubated at 40°C in the dark. The same solution without any test substance was used as the control. The peroxide level of each sample was determined by reading the absorbance at 500 nm in a UV-VIS spectrophotometer after reaction with 0.1 mL of 20 mM FeCl2 and 0.2 mL of 30% ammonium thiocyanate every 12 h. BHA and BHT were used as positive standards.
DPPH free radical scavenging activity
The capacity of volatile oil and oleoresins of tomer to scavenge the DPPH radical was determined by the method reported earlier.Citation[22] For this, 1 mL of freshly prepared solution of DPPH radical solution (0.1 mM in methanol) was mixed thoroughly with 3 mL of methanolic solution of volatile oil and oleoresins (5–20 μg/mL). Then the reaction mixture was left for 30 min in the dark at room temperature to measure the absorbance, which was recorded at 517 nm. In addition, the control (without having any additive) and standards (with BHA and BHT; in place of oil and oleoresins) were also tested. The capability to scavenge the DPPH radical (% inhibition) was calculated using the following equation:
Ferrous ion chelating activity
The method reported by Senevirathne et al.Citation[23] was used to determine the ferrous ion chelating activity of different tomer essential oil and oleoresins. A 200-μL amount of each essential oil/oleoresins was mixed with 0.1 mL of 2 mM FeCl2 and 0.2 mL of 5 mM ferrozine solutions. After a 10 min equilibrium period, the absorbance was recorded at 562 nm. In fact, a complex of Fe2+/ferrozine showed strong absorbance at 562 nm.
Statistical Analysis
Experimental results were the means ± standard deviation of three parallel measurements (data are not shown). Significant differences between means were determined by Student's t-test by using a Microsoft Excel (Microsoft Office, India) statistical analysis program and p < 0.05 was considered as significant.
RESULTS AND DISCUSSION
Chemical Investigations
The seeds of Z. armatum were subjected to hydrodistillation to get 1.2% w/w of the essential oil. GC-MS revealed at least 38 components that could be identified representing 99.8% of the oil (). The essential oil consists of linalool (62%) as the major component followed by limonene (18.1%), E-methyl cinnamate (6.5%), and myrcene (2.3%). Among oleoresins, 33 components were identified in ethanol oleoresin representing about 79.2% of the total weight; 33 components in ethyl acetate oleoresin constituting 88.3% of the oleoresin, and 26 components were identified in isopropyl alcohol oleoresin representing about 86% of the total amount of the oleoresin. The major components identified in oleoresins were linoleic acid, palmitoleic acid, linalool, oleic acid, and palmitic acid (), although the other components present are minor ones.
These findings are in good agreement with the previous reportsCitation[11, Citation24] with some variations in the constituents. The variations in the composition of the essential oil and oleoresins obtained from the same species may be due to different chemotypesCitation[25] or may result from the environmental, developmental, genetic, or some other differences.Citation[26] The yield and composition of essential oil and oleoresins also differ widely with the production conditions,Citation[27] variety, cultivars, or population.Citation[28] Moreover, it has been observed experimentally that the extraction of oleoresins in different solvents yields different components.
Antioxidant Activity
Oxidation of mustard oil
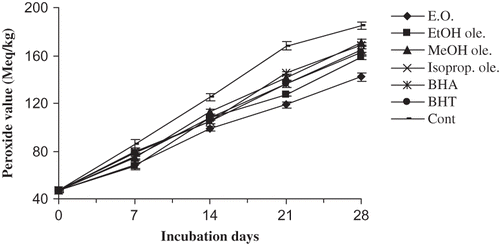
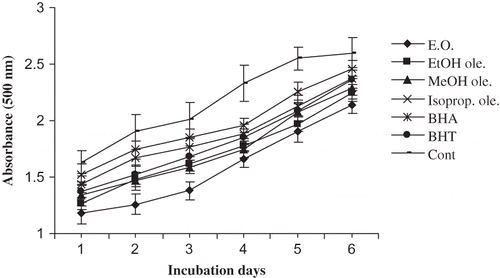
The curves in show PV changes in mustard oil with different additives. It should be mentioned that the PV is a widely used measure of primary lipid oxidation indicating the amount of peroxides formed in fats and oils during oxidation. The stoichiometries for this method are:
where ROOH is a lipid hydroperoxide and ROOR is lipid peroxide.
Mustard oil oxidation was measured at fixed intervals during 28 days of storage. During this time, the PV of the blank sample (control) increased from 46.7 to 185 meq kg−1. The results given in show that all the tested substances had significantly (p < 0.05) reduced the oxidation rate of mustard oil at 70°C in terms of formation of peroxides. However, the best activity was shown by the essential oil followed by ethanol oleoresin. The activities of both these were significantly higher (p < 0.05) than BHA and BHT. The activities of the other two oleoresins were comparable to BHA but lower than BHT.
Primary oxidation products are unstable compounds that produce a number of secondary products, such as alkanes, alcohols, aldehydes, and acids. Many of these secondary oxidation products are highly reactive themselves and may initiate the oxidation chain processes. Hence, it was necessary to determine simultaneously the changes in the concentration of the secondary oxidation products after every 7 days with the measurement of PV.
Table 1 Chemical composition (% MS) of the essential oil and oleoresins of Zanthoxylum armatum
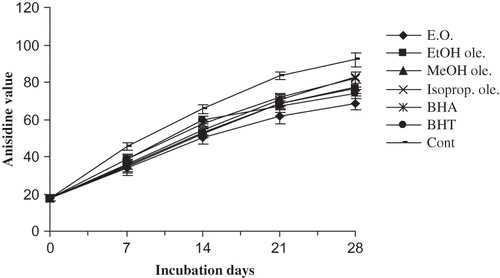
The anisidine value represents the level of aldehydes, principally 2-alkenals, in the oils. shows the anisidine values of various mustard oil samples at different time intervals during the storage. The values obtained are in good correlation with peroxide values of the same samples. The essential oil and ethanol oleoresin had shown significantly higher (p < 0.05) activities than BHA and BHT. However, the activities of methanol and isopropanol oleoresins were found to be comparable to those of BHA but significantly lower (p < 0.05) than that of BHT. Malondialdehyde (formed by the decomposition of peroxides during the oxidation process, is also used as an index of lipid peroxidation) reacts with TBA to form a pink chromogen that is measured spectrophotometrically:
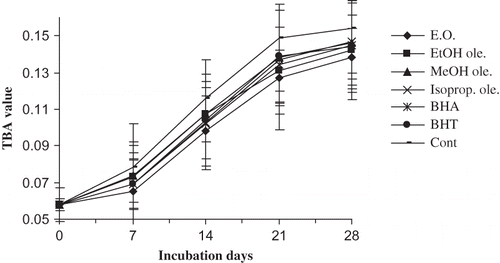
shows that the volatile oil and ethanol oleoresin had significantly (p < 0.05) lowered the TBA value than the control. Moreover, their activities were also significantly (p < 0.05) higher than those of BHA and BHT. The activities of the other two oleoresins were also good but there was no any significant (p < 0.05) difference between them and the synthetic compounds. From Figs. , it can be interpreted that the essential oil and oleoresins of tomer can control both primary and secondary oxidation processes and their antioxidative potentials were found to be better than BHA, and in a few experiments, better than BHT also.
Individual complementary assays
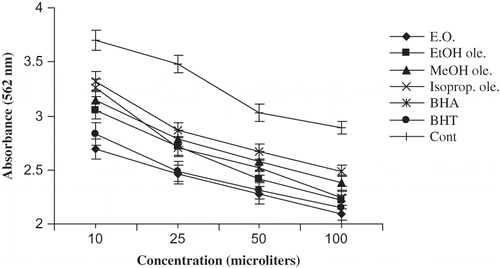
The peroxide level during the initial stages of lipid peroxidation was measured using the emulsified linoleic acid system. The inhibitor activities against lipid peroxidation in linoleic acid caused by additives were evaluated by measuring concentration of ferric thiocyanate. Oxygen is needed for linoleic acid oxidation and converts it to the lipid hydroperoxide:Citation[29]
The hydroperoxide is being very active and attacks other molecules very easily. It is well known that antioxidants perform their inhibition by various mechanisms, such as peroxide decomposition or chain stopping.Citation[30] is concerned with the absorbance values measured for different samples. A low absorbance value indicates a high level of antioxidant activity. The values obtained without an additive were taken for 100% lipid peroxidation. From , it is evident that there was a significant (p < 0.05) difference between the blank and the antioxidants. The activity of essential oil was found to be better than the synthetic ones. Moreover, the results were well correlated with those obtained previously by PV and TBA methods.
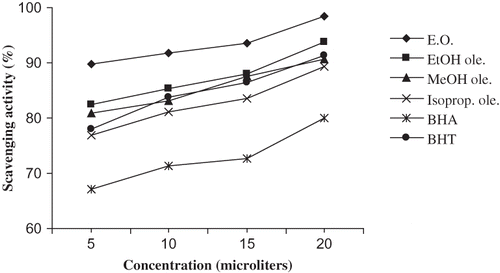
As is clear from , the essential oil, oleoresins, and the reference compounds were all able to reduce the stable radical DPPH to the yellow-colored diphenylpicrylhydrazine. The strongest effect was observed for the essential oil. The scavenging effect of tested substances on DPPH radicals increased with increasing concentrations (5–20 μg/mL). According to , essential oil (20 μg/mL) exhibited 98.5% scavenging activity, which was significantly (p < 0.05) higher than that of the oleoresins, BHA, and BHT at the same concentration. The activity of ethanol oleoresin was also significantly higher (p < 0.05) than BHA and BHT, while the activities of the other two oleoresins were higher than BHA but lower than BHT. Przybylski et al.Citation[31] have reported that extracts isolated with polar solvents could contain higher amounts of compounds, which act as H-donors and showed a higher antioxidant activity. When an antioxidant is added to the radicals, there is a degree of decolorization owing to the presence of the antioxidant, which reverses the formation of radicals:
Figure 5 Radical scavenging effect of Z. armatum essential oil and oleoresins on 2,2′-diphenyl-1-picrylhydazyl radical.
Metal chelating capacity is significant since it reduces the concentration of the catalyzing transition metal in lipid peroxidation. The chelation of ferrous ions by the components of essential oil, oleoresins, and commercial antioxidants was estimated by the ferrozine assay and the results are shown in . Among the tested substances, essential oil showed the highest chelating activity, which is significantly higher (p < 0.05) than that of BHA and BHT. The second highest activity was shown by ethanol oleoresin followed by isopropanol oleoresin, which is significantly higher (p < 0.05) than the value for BHA but lower than BHT. The activity of methanol oleoresin is higher (p < 0.05) than BHA but lower than BHT. The Fe+2 chelating properties of the antioxidant substances may be attributed to their indigenous chelating agents, mainly phenolics. Certain phenolic compounds have properly oriented functional groups, which can chelate metal ions.Citation[32] Moreover, the antioxidative properties of essential oil and oleoresins may be partially related to their iron binding capacity.
According to the data obtained from the present study, essential oil and ethanol oleoresin of tomer seeds were found to be effective antioxidants in different in vitro assays when compared to standard antioxidant compounds. The effectiveness of additives not only depends on their structural features but also on many other factors, which include nature of the lipid system, the temperature, and the binding of fatty acids.Citation[33, Citation34] However, it is very difficult to give a definite explanation for all the results obtained within the scope of the present study. There are many reports that emphasize that the phenolic group plays an important role in antioxidant activity.Citation[14, Citation15, Citation35] Hence, the presence of linalool, a phenolic compound, in major quantity in essential oil and oleoresins of tomer () may be responsible for their antioxidant properties. Moreover, the composition of essential oil and oleoresins is very complex, consisting of various classes of organic compounds; therefore, it is possible that several compounds of different polarity may contribute to the antioxidant activity. It has been reportedCitation[36] that most of the natural antioxidative compounds work synergistically with each other to produce a broad spectrum of antioxidant activities that create an effective defense system against free radical attack.
The observed variations in the antioxidant potential of essential oil and oleoresins of tomer may be due to different polarity of volatile and non-volatile compounds, both possessing antioxidant activity, which are present in the extracts. The yield and antioxidant activity of natural extracts is also closely dependent on the solvent used for extractionCitation[37] and on the methodical principles used for the assessment of antioxidant activity. And finally, heat- and water-induced chemical reactions can also change the activity of numerous compounds with different chemical and physical properties.
CONCLUSIONS
In conclusion, volatile oil and ethanol oleoresin of tomer seeds were found to be effective antioxidants. The results indicate that these are as potent as those of known antioxidants (BHA and BHT). Herbs and spices possessing antioxidant activity, such as tomer, would not only be very useful to maintain food freshness and taste, but also to alleviate diseases by preventing oxidative deterioration.
ACKNOWLEDGMENTS
The authors are thankful to the Head of the Department of Chemistry, DDU Gorakhpur University for providing laboratory facilities. The financial support from the UGC to Pratibha Singh and CSIR for Emeritus Scientist to Dr. Gurdip Singh is also acknowledged.
Notes
*This article is part 73 in a series on essential oils.
REFERENCES
- Barloco , S.M. 1990 . “ Toxicological aspects of antioxidants used as additives ” . In Food Antioxidants; Hudson, B.J.F , 253 – 307 . London : Elsevier .
- Shahidi , F. 1997 . “ Natural antioxidants an overview ” . In Natural Antioxidants: Chemistry, Health Effects and Applications , 64 – 75 . Champaign , IL : AOAC .
- Kordali , S. , Cakir , A. , Mavi , A. , Kilic , H. and Yildirim , A. 2005 . Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkisk Artemisia species . Journal of Agriculture and Food Chemistry , 53 : 1408 – 1416 .
- Atmani , D. , Chaher , N. , Berboucha , M. , Ayouni , K. , Lounis , H. , Boudaoud , H. , Debbache , N. and Atmani , D. 2009 . Antioxidant capacity and phenol content of selected Algerian medicinal plants . Food Chemistry , 112 : 199 – 212 .
- Chanwitheesuk , A. , Teerawutgulrag , A. and Rakariyatham , N. 2005 . Screening of antioxidant activity and antioxidant compounds of some edible plants of Thiland . Food Chemistry , 92 : 491 – 497 .
- Kelen , M. and Tepe , B. 2008 . Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora . Bioresources Technology , 99 : 4096 – 4104 .
- Okmer , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish Eggplant (Solanum melongena L.) cultivars . International Journal of Food Properties , 12 ( 3 ) : 616 – 624 .
- Szabo , M.R. , Radu , D. , Gavrilas , S. , Chambre , D. and Iditoiu , C. 2010 . Antioxidant and antimicrobial activities of selected spice extracts . International Journal of Food Properties , 13 ( 3 ) : 535 – 545 .
- Kalia , N.K. , Singh , B. and Sood , R.P. 1996 . A new amide from Zanthoxylum armatum . Journal of Natural Products , 62 : 311 – 312 .
- Mehta , M.B. , Kharya , M.D. , Srivastava , R. and Verma , K.C. 1981 . Antimicrobial and antihelmintic activities of the essential oil of Zanthoxylum alatum Roxb . Indian Perfumer , 25 ( 2 ) : 19 – 21 .
- Tiwari , M. , Naik , S.N. , Tewary , D.K. , Mittal , P.K. and Yadav , S. 2007 . Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors . Journal of Vector Borne Diseases , 44 : 198 – 204 .
- Kokate , S.D. , Venkatachalam , S.R. and Hassaraj , S.A. 2001 . “ Zanthoxylum armatum extract as mosquito larvicide ” . In Proceedings of National Academy of Sciences, India, Sec B—Biological Sciences , 229 – 232 . Allahabad : National Academy of Sciences, India .
- Singh , G. , Singh , O. P. and Maurya , S. 2002 . Chemical and biocidal investigations on essential oils of some Indian Curcuma sps . Progress in Crystal Growth and Characterization of Materials , 45 : 75 – 81 .
- Singh , G. , Marimuthu , P. , Heluani , C.S. and Catalan , C. 2005 . Antimicrobial and antioxidant potentials of essential oil and acetone extract of Myristica fragrans Houtt. (Aril part) . Journal of Food Science , 70 : 2
- Singh , G. , Kapoor , I.P.S. , Singh , P. , Heluani , C.S. , Lampasona , M.P. and Catalan , C.A.N. 2008 . Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale . Food and Chemical Toxicology , 45 : 3290 – 3302 .
- Kapoor , I.P.S. , Singh , B. , Singh , G. , Isidorov , V. and Szczepaniak , L. 2008 . Chemistry, antifungal and antioxidant activities of cardamom (Amomum subulatum) essential oil and oleoresins . International Journal of Essential Oil Therapeutics , 2 : 29 – 40 .
- European Pharmacopoeia. Part 1. Massoneuve SA: Sainte Ruffine, 1983 p. v.4.5.8.
- Hortwitz , W. 2002 . “ 41.1.16. AOAC Official Methods 965.33, Peroxide value of oils and fats ” . In Official Methods of Analysis of AOAC International , 17th , Gaithersberg , MD : AOAC International .
- 1998 . “ AOCS Official Methods: p-Anisidine value ” . In Official Methods and Recommended Practices of the American Oil Chemists's Society , 5th , 18 – 90 . Illinois : AOCS Press . CD
- Pokorny , J. and Dieffenbacher , A. 1989 . Determination of 2-thiobarbituric acid value: Direct method. (Results of a collaborative study and the standardized method) . Pure and Applied Chemistry , 61 ( 6 ) : 1165 – 1170 .
- Fei Que , M.B. , Mao , L. and Xheng , X. 2007 . In vitro and in vivo antioxidant activities of daylily flowers and the involvement of phenolic compounds . Asia Pacific Journal of Clinical Nutrition , 16 ( 1 ) : 196 – 203 .
- Cuendet , M. , Hostettmann , K. , Potterat , O. and Dyatmiko , W. 1997 . Iridoid glucosides with free radical scavenging properties from Fagraea blumei . Helvetica Chimica Acta , 80 : 1144 – 1152 .
- Senevirathne , M. , Kin , S.H. , Siriwardhana , N. , Ha , J.H. , Lel , K.W. and Jeon , Y.J. 2006 . Antioxidant potential of Ecklonia cava on reactive oxygen species, scavenging, metal chelating, reducing power and lipid peroxidation inhibition . Food Science and Technology International , 12 ( 1 ) : 27 – 38 .
- Jain , N. , Srivastava , S.K. , Aggrawal , L.K.K. , Ramesh , S. and Kumar , S. 2001 . Essential oil composition of Zanthoxylum alatum seeds from northern India . Flavour and Fragrance Journal , 16 : 408 – 410 .
- Chericoni , S. , Flamini , G. , Campeol , E. , Cioni , P.L. and Morelli , I. 2004 . GC-MS analysis of the essential oil from the aerial parts of Artemisia verlotiorum: Variability during the year . Biochemical Systematics and Ecology , 32 : 423 – 429 .
- Sefidkon , F. , Jamzad , Z. and Mirza , M. 2004 . Chemical variations in the essential oil of Satureja sahendica from Iran . Food Chemistry , 88 ( 3 ) : 325 – 328 .
- Blair , J. , Aichinger , T. , Hackal , G. , Hueber , K. and Dachler , M. 2001 . Essential oil content and composition in commercially available dill cultivars in comparision to caraway . Industrial Crop Production , 14 : 229 – 239 .
- Galambosi , B. and Peura , P. 1996 . Agrobotanical features and oil content of wild and cultivated forms of caraway (Carum carvi L.) . Journal of Essential Oil Research , 8 : 389 – 397 .
- Ahmadi , F. , Sadeghi , S. , Modarresi , R. and Mikaeli , A. 2010 . Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth., of Iran . Food and Chemical Toxicology , 48 : 1137 – 1144 .
- Gholivand , M.B. , Rahimi-Nasrabadi , M. , Batooli , H. and Ebrahimabadi , A.H. 2010 . Chemical composition and antioxidant activities of the essential oil and methanol extracts of Psammogeton canescens . Food and Chemical Toxicology , 48 : 24 – 28 .
- Przybylski , R. , Lee , Y.C. and Eskin , N.A.M. 1998 . Antioxidant and radical-scavenging activities of buckwheat seed components . Journal of American Oil Chemists Society , 75 ( II ) : 1595 – 1601 .
- Thompson , M. and Williams , C.R. 1976 . Stability of flavonoid complexes of copper (II) and flavonoid antioxidant activity . Analytica Chimica Acta , 85 : 375 – 381 .
- Yanishlieva , N.V. and Maslarova , E.M. 1998 . Activity and mechanism of action of antural antioxidants in lipids . Recent Research and Development in Oil Chemistry , 2 : 1 – 14 .
- Singh , G. , Maurya , S. , Lampasona , M.P. and Catalan , C. 2006 . Chemical constituents, antifungal and antioxidant potential of Foeniculum vulgare volatile oil and its acetone extract . Food Control , 17 : 745 – 752 .
- Silva , F.A.M. , Boryer , F. , Guimaraes , C. , Lima , J.L.F.C. , Matos , C. and Reis , C. 2000 . Phenolic acids and derivatives: studies on the relationship among structure, radical scavenging activity and physicochemical parameters . Journal of Agriculture and Food Chemistry , 48 : 2122
- Lu , F. and Foo , L.Y. 2001 . Antioxidant activities of polyphenol from sage (Salvia officinalis) . Food Chemistry , 75 : 197 – 202 .
- Maunza , D. , Robert , R. and Sparks , W. 1998 . Antioxidant derived from lentil and its preparation and uses U.S. Patent No. US5760936