Abstract
Propolis has highly biologically active natural substances. Propolis is rich in phenolic compounds, and is becoming increasingly popular because of its components' potential role in contributing to human health. Its composition is variable and depends on several factors. In this study, some individual phenolic compounds were analyzed and investigated in vitro biological activities of ten different Turkish propolis samples. Seventeen different phenolic constituents were measured by reverse phase-high performance liquid chromatography. Total phenolic compounds and ferric reducing antioxidant power were used as antioxidant capacity determinants. The antimicrobial activity was studied by agar diffusion method using six bacteria and two yeasts. All propolis showed strong antioxidant and antimicrobial activity and contained large amounts of antioxidant compounds. Quercetin, benzoic acid, caffeic acid, ferulic acid, and coumaric acid were detected in all propolis samples in high amounts, while vanillic acid, chlorogenic acid, epicathecin, rutin, syringic acid, and o-coumaric acid were found in very small quantities and cathecin was not found in any of them. The methanolic extracts were active against all bacteria and yeasts. They were especially effective against Pseudomonas aeruginosa ATCC®27853. The propolis extracts proved to be a good source of antioxidants and antimicrobial agents that might serve to protect health and fight against several diseases.
INTRODUCTION
Propolis is a resinous natural substance that is used to protect hives from various threats. Propolis is collected from tree buds or seeps from the bark of other trees. Propolis has been used in folk medicine in many countries and there is growing interest in using it in biologically active supplements.[Citation1,Citation2] Many studies reported that propolis contains a variety of flavonoids and phenolic acids (both benzoic and cinnamic acid derivatives) and represents a wide range of biological effects, including antibacterial, anti-inflammatory, anti-allergic, and anti-thrombotic activities, which makes it a good antioxidant additive and increases its available potential in ethnomedicine.[Citation3–5 Citation Citation5 Especially, ethanolic propolis extracts are produced and used as antioxidant capsules, free throat spray, ingredients in cosmetics and toothpaste, and antibacterial, antiviral, antioxidant, anticancer, and anti-inflammatory agents.[Citation6–8 Citation Citation8 In recent years, scientists have studied propolis samples in various countries to evaluate their chemical compositions and biological properties.[Citation9–11 Citation Citation11 The results revealed that the propolis composition depends on geographical variations.
Turkey, which is the fourth largest honey producing country in the world, has a rare mix of suitable conditions for beekeeping. In recent years, the use of propolis as folk medicine has been increasing. Components vary in terms of the geographical characteristics of the regions where it is collected.[Citation12] However, there are only a few reports about phenolic composition and biological activities of Turkish propolis.[Citation13–15 Citation Citation15 Turkey has both European and Asian flora characteristics, enriching the bee products, such as honey, pollen, and propolis. The current study was designed to assess the phenolic composition, including phenolic acids and flavonoids, and in vitro biological activities, in terms of antioxidant, antibacterial, and antifungal activities of ten Turkish propolis samples, as well as to evaluate their nutritional and medicinal potentials.
MATERIALS AND METHODS
Chemicals and Instrumentation
The phenolic standards (purity >99.0%) gallic acid, protocathechuic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, chlorogenic acid, syringic acid, epicatechin, p-coumaric acid, ferulic acid, benzoic acid, o-coumaric acid, trans-cinnamic acid, abscisic acid, catechin, rutin, quercetin, and propylparaben as internal standards (IS) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Merck (Darmstadt, Germany); methanol, acetic acid, and acetonitrile from Merck (Darmstadt, Germany); Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), TPTZ (2,4,6-tripyridyl-s-triazine), and Folin-Ciocalteu's phenol reagent from Fluka Chemie GmbH (Buchs, Switzerland); and polytetrafluoroethylene membranes (porosity 0.2 μm) for the filtration of the extracts were obtained from Sartorius (Goettingen, Germany).
High performance liquid chromatography (HPLC) (Agilent 1000, Agilent Technologies, Waldbronn, Germany) analysis of phenolic compounds was performed on a reverse phase Agilent Zorbax Eclipse XDB-C18 column (Agilent Technologies, Waldbronn, Germany) (4.6 × 150 mm, 5 μm), using a gradient program with two solvents system (A: 0.5% acetic acid in acetonitrile:water [1:1]; B: 2% acetic acid in water) at a constant solvent flow rate of 1.2 ml/min. Injection volume was 20 μl. The signals were detected at 280 nm by UV-VIS detection.
An ATI-Unicam UV-2 UV-Vis spectrophotometer (ATI Unicam, Cambridge, UK) was used in all absorbance measurements. All solutions were prepared with deionized water purified in an Elgacan® C114 Ultra Pure Water System Deioniser (The Elga Group, Buckinghanshire, England).
Samples and Preparation of Extracts
Ten samples of propolis were gathered from different regions of Anatolia in Turkey by means of the Union of Turkey Bee Raisers (TAB) as the raw product of the whole season of 2008. The crude propolis samples were stored at +4°C in a refrigerator. An amount of 5 g of propolis was weighed and added to 100 ml of methanol. Then each of the samples was continuously stirred with a shaker at room temperature for 24 h. The suspension was removed by centrifuging at 10,000 g for 15 min. Then, the supernatant was concentrated in a rotary evaporator under reduced pressure at 40°C and the residue resolved in a minimal volume of 98% ethanol and was kept at 4°C until used. For HPLC analysis, 100 μl samples were taken from the stock solution and the final volumes were completed to 1 ml for necessary dilution. Seventeen standards of phenolic compounds were used for analyses as follows: gallic acid, proto-catechuic acid, p-OH benzoic acid, catechin, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, epicatechin, p-coumaric acid, ferulic acid, benzoic acid, rutin, o-coumaric acid, cis- and trans-abscisic acid, trans-cinnamic acid, and quercetin. Besides, an IS (internal standard) technique was applied to the analysis to increase the repeatability and propylparaben was the suitable IS.[Citation16]
Determination of Phenolic Compounds by HPLC
After filtration and centrifugation of methanolic propolis solution, pH was adjusted to 1.0 with HCl then solid phase extraction was applied by using supelcleanTM LC-18 SPE tubes (Bellefonte, PA, USA) to separate phenolic compounds. The phenolic compounds adsorbed on the column were eluted with methanol. The solvents of the methanolic fractions were evaporated to dryness under reduced pressure in a rotary evaporator at 40°C. The residue was redissolved in methanol for HPLC analysis.
Determination of Antioxidant Capacity
Total phenolic contents were determined by the Folin-Ciocalteau procedure[Citation17] using gallic acid as the standard. Briefly, 0.1 ml of various concentrations of gallic acid and methanolic samples (1 mg/ml) were diluted with 5.0 ml distilled water, 0.5 ml of 0.2 N Folin-Ciocalteu reagent was added, and the contents were vortexed. After 3 min incubation, 1.5 ml of Na2CO3 (2%) solution was added and, after vortexing, the mixture was incubated for 2 h at 20°C with intermittent shaking. The absorbance was measured at 760 nm at the end of the incubation period. The concentration of total phenolic compounds was calculated as mg of gallic acid equivalents per g of 100 g sample, by using a standard graph.
The antioxidant activities of the samples were determined by ferric reducing antioxidant power (FRAP) assay.[Citation18] The method is based on the measurement of the iron reducing capacities of the propolis. Working FRAP reagent was prepared as required by mixing 25 ml of 0.3 M acetate buffer at pH 3.6 with 2.5 ml of 10 mM/l 2,4,6-tripyridyl-S-triazine (TPTZ) solution in 40 mM/l HCl and 2.5 ml of 20 mM/l FeCl3.6H2O. Then, 100 μl of the sample were mixed with 3 ml of freshly prepared FRAP reagent. The reaction mixture was then incubated at 37°C for 4 min. After that, the absorbance was determined at 593 nm against a blank that was prepared using distilled water and incubated for 1 h instead of 4 min. A calibration curve was used, using an aqueous solution of ferrous sulphate FeSO4.7H2O concentrations in the range of 100–1000 μM, r 2 = 0.97. In order to make a comparison, Trolox® was also tested under the same conditions as a standard antioxidant compound. FRAP values were expressed as μM Trolox equivalent of g propolis sample.
Test Strains and Culture Media
Strains of bacteria and fungi were obtained from ATCC (American Type Culture Collection, Rockville, MD, USA). Antimicrobial activities of samples were assayed against S. aureus ATCC®25923, E. coli ATCC®25922, S. typhimurium ATCC®14028, B. cereus ATCC®10876, L. monocytogenes ATCC®7677, P. aeruginosa ATCC®27853, A. nigerATCC®9642, and C. albicans ATCC®10231. The species of bacteria were grown in Muller Hinton Agar (Oxoid Ltd., Basingstoke, Hampshire, UK) and Muller Hinton Broth (Merck Co., Darmstadt, Germany). C. albicans and A. niger were grown in Saboraud Dextrose Broth (Difco Laboratories, Detroit, MI, USA) and Saboraud Dextrose Agar (Oxoid Ltd., Basingstoke, Hampshire, UK). The concentrations of bacterial suspensions were adjusted to 108 cells/ml, while those of fungal suspensions were adjusted to 107 cells/ml.
Antifungal Assay and Antibacterial Assay
Antibacterial and antifungal activities were measured using methods of diffusion disc plates on agar. In order to test antibacterial and antifungal activity, the fractions of propolis samples were dissolved in methanol. Mueller Hinton Agar medium (Oxoid Ltd., Basingstoke, Hampshire, UK) (20 ml) for bacteria and Sabouraud Dextrose Agar (Oxoid Ltd.) (20 ml) for fungus were poured into a 15-cm Petri dish. All bacterial strains were grown in Mueller Hinton Broth medium (Merck Co., Darmstadt, Germany) for 24 h at 37°C, and C. albicans was grown in Sabouraud Dextrose Broth (Difco Laboratories, Detroit, MI, USA) at 27°C for 48 h. Growth was adjusted to 600 nm of 0.1 by dilution with Mueller Hinton Broth medium (Merck Co., Darmstadt, Germany) and Sabouraud Dextrose Broth medium (Difco Laboratories) for bacteria and fungus, respectively. Suspension (100 μl) with approximately 108 microorganisms per milliliter was placed in Petri dishes. Then, sterile paper discs (6 mm in diameter) were placed on the agar to load 15 μl of each plant and honey samples (20 mg/ml). One hundred units of nystatin for fungus and Ampicillin and Cephazolin for bacteria, all obtained from a local pharmacy, were used as positive controls and alcohol as a negative control. Inhibition zones were determined after incubation at 27°C for 48 h. All tests were made in triplicate.
Minimum Inhibition Concentration
The agar dilution method was used for the antibacterial screening with slight modifications.[Citation19] Instead of 96 well microtiter plates, 24 well tissue culture (Corning Costar Co., Corning, NY, USA) plates were used. The crude extracts were dissolved in methanol and physiological Tris-buffer (1:4) and mixed with an equal amount of 3% agar solution at 45°C to a final concentration of 10, 5, 2.5, and 1.25 mg of extract/ml. An amount of 400 μl from the solutions was transferred into each well of the tissue culture (Corning) plate. After solidification, each well was inoculated with 10 μl of freshly prepared bacterial suspension of 108 bacterial/ml and incubated at 37°C for 24 h. Ampicillin and Cephazolin for bacteria and Nystatin for fungi obtained from a local pharmacy were used at (1.25–10 mg/ml) as positive controls for fungus nystain. The microbial growth was assessed by a stereo microscope after the incubation period. All tests were made in triplicate.
Statistical Analysis
Results are presented as mean values of two replicates. Data were tested using SPSS for Windows Release 10 (SPSS Inc., Chicago, IL, USA). Significance of the analysis of the results was based on the Kruskal-Wallis test and Pearson correlation. Significant differences were statistically considered at the level of p < 0.05.
RESULTS AND DISCUSSION
HPLC Analyses
Table 1 HPLC analyses of phenolic constituents of the methanolic propolis extracts from different regions of Turkey (mg/100 g propolis)
Figure 1 HPLC chromatogram of phenolic standards searched in Turkish propolis samples detected at 280 nm and propyl paraben was used as an internal standard. Zorbax Eclipse XDB-C18 column (Agilent Technologies, Waldbronn, Germany) (4.6 × 150 mm, 5 μm), gradient eluent acetic acid/acetonitrile/water, flow rate 1.2 mL/min. Peak identification: (1) gallic acid, (2) proto-catechuic acid, (3) p-OH benzoic acid, (4) catechin, (5) chlorogenic acid, (6) vanillic acid, (7) caffeic acid, (8) syringic acid, (9) epicatechin, (10) p-coumaric acid, (11) ferulic acid, (12) benzoic acid, (13) rutin, (14) o-coumaric acid, (15) cis, trans-abscisic acid, (16) trans-cinnamic acid, (17) quercetin, and (18) propylparaben.
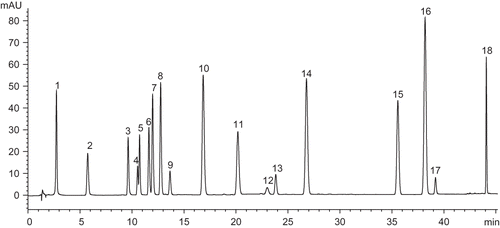
However, it is interesting to note that cathecin was not detected in these propolis samples. When comparing the results with other propolis studies, catechin were not detected in any of the ten propolis samples from Venetian.[Citation9] According to the result of capillary electrophoresis, a small amount of catechin was determined in Italian propolis.[Citation25] In a previous study, the authors had found that ferulic and caffeic acid is the major phenolic acid in chestnut propolis.[Citation13] The propolis is classified into two groups according to the differences of their components: one is a Brazilian-type rich in p-coumaric acid derivatives, the other is a European type rich in flavonoids.[Citation26] This present study reveals that propolis samples taken from different areas in Turkey possess similar characteristics from both groups.
Table 2 Collection sites, total polyphenol, and FRAP activity in methanolic propolis from different regions of Turkey
Total Phenolics and Antioxidant Activity
Total phenolic content was determined in comparison with standard gallic acid and the results expressed in terms of mg GAE/g propolis. Total phenolic content of methanolic propolis samples were found to be 115–210 mg gallic acid/g of methanolic propolis extract by using Folin-Ciocalteu method (). All the propolis samples, except Artvin sample (P10), possessed a high level of phenolic compound. Total phenolic contents found in propolis from Santiago del Estro, Argentina were between 92–170 mg/g.[Citation27] It was reported that the amount of total phenolic collected from 49 different areas in Brazil was 0.41–3.90 g GAE/100 ml ethanolic extract of propolis.[Citation28] Total phenolic contents of 20 samples from 12 different botanical origins were reported to be between 43–302 mg/g of ethanolic extract of propolis.[Citation8] All propolis samples contain an extensive number of phenolic compounds, and each sample has a distinctive profile. The amount and type of phenolic agents depend on the floral origin of propolis and they exhibit a wide range of biological effects and act as natural antioxidants and antimicrobials.[Citation5,Citation29]
In this study, the authors investigated in vitro antioxidant activity of methanolic propolis extracts from various Anatolian areas from Turkey. The FRAP method was used to determine antioxidant capacities involving the reduction of ferric tripyridyltriazine (Fe-(III)-TPTZ) complex to a blue-colored Fe (II)-TPTZ by sample antioxidants. The reduction of ferric iron in FRAP reagent resulted in the formation of a blue product (ferrous–TPTZ complex) whose absorbance can be read at 593 nm in the samples with those containing Trolox at a known concentration of 1.000 μM. The increased absorbance is an indication of higher reducing power in this method. Because the total reducing power is the sum of the reducing powers of individual compounds present in a sample, a FRAP test is considered to be a good indicator for total antioxidant power. Total antioxidant activity as FRAP values of methanolic propolis samples are shown in . FRAP values of propolis methanolic extracts were in the range of 54.38 to 85.56 μM Fe (II)/g propolis. The FRAP values of Southern Eskisehir (P4), Izmir (P7), and Sinop (P9) propolis were significantly higher than others (p < 0.05). On the other hand, significantly different values of FRAP and total phenols between Southern and Northern regions of Eskisehir and Kastamonu have been found (p < 0.05). A high positive correlation between the phenolic content and FRAP values of the propolis samples (r 2: 0.73) has been found. This positive correlation has been firmly reported in several investigations.[Citation28–30 Citation Citation30
Antimicrobial Activity
The methanolic extracts were screened for antimicrobial activity against five bacteria strains and two yeast strains by disc diffusion method. The disc diffusion method gives the opportunity to determine an approximate MIC indicating the degree of potential antimicrobial activity compared with that of positive controls, ampicillin, and ciprofloxacin. The screening results and MIC values of the propolis samples were summarized in and . The screening results were interpreted in terms of diameter of inhibition zone.
Table 3 Results of antimicrobial screening of the methanolic propolis extracts determined by the agar diffusion method (inhibition zone in mm)
Table 4 Results of antimicrobial screening of the methanolic propolis extracts determined by the agar-well diffusion method (minimum inhibitory concentration [mic], in mg/mL)
All propolis extracts were active against six bacteria and two yeasts, especially P. aeruginosa and L. monocytogenes were inhibited to a greater extent. P8 and P10 propolis extracts showed the lowest inhibition values of 1.25 μg/ml against P. aeruginosa. Some researches reported that ethanolic propolis extracts inhibited P. aeruginosa. All propolis also showed medium antifungal activity against the tested C. albicans, which correlates well with the literature data.[Citation10,Citation14] Consequently, antimicrobial screening clearly indicated that Eregli propolis had higher efficiency than other propolis (p < 0.05). Although the P10 propolis sample had the lowest phenolic substances, the sample showed good antimicrobial activity. The explanation of this may be that the types of phenolics in the sample may vary highly as their antimicrobial activities do. It is well known that the type and position of the substituent of phenolics, such as flavonoids and phenolic acids, considerably affect their biological activity. It is clear that both the quantity of phenolic compounds and their molecular structure are important for biological activity.
CONCLUSIONS
It is apparent that propolis contains an extensive number of nutraceutical agents, and each propolis sample tends to have a distinctive profile. The results may justify that propolis can be used as a source of natural antioxidants. The concentration and type of polyphenolic substances depend on the floral origin of propolis, and they are the major factors responsible for biological activities, including antioxidant and antimicrobial. Propolis may protect humans from deleterious oxidative stress as well as antioxidants. Because of their high phenolic constituents, they may also possess anticancer activities. The polyphenolic-rich natural product can be an attractive source of nutraceuticals and medicinal ingredients. Actually, propolis has a valuable source of phenolic compounds and it will be much more valuable in the future than today.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Bahri Yilmaz, president of the Union of Turkey Bee Keepers (TAB) and Mr. Selahattin Güney for providing the authentic propolis samples.
REFERENCES
- Caravaca , G.A.M. , Romero , M.G. , Román , D. , Carretero , A.A. , Segura , A. and Gutiérrez , F. 2006 . Advances in the analysis of phenolic compounds in products derived from bees . Journal of Pharmaceutical and Biomedical Analysis , 41 : 1220 – 1234 .
- Gunduz , C. , Biray , C. , Kosova , B. , Yılmaz , B. , Eroglu , Z. , Omay , F.S.B. and Cogulu , O. 2005 . Evaluation of Manisa propolis effect on leukemia cell line by telomerase activity . Leukemia Research , 29 : 1343 – 1346 .
- Marcucci , M.C. , Ferreres , F. , Vıguera , C.G. , Bankova , V.S. , Castro , S.L.D. , Dantas , A.P. , Valente , P.H.M. and Paulino , N. 2001 . Phenolic compounds from Brazilian propolis with pharmacological activities . Journal of Ethnopharmacology , 74 : 105 – 112 .
- Kumazawa , S. , Hamasak , T. and Nakayama , T. 2004 . Antioxidant activity of propolis of various geographic origins . Food Chemistry , 84 : 329 – 339 .
- Pyrzynska , K. and Biesaga , M. 2009 . Analysis of phenolic acids and flavonoids in honey . Trends in Analytical Chemistry , 28 : 893 – 902 .
- Santos , F.A. , Bastos , E.M.A. , Uzeda , M. , Carvalho , M.A.R. , Farias , L.M. , Moreira , E.S.A. and Braga , F.C. 2002 . Antibacterial activity of Brazilian propolis and fractions against oral anaerobic bacteria . Journal of Ethnopharmacology , 80 : 1 – 7 .
- Nolkemper , S. , Reichling , J. , Sensch , K.H. and Schnitzler , P. 2010 . Mechanism of herpes simplex virus type 2 suppression by propolis extracts . Phytomedicine , 17 : 132 – 138 .
- Ahn , M.R. , Kumazawa , S. , Usui , Y. , Nakamura , J. , Matsuka , M. , Zhu , F. and Nakayama , T. 2007 . Antioxidant activity and constituents of propolis collected in various areas of China . Food Chemistry , 101 : 1383 – 1392 .
- Gregoris , E. and Stevanato , R. 2010 . Correlations between polyphenolic composition and antioxidant activity of Venetian propolis . Food and Chemical Toxicology , 48 : 76 – 82 .
- Mohammadzadeh , S. , Shariatpanahi , M. , Hamedi , M. , Ahmadkhaniha , R. , Samadı , N. and Ostad , S.N. 2007 . Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis . Food Chemistry , 103 : 1097 – 1103 .
- Vatansever , H.S. , Sorkun , K. , Gurhan , S.I.D. , Kurt , F.O. , Turkoz , E. , Gencay , O. and Salih , B. 2010 . Propolis from Turkey induces apoptosis through activating caspases in human breast carcinoma cell lines . Acta Histochemica , 112 : 546 – 556 .
- Kolaylı , S. , Aliyazıcıoğlu , R. and Ulusoy , E. 2008 . Karaoğlu, Ş. Antioxidant and antimicrobial activities of selected Turkish honeys . Hacettepe Journal of Biology and Chemistry , 36 : 163 – 172 .
- Sarıkaya , A.O. , Ulusoy , E. , Ozturk , N. , Tunçel , M. and Kolaylı , S. 2009 . Antioxidant activity and phenolic acid content of chestnut honey and propolis . Journal of Food Biochemistry , 33 : 470 – 481 .
- Uzel , A. , Sorkun , K. , Önçap , Ö. , Çoğulu , D. , Gençbay , Ö. and Salih , B. 2005 . Chemical compositions and antimicrobial activities of four different Anatolian propolis samples . Microbiology Research , 160 : 189 – 195 .
- Popova , M. , Silici , S. , Kaftanoglu , O. and Bankova , V. 2005 . Antibacterial activity of Turkish propolis and its qualitative and quantitative chemical composition . Phytomedicine , 12 : 221 – 228 .
- Öztürk , N. , Tunçel , M. and Tunçel , N.B. 2007 . Determination of phenolic acids by a modified HPLC: Its application to various plant materials . Journal of Liquid Chromatography and Related Technologies , 30 : 587 – 596 .
- Slinkard , K. and Singleton , V.L. 1977 . Total phenol analyses: Automation and comparison with manual methods . American Journal of Enology and Viticulture , 28 : 49 – 55 .
- Benzie , I.F. and Szeto , Y.T. 1999 . Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay . Journal of Agricultural and Food Chemistry , 47 : 633 – 636 .
- Vanden Berghe , D.A. and Vlietinck , A.J. 1991 . “ Screening methods for antibacterial and antiviral agents from higher plants ” . In Methods in Plant Biochemistry , Vol 6 , 47 – 69 . New York : Academic Press .
- Herken , E.N. , Erel , O. , Guzel , S. , Celik , H. and Senol , Ibanoglu . 2009 . Total antioxidant, phenolic compounds, and total oxidant status of certified and uncertified Turkey's honeys . International Journal of Food Properties , 12 : 461 – 468 .
- Kolayli , S. , Kara , M. , Tezcan , F. , Erim , F.B. , Sahin , H. , Ulusoy , E. and Aliyazicioglu , R. 2010 . Comparative study of chemical and biochemical properties of different melon cultivars: standard, hybrid, and grafted melons . International Journal of Agricultural and Food Chemistry , 58 : 9764 – 9769 .
- Gungor , N. and Sengul , M. 2008 . Antioxidant activity, total phenolic content and selected physicochemical properties of white mulberry (Morus alba L.) fruits . International Journal of Food Properties , 11 : 44 – 52 .
- Itagaki , S. , Kurokawa , T. , Nataka , C. , Saita , Y. , Oikawa , S. , Kobayaski , M. , Hirano , T. and Iseki , T. 2009 . In vitro and in vivo antioxidant properties of ferulic acid: A comparative study with other natural oxidation inhibitors . Food Chemistry , 104 : 466 – 471 .
- Boots , A.W. , Haenen , G.R. and Bast , A. 2008 . Health effects of quercetin: From antioxidant to nutraceutical . European Journal of Pharmacology , 585 : 325 – 337 .
- Volpi , N. 2004 . Separation of flavonoids and phenolic acids from propolis by capillary zone electrophoresis . Electrophoresis , 25 : 1872 – 1878 .
- Fujimoto , T. , Nakamura , J. and Matsuka , M. 2001 . Diversity of propolis, Part 1 . Propolis from the world. Honeybee Science , 22 : 9 – 16 .
- Chaillou , L.L. and Nazareno , M.A. 2009 . Bioactivity of propolis from Santiago del Estero, Argentina, related to their chemical composition . LWT—Food Science and Technology , 42 : 1322 – 1427 .
- Silva , J.F.M. , Souza , M.C. , Matta , S.R. , Andrade , M.R. and Vidal , F.V.N. 2006 . Correlation analysis between phenolic levels of Brazilian propolis extracts and their antimicrobial and antioxidant activities . Food Chemistry , 99 : 431 – 435 .
- Küçük , M. , Kolayli , S. , Karaoğlu , Ş. , Ulusoy , E. , Baltacı , C. and Candan , F. 2007 . Biological activities and chemical composition of three honeys of different types from Anatolia . Food Chemistry , 100 : 526 – 534 .
- Socha , R. , Juszczak , L. , Pietryzk , S. and Fortuna , T. 2009 . Antioxidant activity and phenolic composition of herbhoneys . Food Chemistry , 103 : 568 – 574 .