Abstract
The effects of three extraction techniques (shaking, soaking, and stirring) and two solvents (80% methanol and 80% ethanol) on the antioxidant attributes of extracts from seeds of mungbean have been investigated. The yield of mungbean extracts varied between 6.90 and 9.65 g/100 g of dry matter. Mungbean extracts contained a considerable amount of phenolics (0.78–1.12 g GAE/100 g) and flavonoids (1.23–1.78 g CE/100 g). An appreciable level of reducing power (1.46–2.18) at 10 mg/mL extract concentration, inhibition of linoleic acid peroxidation (85.2–90.4%), and DPPH radical scavenging activity (IC50 value 16.4–42.9 μg/mL) were also documented. Overall, the efficacy of an extraction system in isolating potent antioxidant components from mungbean seeds followed the order: shaking, 80% methanol > shaking, 80% ethanol > stirring, 80% methanol > stirring, 80% ethanol > soaking, 80% ethanol > soaking, 80% methanol. The yield and antioxidant activity of the mungbean extracts varied significantly (p < 0.05) as function of extraction techniques and solvents employed.
INTRODUCTION
Currently, the usage of synthetic antioxidants in foods is limited due to their perceived carcinogenic potential and safety concerns. On the other hand, there is a continuing interest for the exploration of some effective, safer, and viable natural antioxidants.Citation[1] Nature has blessed plants with a multitude of substances, such as carotenoids, flavonoids, and phenolic acids, possessing antioxidant activities.Citation[2] Consumption of vegetables, legumes, and beans, being potential sources of antioxidant nutrients, is linked with the reduced prevalence of so called life-style diseases, such as cardiovascular disorders, aging, inflammation, and certain cancers.Citation[3, Citation4]
A native of the Indo-Pak region, mungbean, also known as green bean, is the seed of [Vigna radiata (L.) Wilczek]. As a short season summer-growing legume crop, mungbean has been widely cultivated for its edible seeds and pods in the tropical and subtropical regions of the world, especially in Asian countries.Citation[1, Citation5] It is also commonly grown in different regions of Pakistan on an estimated area of 2,019,700 ha, yielding approximately 1,057,400 tons of beans.Citation[6] Traditionally, mungbean is often consumed as a seed, sprout, boiled, or cooked with vegetables or meat. Moreover, being a rich source of protein and other high-value components, it is also used as an important ingredient in various processed products, such as cold jellies, noodles, cakes, and brews.Citation[1, Citation7]
Antioxidant extracts from a plant material can be isolated using different extraction processes. The yields and antioxidant properties of the resultant extracts noticeably vary depending upon the efficacy and dynamism of the extraction methods.Citation[8, Citation9] It is generally considered that pure or aqueous mixtures of methanol or ethanol are more efficient towards recovering phenolic antioxidant components from plant-based materials.Citation[10– Citation13] Similarly, different extraction devices, e.g., rotary shaker, multi-wrist shaker, sonicator, soxhlet, and supercritical fluid-extractor, etc., are in practice for extraction of antioxidant components from plant matrices.Citation[14]
In view of the high nutritive value and functional food potential of mungbean seed, it is important to ascertain an effective method for the isolation of antioxidants from this valuable legume crop. So far, negligible studies have been reported in the literature on the extraction of mungbean antioxidants involving the use of different techniques and solvents leading towards exploring an optimum extraction method. The main objective of this study was to evaluate and quantify the effects of different techniques, namely, shaking, stirring, and soaking as well as two solvents (80% methanol and 80% ethanol) on the recovery of potent antioxidant components from mungbean seeds, so as to devise an optimized extraction protocol.
MATERIAL AND METHOD
Material
The seeds of mungbean (cv. AZRI-Mung-06) were obtained from the Ayub Agricultural Research Institute, Faisalabad, Pakistan. The chemicals and reagents, namely, Folin-Ciocalteu regent, 2,2-diphenyl1-picrylhydrazyl (DPPH) free radical, linoleic acid, β-carotene, and butylated hydroxytoluene (BHT), were obtained from Sigma Chemical Company (St. Louis, MO, USA). All other reagents and chemicals used were of analytical grade from Merck (Darmstadt, Germany).
Preparation of Mungbean Antioxidant Extracts
The hull-free mungbean seeds were ground into a fine powder using a commercial coffee grinder. The material that passed through the 80-mesh sieve was used for extraction of antioxidant components. The extraction of mungbean antioxidant components was performed by three extraction techniques, i.e., shaking, soaking, and stirring, using as well as two solvents (80% methanol and 80% ethanol). In orbital shaking, 20 g of mungbean ground seed material was taken in a 500-mL Erlenmeyer flask and extracted individually with 200 mL of 80% methanol (methanol:water, 80:20 v/v) and 80% ethanol (ethanol:water, 80:20 v/v) for 8 h at room temperature using an orbital shaker (Gallenkamp, Leicestshire, UK). In the soaking method, the material (20 g) was soaked separately in 200 mL of 80% methanol and 80% ethanol in a 500-mL Erlenmeyer flask for 8 h. Extraction using stirring was carried out by mixing the ground material independently with 200 mL of 80% methanol and 80% ethanol in a 500-mL Erlenmeyer flask followed by magnetic stirring on a stirring plate (Utech Products Inc., Albany, NY, USA) using polyvinylidene fluoride coated 50 mm magnetic bar. After complete extraction in each case, the solid residue was separated from the extract by filtering through Whatman No. 1 filter paper. The residues were re-extracted twice using fresh solvent, and the three extracts were pooled. The excess solvent was then removed by distilling off under reduced pressure at 45°C, using a rotary vacuum evaporator (EYELA, SB-651, Rikakikai Co. Ltd., Tokyo, Japan). The crude concentrated extracts obtained were stored at −4°C until used for further analyses.
Antioxidant Activity Evaluation of Mungbean Extracts (ME)
Determination of total phenolic contents (TPC)
Quantification of TPC was based upon spectrophotometric measurement following Folin-Ciocalteu reagent methodCitation[15] with slight modifications. The extract (50 mg) was mixed with 7.5 mL of deionized water and 0.5 mL of Folin-Ciocalteu reagent in a test tube. After thorough mixing, the mixture was kept at room temperature for 10 min, followed by an addition of 1.5 mL of freshly prepared 20% aqueous sodium carbonate (w/v) solution. The mixture was then incubated in a water bath at 40°C for 20 min. After cooling the mixture, the absorbance was read at 755 nm using a Hitachi spectrophotometer (Model U-2001, Hitachi Instruments Inc., Tokyo, Japan). The amount of TPC was calculated using gallic acid calibration curve and expressed as gallic acid equivalents (GAE) g/100 g of dry matter.
Determination of total flavonoid contents (TFC)
Total flavonoid contents were determined using a previously described spectrophotometric method.Citation[16] A known quantity of aqueous extract (0.01 g/mL of dry matter) was taken in a 10-mL test tube, then 4 mL of distilled water and 0.3 mL of 5% aqueous NaNO2 were added sequentially. After 5 min, 0.3 mL of 10% AlCl3 and 2 mL of 1 M of aqueous NaOH were added and then the final volume was made up to 10 mL by distilled water. The test tube reaction mixture was shaken well and absorbance was noted at 510 nm using a spectrophotometer. The contents of total flavonoids were calculated as catechin (CE) equivalents g/100 g of dry matter.
DPPH radical scavenging assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging capacity of ME was assessed spectrophotometrically following a method reported by Negi et al.Citation[17] with minor modifications. The plant extracts (variable concentrations ranging from 0.2 to 5.0 μg/mL of methanol), were mixed with 1.0 mL of DPPH solution (90 μM), and final volume of the resulting mixture was made up to 4 mL with 95% methanol. The resulting solutions were incubated for 1 h at room temperature and absorbance was recorded against a control at 515 nm using a spectrophotometer. A control was prepared similarly as the sample, however, without extract addition. Butylated hydroxyl toluene (BHT) was used as a positive control. The percent of scavenging of DPPH free radical was calculated by the following formula:
Antioxidant activity in linoleic acid system
Measurement of the percent inhibition of linoleic acid peroxidation was also used to assess the antioxidant activity of mungbean extracts.Citation[18] Ethanolic solution of extract, containing 0.25 mg/0.5 mL of dry matter, was mixed with a solution mixture containing 0.13 mL linoleic acid, 10 mL ethanol (99.8%), and 10 mL of 0.2 M sodium phosphate buffer (pH = 7) in a 25-mL volumetric flask. The mixture was diluted to 25 mL with distilled water and incubated at 40°C for 15 days. Magnitude of oxidation was measured by the peroxide value following the ammonium thiocyanate method.Citation[19] For this purpose, 10 mL of 75% ethanol, 0.2 mL of an aqueous solution of 30% ammonium thiocyanate, 0.2 mL of sample solution, and 0.2 mL of ferrous chloride (FeCl2) solution (20 mM in 3.5% HCl) were added in sequence to the volumetric flask. After 3 min of stirring, absorbance of the mixture was recorded spectrophotometrically at 500 nm. A control was prepared having all reagents except the extracts. Synthetic antioxidants, butylated hydroxytoluene (BHT) and ascorbic acid (200 mg L−1), were used as positive control. The percent inhibition of linoleic acid oxidation was calculated by the following formula:
Determination of reducing power
The determination of reducing potential of ME was made following the method of Yen et al.Citation[18] Briefly, variable extract concentrations (2.5–10.0 mg/mL of distilled water) were mixed with 5.0 mL of 0.2 M sodium phosphate buffer (pH 6.6) and 5.0 mL of 1.0% aqueous solution of potassium ferricyanide. The mixture produced was incubated at 50°C for 20 min in a water bath followed by the addition of 5 mL of 10% trichloroacetic acid. The mixture was then centrifuged (980 g) for 10 min at 5°C using a refrigerated centrifuge machine (CHM-17, Kokusan Denki, Tokyo, Japan). The upper layer of the centrifuged solution (5.0 mL) was recovered and further diluted with 5.0 mL of distilled water and 1.0 mL of ferric chloride (0.1%). Finally, the absorbance of the resulting solution was recorded at 700 nm using a spectrophotometer.
Statistical Analyses
All the analyses were carried out in triplicate and the data thus generated were subjected to analysis of variance (ANOVA) using STATISTICA 5.5 statistical software (Stat Soft Inc., USA). Statistical significance differences were denoted at a probability value P < 0.05. Data were reported as means ± SD for triplicate determinations.
RESULTS AND DISCUSSION
The extract yields of mungbean seed-derived antioxidant components ranged from 6.90 to 9.65% (). The maximum yield (9.65%) was obtained with 80% ethanol using a stirring technique, while the minimum (6.90%) with 80% methanol was via a soaking method. The stirring technique, involving 80% ethanol as solvent, was found to be the most effective mean for extraction of antioxidant components from mungbean seeds. In agreement with the present findings, Abdille et al.Citation[10] and Peschel et al.Citation[9] reported that methanol and ethanol (in pure form or as aqueous mixtures) have had superior efficacy towards extraction of antioxidant compounds from plant-based materials. As is revealed by some previous studies, the yield of plant antioxidant fractions is mainly affected and enhanced by the use of proper extraction procedure as well as the ability of extraction solvent to dissolve indigenous components.Citation[13, Citation19]
Table 1 Percent yield, total phenolic, and total flavonoid contents of mungbean extracts as affected by extraction technique/solvent
Total phenolic contents (TPC) of mungbean extracts (ME) varied between 0.78 and 1.12 GAE g/100 g dry matter (). TPC of 80% methanolic extract, produced by a shaking method, was found to be the highest (1.12 g/100 g), while the lowest (0.78 g/100 g) was for 80% methanolic extract produced by a stirring method. The present results showed significant (P < 0.05) variations for the phenolic contents of ME as function of extraction procedures and solvents employed. Similar to the authors’ results, Sun and HoCitation[20] also found that antioxidant activity and phenolics concentrations of buckwheat extracts were influenced by the extraction system. The amounts of total phenolics, determined in the present analysis of mungbean, were noted to be considerably lower than those previously reported for ‘Ghana’ and ‘Arriba’ beans (Ecuador) 1.42 and 1.72 (g/100 g), respectively,Citation[21] and common bean (1.12–1.69 g/100 g).Citation[22]
Total flavonoid contents (TFC) of mungbean extracts ranged from 1.23 to 1.78 CE g/100 g of dry matter (). TFC in 80% methanolic extract of a soaking method was found to be the highest (1.78 g/100 g), whereas the least (1.23 g/100 g) in 80% ethanolic extract of soaking method. These results revealed significant (P < 0.05) variations for TFC of mungbean extracts in relation to the extraction methods and solvents used. According to Oomah et al.,Citation[23] mungbean contained considerable amounts of flavonoids, which might be correlated to the high anthocyanins contents.
The data depicting the ability of ME to scavenge DPPH free radical is given in . DPPH, a stable, organic free radical having deep violet/purple color, shows absorption maxima at 515–528 nm. Upon receiving proton from any hydrogen donor, mainly from phenolics, it loses its chromophore and becomes yellow. It is generally accepted that as the concentration of phenolic compounds or the degree of hydroxylation of phenolic compounds increases, DPPH radical scavenging capacity and, thus, antioxidant activity of the plant materials also increases. The tested ME were found to be strongly effective in reducing the stable DPPH radical (purple-colored) to yellow-colored DPPH product. The scavenging capacity of ME (in terms of IC50) was noted to be increased in a concentration dependent manner. The lowest activity (IC50 42.9 μg/mL) was observed for 80% ethanol extract obtained by the stirring method whereas the highest activity (IC50 16.4 μg/mL) was observed for 80% ethanol extract by the shaking method (). The lower the value of IC50, the higher will be the ability of the extract to act as a free radical scavenger. The present free radical scavenging capacity of ME was found to be comparable with that of cocoa bean.Citation[24]
Table 2 Inhibition of linoleic acid peroxidation and DPPH free radical scavenging activity of mungbean extracts as affected by extracting technique/solvent
Antioxidant activity of ME was also examined by assessing their efficiency towards inhibition of linoleic acid (C18:2) peroxidation. In this test, linoleic acid acts as a substrate and forms peroxides on oxidation, which oxidizes ferrous (Fe2+) to Fe3+ (ferric) ions, the latter forms a complex with thiocyanate. The absorption intensity of the resulting colored complex is measured at 500 nm.Citation[13] It is evident from the data presented in that 80% methanol extract of mungbean, produced by a stirring method, exhibited the highest inhibition of linoleic acid oxidation (90.4%), reflecting superior antioxidant activity. On the other hand, 80% ethanol extract, prepared by a soaking method, offered the lowest inhibition (85.2%). The ability of ME to inhibit linoleic acid oxidation might be ascribed to the presence of appreciable amounts of phenolics and flavonoids, which are well accepted to act as potent antioxidants of legumes.Citation[3]
The authors’ results are in agreement with those of Choi et al.,Citation[25] who investigated a high level of percent inhibition of linoleic acid peroxidation (92%) for methanolic extract of mungbean. In a previous study, Anwar et al.Citation[1] also reported that extracts from different mungbean cultivars effectively inhibited the linoleic acid oxidation, ranging from 49.8 to 89.2% as against 89.4% for BHT. The variations in the percent inhibition of linoleic acid oxidation data between the present analysis of mungbean and reported data might have been due to varied efficacy of extraction methods and solvents employed.
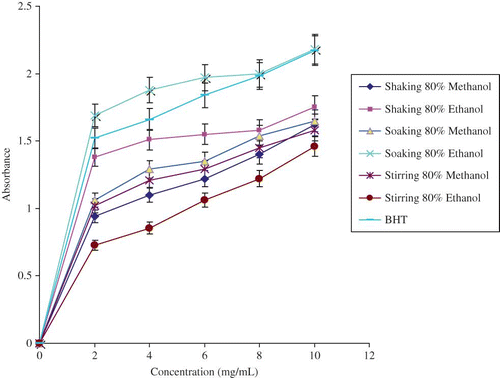
Measurement of reducing potential can also be used to express the antioxidant activity of plant extracts. Ferric ions are reduced to ferrous ions with change in color from yellow to bluish green. The intensity of the color depends on the reducing potential of the antioxidant compounds present in the extract. The greater the intensity of the color of the reaction mixture, the higher will be the absorption, corresponding to higher antioxidant activity of extracts. The extent of reducing potential can be directly correlated to the amount of phenolic compounds.Citation[26] The tested ME, having concentrations between 2–10 mg/mL of reaction solvent, exhibited a strong reducing potential. As is evident from , reducing power of the tested extracts increased in a concentration dependent manner. The reducing power for the extract (typical concentration of 10 mg/mL) of a soaking (with 80% ethanol) method was found to be the maximum (2.18), whereas the minimum (1.46) was for the extracts by a stirring technique (80% ethanol) (). The present trends of reducing potential of ME were quite similar with those examined by Anwar et al.Citation[1] for different cultivars of mungbean, ranging between 1.79 and 1.96.
CONCLUSION
The results of this study revealed that both the extraction techniques and extraction solvents significantly affected the yield and antioxidant properties of mungbean extracts. Generally speaking, of the extraction solvents used, 80% methanol was found to be more promising for extraction of mungbean antioxidant components. Regarding the effects of extraction techniques on the recovery of antioxidant components from mungbean, with few exceptions, the results mainly revealed the superior efficacy of the shaking process, followed by stirring, while least amounts of antioxidants were recovered by the soaking technique. It could be concluded that an optimum extraction protocol/system might be selected for isolation of potent mungbean antioxidant components. A detailed study is further recommended to fully characterize and quantify the individual phenolic acids and flavonoids components of mungbean seeds using state-of-the-art chromatographic approaches coupled with some in vivo antioxidant activity trials of the whole extracts produced.
REFERENCES
- Anwar , F. , Latif , S. , Przybylski , R. , Sultana , B. and Ashraf , M. 2007 . Chemical composition and antioxidant activity of seeds of different cultivars of mungbean . Journal of Food Science , 72 : S503 – S510 .
- Thitilertdecha , N. , Teerawutgulrag , A. and Rakariyatham , N. 2008 . Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts . Journal of Food Science and Technology , 41 : 2029 – 2035 .
- Madhujith , T. , Naczk , M. and Shahidi , F. 2004 . Antioxidant activity of common beans (Phaseolus vulgaris L.) . Journal of Food Lipids , 11 : 220 – 233 .
- Duenas , M. , Hernandez , T. and Estrella , I. 2006 . Assessment of in vitro antioxidant capacity of the seed coat and the cotyledon of legumes in relation to their phenolic contents . Food Chemistry , 98 : 95 – 103 .
- Thomas , M. , Robertson , M.J. , Fukaic , S. and Peoples , M.B. 2004 . The effect of timing and severity of water deficit on growth, development, yield accumulation and nitrogen fixation of mungbean . Journal of Field Crop Research , 86 : 67 – 80 .
- Economic Survey of Pakistan 2008--2009. Ministry of Food and Agriculture, Federal Bureau of Statistics, Islamabad, Government of Pakistan.
- Mendoza , E.M.T. , Adachi , M. , Bernardo , A.E.N. and Utsumi , S. 2001 . Mungbean [Vigna radiata (L.) Wilczek] globulins: Purification and characterization . Journal of Agriculture and Food Chemistry , 49 : 1552 – 1558 . 2001
- Cheung , L.M. , Cheung , P.C.K. and Ooi , V.E. 2003 . Antioxidant activity and total phenolics of edible mushroom extracts . Food Chemistry , 81 : 249 – 255 .
- Peschel , W. , Sanchez-Rabaneda , F. , Dn , W. , Plescher , A. , Gartzia , I. , Jimenez , D. , Lamuela-Raventos , R. , Buxaderas , S. and Condina , C. 2006 . An industrial approach in the search of natural antioxidants from vegetable and fruit wastes . Food Chemistry , 97 : 137 – 150 .
- Abdille , M.H. , Singh , R.P. , Jayaprakasa , G.K. and Jens , B.S. 2005 . Antioxidant activity of the extracts from Dillenia indica fruits . Food Chemistry , 90 : 891 – 896 .
- Rehman , Z.U. 2006 . Citrus peel extract—A natural source of antioxidant . Food Chemistry , 99 : 450 – 454 .
- Li , Y. , Guo , C. , Yang , J. , Wei , J. , Xu , J. and Cheng , S. 2006 . Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract . Food Chemistry , 96 : 254 – 260 .
- Sultana , B. , Anwar , F. and Przybylski , R. 2007 . Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees . Food Chemistry , 104 : 1106 – 1114 .
- Luthria , L.D. , Biswas , R. and Natarajan , S. 2007 . Comparison of extraction solvents and techniques used for the assay of isoflavones from soybeans . Food Chemistry , 105 : 325 – 333 .
- Okmen , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish Eggplant (Solanum melongena L.) cultivars . International Journal of Food Properties , 12 ( 3 ) : 616 – 624 .
- Dewanto , V. , Wu , X. and Liu , R.H. 2002 . Processed sweet corn has higher antioxidant activity . Journal of Agriculture and Food Chemistry , 50 : 4959 – 4964 . 2002
- Negi , P.S. , Jayaprakasha , G.K. and Jena , B.S. 2010 . Evaluation of antioxidant and antimutagenic activities of the extracts from the fruit Rinds of Garcinia cowa . International Journal of Food Properties , 13 ( 6 ) : 1256 – 1265 .
- Yen , G. , Duh , E.D. and Lister , C.E. 2000 . Antioxidants of anthraquinones and anthrone . Food Chemistry , 70 : 307 – 315 .
- Siddhuraju , P. and Becker , K. 2003 . Antioxidant properties of various extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves . Journal of Agriculture and Food Chemistry , 51 : 2144 – 2155 .
- Sun , T. and Ho , C. 2005 . Antioxidant activities of buckwheat extracts . Food Chemistry , 90 : 743 – 749 .
- Wollgast , J. and Anklam , E. 2000 . Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification . Food Research International , 33 : 423 – 447 .
- Elizabeth , N.R. , Annete , H. , Francisco , R.G. , Javier , F.R. , Graciela , Z. and Alberto , J.G. 2007 . Antioxidant and antimutagenic activity of phenolic compounds in three different colour groups of common bean cultivars (Phaseolus vulgaris L.) . Food Chemistry , 103 : 521 – 527 .
- Oomah , B.D. , Cardador-Martinez , A. and Loarca-Pin , G. 2005 . Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L.) . Journal of Science and Food Agriculture , 85 : 935 – 942 .
- Othman , A. , Ismail , A. , Ghani , N.A. and Adenan , I. 2007 . Antioxidant capacity and phenolic contents of coca bean . Food Chemistry , 100 : 1523 – 1530 .
- Choi , J. , Heon-Sang , J. and Lee , J. 2007 . Antioxidant activity of methanolic extracts from some grains consumed in Korea . Food Chemistry , 103 : 130 – 138 .
- Siddhuraju , P. , Mohan , P.S. and Becker , K. 2002 . Studies on the antioxidant activity of Indian laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flower and fruit pulp . Food Chemistry , 79 : 61 – 67 .