Abstract
The aim of this study was to quantify specific phytosterols/-stanols (campesterol, β-sitosterol, stigmasterol, β-sitostanol, and campestanol) and tocols (tocopherol and tocotrienol) in the vegetable oils (corn oil, sunflower oil, blended oil, and palm oil) available in the Egyptian market. Gas-liquid chromatography and high performance liquid chromatography procedures were applied to 12 vegetable oils. The best source of phytosterols was the corn oil (4814 μg/g oil) samples while palm oil samples had the lowest phytosterols level (660 μg/g). The mean value of total phytosterols was 2872 and 3443 μg/g for sunflower and blended oil samples, respectively. β-Sitosterol was the dominant phytosterol (ca. 70% of the total phytosterols), followed by campesterol (ca. 16%) and stigmasterol (ca. 9.4%), while β-sitostanol and campestanol were detected in some oils in small amounts or traces. The levels of mean total tocols in corn, sunflower, blended, and palm oil samples were 891.4, 716.1, 707.5, and 311.8 μg/g, respectively. In sunflower and blended oil samples, α-tocopherol was the main tocol, which accounted for more than 80% of total tocols, while α-tocopherol was found in lower levels in corn and palm oils accounting for ca. 40% of total tocols.
INTRODUCTION
Vegetable oils and derived products are a major source of phytosterols (ST) and tocols (TO) in the diet. They contribute to 20 and 41% of the total α-tocopherol equivalent intake in the US and in Finland, respectively,[Citation1, Citation2] and to 26, 39, and 50% of the total ST intake in Holland, Finland, and Spain, respectively.[Citation3–5 Citation Citation5 Phytosterols are isoprenoid compounds with a sterol nucleus and an alkyl chain. Most plant sterols have a double bond in position C–5 in the nucleus, while others are totally saturated and called stanols.[Citation6] In this work, the term “phytosterols, ST” is used for both sterols and stanols. Together with cholesterol, ST are chemically classified as 4-desmethyl sterols, wherein more than 250 types of ST have been reported.[Citation7] The most common ST are sitosterol (24α-ethylcholest-5en-3β-ol), campesterol (24α-methyl-5-cholesten-3β-ol), and stigmasterol (5,22-cholestadien-24α-ethyl-3βol). Phytosterols exist in four different forms in foods: free, esterified to fatty acids, sugar moieties, or phenolic acid.[Citation7]
Data on the ST content of many edible oils have been published;[Citation8, Citation9] however, more information about the ST profile in commercial vegetable oils is still needed. Some of the available data on the ST composition of edible oils are for crude oilseed extracts.[Citation10] These data may not apply to commercially available refined oils due to the effects of processing on ST content and composition. For example, refining has been reported to decrease the ST levels of crude oils. On the other hand, in many old reports ST values were from colorimetric, not chromatographic, analysis. Differences in analytical methods might also confound inter-study comparison of values for different oils. In addition, many older studies involved thin-layer chromatographic separation and/or colorimetric quantification of sterols and lacked validation of quantitative recovery.[Citation11] Recently, gas chromatography with flame ionization detection is most frequently applied in ST analysis.[Citation12–16 Citation Citation Citation Citation16
Several reviews on the health effects of ST were recently published.[Citation17, Citation18] Most of them focus on the clinical trials of the cholesterol-lowering properties with ST and the preventive impacts this may have on coronary heart disease. A preventive impact on the development of colon cancer has been suggested through findings of animal and in vitro studies. Moreover, ST has been widely studied for their hypocholesterolemic, anticarcinogenic, and other health impacts.[Citation19] Unlike sterols that compete with cholesterol for absorption and are themselves present in serum, stanols are virtually unabsorbable. Still, they are capable of decreasing total and low density lipoprotein (LDL) cholesterol as well as ST serum levels.[Citation7, Citation20] To study the relation between ST intake and health outcome, validated databases are needed. Earlier studies have made an attempt to quantify ST intake; they show that ST are usually consumed in the range of 100–400 mg/day.[Citation21, Citation22] It is, however, likely that these estimates would change with the choice of database.
Tocopherols and tocotrienols, together abbreviated as tocols (TO), are a group of fat soluble antioxidants with a chromanol ring and a hydrophobic side chain (phytyl in the case of tocopherols, isoprenyl in the case of tocotrienols). Tocopherols (α-, β-, γ-, and δ-tocopherol) and the corresponding tocotrienols differ by the number and positions of methyl substituents on the phenolic part of the chromanol ring. Despite similar absorption from the gastrointestinal tract, α-tocopherol is preferentially resecreted by incorporation into lipoproteins by the hepatic α-tocopherol transfer protein, resulting in the highest plasma and tissue levels. Therefore, the Institute of Medicine defined natural α-tocopherol as the only TO contributing toward meeting the vitamin E requirement.[Citation23] Analysis of TO is usually performed by normal phase high performance liquid chromatography (NP-HPLC). Vegetable oils contain TO mainly in unconjugated form and can be directly injected into the NP-HPLC system after dilution.[Citation14, Citation15] Many industrial fats, however, contain water, emulsifiers, and possibly added α-tocopherol esters. Thus, their analysis requires saponification in order to avoid degradation of TO in alkaline solution.[Citation24]
The market situation of vegetable oils in Egypt has changed dramatically. Cottonseed oil and hydrogenated vegetable oils have disappeared almost completely from the market, and items like sunflower oil, corn oil, blended oils, and palm oil have found their way into the shelves of retail stores. The aim of this work was to provide up-to-date and validated data on the levels of the individual TO and ST in the main vegetable oils currently available on the Egyptian market in order to enable an update of the Egyptian food composition database. To the best of knowledge, this is the first database for ST and TO in the vegetable oils consumed in Egypt. Valid data would represent a welcome addition since they could be used to generate data on the health impacts of bioactive ST and TO.
MATERIALS AND METHODS
Vegetable Oil Samples and Chemicals
Commercially available vegetable oil samples (corn, sunflower, blended oil [mixture of soybean, sunflower, and palm oils], and palm) from different producers readily available in the Egyptian market (Arma, Savola, and Mazola) were bought in different retail outlets in Zagazig (Egypt) in October 2008. Since cottonseed oil is hardly found in the Egyptian market, these four edible oils represent the main oils found in the market and were advertised as suitable for different kinds of edible application (cooking, frying, etc.). Multiple samples (three samples from the same oil type) were assayed to evaluate potential variability in the ST and TO distribution. Standards used for ST characterization were purchased from Supelco (Bellefonte, PA, USA). Standards used for TO were purchased from Merck (Darmstadt, Germany). Reagents and chemicals used were of the highest purity available.
Gas Liquid Chromatography (GLC) Analysis of Fatty Acids
Fatty acids were transesterified into methyl esters (FAME) using N-trimethylsulfoniumhydroxide (Macherey-Nagel, Düren, Germany) according to the procedure reported by Ramadan and Mörsel.[Citation15] FAME were identified on a Shimadzu GC-14A equipped with flame ionization detector (FID) and C-R4AX chromatopac integrator (Kyoto, Japan). The flow rate of the carrier gas helium was 0.6 mL/min and the split value with a ratio of 1:40. A sample of 1 μL was injected on a 30 m × 0.25 mm × 0.2 μm film thickness Supelco SP™-2380 (Bellefonte, PA, USA) capillary column. The injector and FID temperature was set at 250°C. The initial column temperature was 100°C programmed by 5°C/min until 175°C and kept 10 min at 175°C, then 8°C/min until 220°C and kept 10 min at 220°C. A comparison between the retention times of the samples with those of authentic standard mixture (Sigma, St. Louis, MO, USA; 99% purity specific for GLC), run on the same column under the same conditions, was made to facilitate identification.
Gas Liquid Chromatography (GLC-FID) Analysis of ST
Separation of ST was performed after saponification of the oil samples without derivatization according to Ramadan, Zayed, and El-Shamy.[Citation16] Oils (250 mg) were refluxed with 5 mL of ethanolic potassium hydroxide solution (6%, w/v) and a few anti-bumping granules for 60 min. The unsaponifiables were first extracted 3 times with 10 mL of petroleum ether, the extracts were then combined and washed 3 times with 10 mL of neutral ethanol/water (1:1, v/v), and then dried overnight with anhydrous sodium sulphate. The extract was evaporated in a rotary evaporator at 25°C under reduced pressure and then ether was completely evaporated under nitrogen. GLC analyses of unsaponifiable residues were carried out using a Mega Series (HRGC 5160, Carlo Erba Strumentazione; Milan, Italy) equipped with FID. The following parameters were performed: DB 5 column (J&W Scientific, Falsom, CA, USA) packed with 5% phenylmethylpolysiloxan, 30 m length, 0.25 mm i.d., 1.0 μm film thickness; carrier gas (helium) flow 38 mL/min (split-splitless injection was used). Detector and injector were set at 280°C. The oven temperature was kept constant at 310°C and the injected volume was 2 μL. The repeatability of the analytical procedure was tested and the relative standard deviation of three repeated analyses of a single sample was <5%. Quantitative analyses were performed with a Shimadzu (C-R6A Chromatopac; Kyoto, Japan) integrator.
Normal Phase High Performance Liquid Chromatography (NP-HPLC) Analysis of TO
NP-HPLC was selected to avoid extra sample treatment (e.g., saponification) according to Ramadan and Mörsel.[Citation14] Analysis was performed with a solvent delivery LC-9A HPLC (Shimadzu, Kyoto, Japan). The chromatographic system included a model 87.00 variable wavelength detector and a 250 × 4 mm i.d. LiChrospher-Si 60, 5 μm column (Knauer, Berlin, Germany). Separation of TO was based on isocratic elution when the solvent flow rate was maintained at 1 mL/min at a column back-pressure of about 65–70 bar. The solvent system selected for elution was isooctane/ethyl acetate (96:4, v/v) with detection at 295 nm. Twenty μL of the diluted solution of oil in the mobile phase were directly injected into the HPLC column. Tocols were identified by comparing their retention times with those of authentic standards. All work was carried out under subdued light conditions. All the experiments were repeated at least three times when the variation on any one was routinely less than 5%. All experimental procedures were performed in duplicate and their mean values (± standard deviation) were given.
RESULTS AND DISCUSSION
Fatty Acid Profile of Vegetable Oils
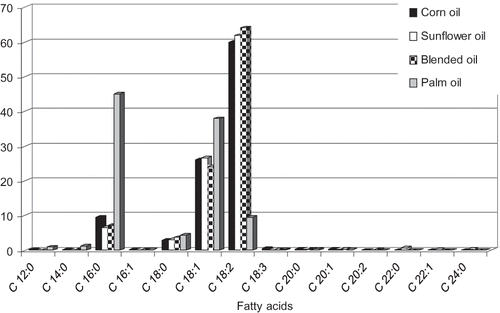
Vegetable oil samples in this work were characterized for their fatty acid composition. In corn, sunflower, and blended oils, the major omega-6 fatty acid is linoleic acid, representing more than 59% of the total fatty acids, followed by oleic acid, palmitic acid, stearic acid, and linolenic acid, respectively. In palm oil, palmitic acid (45%) followed by oleic acid (37%) were the main fatty acids. As shown in , blended oil recorded the highest level of polyunsaturated fatty acids (PUFA, 64%), followed by sunflower oil (61%) and corn oil (60%), respectively. Saturated fatty acids accounted for more than 50% of total fatty acids in palm oil followed by monounsaturated fatty acids (38.4%), while PUFA recorded the lowest amount (9.7%) in this oil.
Levels and Profile of Phytosterols/-stanols in the Vegetable Oils
Vegetable oils are generally regarded as important sources for ST since, in general, they contain relatively higher ST amounts than fruits, vegetables, and fruits.[Citation20, Citation25] Phytosterols/-stanols (ST) concentrations for all oils are listed in . In total, 12 oil items were analyzed. Corn oil samples had the highest levels of total ST (4814 μg/g) and palm oil samples had the lowest ST content (660 μg/g). The mean values of total ST content were 2872 and 3443 μg/g for sunflower and palm oil samples, respectively. The total ST contents determined in this work were generally in line with those listed in the literature.[Citation11] Recently, comparable levels of total ST contents were found in sunflower oil consumed in Spain and the Netherlands (492 and 436 mg/100 g).[Citation5] Corn oil proved to be the best source of ST, followed by blended oil and sunflower oil as also reported by others.[Citation11]
Vegetable oils are complex media because they contain several different phytosterols/-stanols. β-Sitosterol is among the most representative ST along with campesterol, stigmasterol, and other various types of minor ST.[Citation26] As expected, the predominant ST in all vegetable oil samples were β-sitosterol, stigmasterol, and campesterol. Low levels of sitostanol and campestanol were present in all of the samples but a relatively higher concentration of sitostanol was found in corn (130 μg/g). In corn oil, the percentages of individual PS were β-sitosterol (54.9%), campesterol (13.3%), and stigmasterol (6.2%) being the most abundant ST. These data are similar to those reported by Cercaci et al.[Citation27] Generally, in the samples under investigation, it could be noted that β-sitosterol was the dominant ST (ca. 70% of the total concentration of all five ST), followed by campesterol (ca. 16%) and stigmasterol (ca. 9.4%), while β-sitostanol (ca. 1.4%) and campestanol (ca. 2.1%) were detected in some oils in small amounts ().
Table 1 Levels of phytosterols/-stanols (μg/g) in vegetable oils consumed in Egypt
In the comparison of the analyzed ST levels in vegetable oils in the Egyptian market to that of recent ST databases for vegetable oils found in the western countries,[Citation6, Citation11] it could be observed that the ST levels in corn oil from the Egyptian market is significantly lower than the amounts of sterols in the corn oil in the western market (). On the other hand, the ST concentrations in palm oil from the Egyptian market are higher than the amounts of ST in the palm oil in the western market. Concerning sunflower oil, the ST levels resemble that of Normén et al.[Citation11] and Schwartz et al.[Citation6] Several factors may affect the ST content, including plant variety, growing season, shipping, storage, and preparation.
Associations between ST intake and cancer or coronary heart disease (CHD) risk to date were difficult to assess because of the lack of data covering ST levels in foods. New databases[Citation6] have been recently published. The aim was to create databases that could be used in epidemiological studies. Due to the high contribution of ST from different vegetable oils, databases seem, therefore, to be of the highest importance. In addition, due to the high bioavailability of ST in fatty food items, it is thus recommended that these should be included in sufficient numbers in ST databases.[Citation11]
Levels and Profile of Tocols in the Vegetable Oils
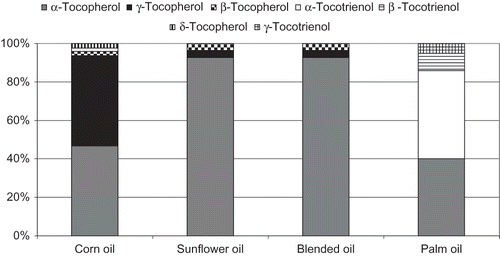
Tocol levels in the vegetable oil samples are given in . α-Tocopherol and γ-tocopherol proved to be the main TO in vegetable oils and fats.[Citation28] The main function of α-tocopherol is that of a radical-chain breaking antioxidant in membranes and lipoproteins, as well as in foods. Despite lower plasma concentrations, other TO are still capable of exerting antioxidant and biological activities. γ-Tocopherol has been reported to be more potent than α-tocopherol in decreasing platelet aggregation, LDL oxidation, and delaying intra-arterial thrombus formation.[Citation29] Likewise, tocotrienols are discussed in the context of reducing the risk of breast cancer and have been shown to inhibit cholesterol biosynthesis.[Citation30] Hence, concurrent administration of various TO may result in increased antioxidant, antitumor, and hypocholesterolemic potential. In order to take into account the biological activities of other TO, some nutrient databases still include them in the form of α-tocopherol equivalents.[Citation6, Citation31]
Table 2 Average levels of ST (μg/g) in vegetable oils consumed in Egypt compared with ST levels in vegetable oils consumed in some western countries
Table 3 Levels of tocols (μg/g) in vegetable oils consumed in Egypt
Vegetable oils contain TO mainly in unconjugated form and can be directly injected into the normal phase HPLC system after dilution.[Citation6, Citation14, Citation32] Rapeseed, corn, and sunflower oils contained high levels of tocopherols, whereas palm oil is the best source of tocotrienols. Wheat germ oil was found to be by far the best source of α-tocopherol, followed by sunflower and olive oils. With the exception of wheat germ oil in which β-tocopherol turned out to be the second most abundant tocopherol, both β- and δ-tocopherol proved to be minor constituents. γ-Tocopherol occurred in the highest levels in camelina, linseed, cold-pressed rapeseed, and corn oils.[Citation6] In this investigation, the levels of mean total TO in corn, sunflower, blended, and palm oil samples were 891.4, 716.1, 707.5, and 311.8 mg/kg, respectively. It was clear that corn, sunflower, and blended oil samples contained high levels of TO, while palm oil samples contained the lowest level. The levels of individual TO in the investigated vegetable oils are given in . In sunflower and blended oil samples, α-tocopherol was the main TO, which accounted for more than 80% of total TO, while α-tocopherol found in lower levels in corn and palm oils accounted for more than 40% of the total TO (). γ-Tocopherol was the major TO in corn oil (417.2 mg/kg), wherein β- and δ-tocopherol were present in minor amounts or traces. In the recent TO database for vegetable oils and industrial fats purchased in Finland,[Citation6] total tocopherols in sunflower oil was 630 μg/g, while in corn oil the total tocopherols amount was 660 μg/g and total tocotrienols level was 25. Palm oil samples were characterized by its high levels of tocotrienols, wherein α-tocotrienol was the major tocotrienol followed by β-tocotrienol and γ-tocotrienol, respectively.
CONCLUSIONS
In Egypt, vegetable oil production is centralized with few manufacturing companies and a few small ones processing cold-pressed healthier oils. Therefore, although the selection of oil brands in supermarkets may be different, the overall market situation in Zagazig is representative for that in all of Egypt. Epidemiological studies on ST and TO consumption and health impact are difficult to find, however, because few databases are found. The aim of the present study was to survey for the first time the ST and TO composition of a variety of the main commercially available edible oils in the Egyptian market. The TO and ST levels of oil samples were determined using validated NP-HPLC and GLC-FID methods. There is a wide spread of concentrations of PS and TO in the edible oils consumed in Egypt. However, no significant differences were observed among the samples from the same oil type. This may be due to using the same plant source as it is known that about 90% of vegetable oils found in the Egyptian market is imported. Corn oil, one of the most commonly used oils in Egypt, proved to be the best source of both TO and ST. Since this survey database is meant to be used in other prospective cohort studies, a slight overestimation of ST and TO levels may be less important since the database only needs to rank the ST and TO intake of people in order to study associations with morbidity and mortality.
ACKNOWLEDGMENTS
The author would like to thank the Science and Technology Development Fund (STDF, Project ID 21) and the Egyptian Ministry of Higher Education for the financial support.
REFERENCES
- Murphy , S.P. , Subar , A.F. and Block , G. 1990 . Vitamin E intakes and sources in the United States . American Journal of Clinical Nutrition , 52 : 361 – 367 .
- Heinonen , M. and Piironen , V. 1991 . The tocopherol, tocotrienol, and vitamin E content of the average Finnish diet . International Journal for Vitamin and Nutrition Research , 61 : 27 – 32 .
- Normén , A.L. , Brants , H.A.M. , Voorrips , L.E. , Andersson , H.A. , van den Brandt , P.A. and Goldbohm , R.A. 2001 . Plant sterol intakes and colorectal cancer risk in the Netherlands cohort study on diet and cancer . American Journal of Clinical Nutrition , 74 : 141 – 148 .
- Valsta , L.M. , Lemström , A. , Ovskainen , M.-L. , Lampi , A.-M. , Toivo , J. , Korhonen , T. and Piironen , V. 2004 . Estimation of plant sterol and cholesterol intake in Finland: Quality of the new values and their effect on intake . British Journal of Nutrition , 92 : 671 – 678 .
- Jiménez-Escrig , A. , Santos-Hidalgo , A.B. and Saura-Calixto , F. 2006 . Common sources and estimated intake of plant sterols in the Spanish diet . Journal of Agricultural and Food Chemistry , 54 : 3462 – 3471 .
- Schwartz , H. , Ollilainen , V. , Piironen , V. and Lampi , A.-M. 2008 . Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats . Journal of Food Composition and Analysis , 21 : 152 – 161 .
- Piironen , V. , Lindsay , D.G. , Miettinen , T.A. , Toivo , J. and Lampi , A.-M. 2000 . Plant sterols: Biosynthesis, biological function and their importance to human nutrition . Journal of the Science of Food and Agriculture , 80 : 939 – 966 .
- Jeong , T.M. , Itoh , T. , Tamura , T. and Matsumoto , T. 1974 . Analysis of sterol fractions from twenty vegetable oils . Lipids , 9 : 921 – 927 .
- Weihrauch , J.L. and Garndner , J.M. 1978 . Sterol content of foods of plant origin . Journal of the American Dietetic Association , 73 : 39 – 47 .
- Johansson , A. 1979 . The content and composition of sterols and sterol esters in sunflower and poppy seed oils . Lipids , 1 : 285 – 291 .
- Normén , L. , Ellegård , L. , Brants , H. , Dutta , P. and Andersson , H.A. 2007 . A phytosterol database: Fatty foods consumed in Sweden and the Netherlands . Journal of Food Composition and Analysis , 20 : 193 – 201 .
- Toivo , J. , Phillips , K. , Lampi , A.-M. and Piironen , V. 2001 . Determination of sterols in foods: Recovery of free, esterified, and glycosidic sterols . Journal of Food Composition and Analysis , 14 : 631 – 643 .
- Lampi , A.-M. , Piironen , V. and Toivo , J. 2004 . “ Analysis of phytosterols in foods ” . In Phytosterols as Functional Food Components and Nutraceuticals , Edited by: Dutta , P.C. 33 – 73 . New York : Marcel Dekker, Inc .
- Ramadan , M.F. and Mörsel , J.-T. 2002 . Direct isocratic normal phase assay of fat-soluble vitamins and β-carotene in oilseeds . European Food Reasearch and Technology , 214 : 521 – 527 .
- Ramadan , M.F. and Mörsel , J.-T. 2003 . Oil goldenberry (Physalis peruviana L.) . Journal of Agricultural and Food Chemistry , 51 : 969 – 974 .
- Ramadan , M.F. , Zayed , R. and El-Shamy , H. 2007 . Screening of bioactive lipids and radical scavenging potential of some solanaceae plants . Food Chemistry , 103 : 885 – 890 .
- Ostlund Jr , R.E. 2002 . Phytosterols in human nutrition . Annual Review of Nutrition , 22 : 533 – 549 .
- Normén , L. , Trautwein , E. and Frohlich , J. 2003 . “ The role of phytosterols in cholesterol-lowering in humans ” . In Phytosterols , Edited by: Dutta , P. New York : Marcel Dekker Inc .
- Awad , A.B. , Downie , A. , Fink , C.S. and Kim , U. 2000 . Dietary phytosterol inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice . Anticancer Research , 20 : 821 – 824 .
- Piironen , V. , Toivo , J. and Lampi , A.-M. 2000 . Natural sources of dietary plant sterols . Journal of Food Composition and Analysis , 13 : 619 – 624 .
- De Vries , J. , Jansen , A. , Kromhout , D. , Van De Bovenkamp , P. , Van Staveren , W. , Mensink , R. and Katan , M. 1997 . The fatty acid and sterol content of food composites of middle-aged men in seven countries . Journal of Food Composition and Analysis , 10 : 115 – 141 .
- Strom , S.S. , Yamamura , Y. , Duphorne , C.M. , Spitz , M.R. , Babaian , R.J. , Pillow , P.C. and Hursting , S.D. 1999 . Phytoestrogen intake and prostate cancer: A case-control study using a new database . Nutrition and Cancer , 33 : 20 – 25 .
- Institute of Medicine . The National Academy of Sciences, Washington DC, USA. 2000 . Vitamin E . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotinoids , : 186 – 283 .
- Ryynänen , M. , Lampi , A.-M. , Salo-Vänänen , P. , Ollilainen , V. and Piironen , V. 2004 . A small-scale sample preparation method with HPLC analysis for determination of tocopherols and tocotrienols in cereals . Journal of Food Composition and Analysis , 17 : 749 – 765 .
- Alvarez-Chavez , L.M. , Valdivia-Lopez , M.L. , Aburto-Juarez , M.L. and Tecantea , A. 2008 . Chemical characterization of the lipid fraction of Mexican Chia seed (Salvia hispanica L.) . Inter. Journal of Food Properties , 11 : 687 – 697 .
- Lampi , A.-M. , Juntunen , L. , Toivo , J. and Piironen , V. 2002 . Determination of thermo-oxidation products of plant sterols . Journal of Chromatography B , 777 : 83 – 92 .
- Cercaci , L. , Rodriguez-Estrada , M.T. , Lercker , G. and Decker , E.A. 2007 . Phytosterol oxidation in oil-in-water emulsions and bulk oil . Food Chemistry , 102 : 161 – 167 .
- Bonvehi , J.S. , Coll , F.V. and Rius , I.A. 2000 . Liquid chromatographic determination of tocopherols and tocotrienols in vegetable oils, formulated preparations, and biscuits . Journal of AOAC International , 83 : 627 – 634 .
- Saldeen , T. , Li , D. and Mehta , J.L. 1999 . Differential effects of alpha- and gamma-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thrombogenesis . Journal of the American College of Cardiology , 34 : 1208 – 1215 .
- Schwenke , D.C. 2002 . Does lack of tocopherols and tocotrienols put women at increased risk of breast cancer? . Journal of Nutritional Biochemistry , 13 : 2 – 20 .
- Bramley , P.M. , Elmadfa , I. , Kafatos , A. , Kelly , F.J. , Manios , Y. , Roxborough , H.E. , Schuch , W. , Sheehy , P.J.A. , Wagner , K.-H. and Vitamin , E. 2000 . Journal of the Science of Food and Agriculture , 80 : 913 – 938 .
- Gursoy , N. , Tepe , B. and Sokmen , M. 2010 . Evaluation of the chemical composition and antioxidant activity of the peel oil of Citrus Nobilis . International Journal of Food Properties , 13 : 983 – 991 .