Abstract
The influence of different winemaking techniques usually used to elaborate red wines (Ganimede, prefermentative maceration, pectolytic enzymes and tannins addition, oak chips addition, delestage, and conventional procedure) on the metal content was evaluated. Linear discriminant analysis was also used to characterize the respective wines based on the metal content. By using iron, manganese, and calcium as chemical descriptors, the six winemaking techniques were perfectly discriminated. As the phenolic composition is an important quality factor in red wines, a correlation study between phenolic compounds and metals was also performed. A good correlation has been found for iron, calcium, and potassium with total polyphenols, tannins, and flavanols reactive. Iron, zinc, calcium, and potassium showed a significant correlation with anthocyanin compounds. Furthermore, higher concentrations of iron and calcium, but lesser ones of potassium, permitted to obtain red wines with a greater chromatic intensity.
INTRODUCTION
Wine is one of the most widely consumed beverages in the world and the evaluation of its quality is important for manufacturers, merchants, and consumers. Wine is a very complex medium containing a great variety of organic and inorganic substances in an ethanol-water solution. The principal dissolved species are inorganic ions, organic acids, polyphenols, polyhydroxyalcohols, proteins, amino acids, and polysaccharides. Furthermore, red wines have a greater quantity of dissolved substances than white wines, particularly in what concerns polyphenols and mineral composition.Citation[1, Citation2]
The study of phenolic compounds in red wines is of great interest since they not only contribute to the sensory characteristics of wines like color, body, bitterness, and astringency, but also act as antioxidants by both free-radical scavenging and metal chelation.Citation3–5 Metals play an important role on the nutritional (zinc and copper are essential nutrients that act as cofactors of enzymes) and organoleptic characteristics (flavor enhancers) of the wine. However, they can also induce spoilage reactions in the wine like precipitation of hydrogen tartrate (potassium) or tartrate (calcium) salts, rate enhanced of oxidation (copper and iron), haze formation (copper and iron), and flavor negative modification (zinc and iron). Furthermore, some essential elements like copper cause toxic effects for the human health when they are present in high concentrations, justifying the establishment by the International Organization of Vine and Wine (OIV) of a legal limit in wines (1 mg/l).
Although the content of metals in wines is not directly responsible for the final quality of the product, it influences the sensory characteristics as mentioned above. Furthermore, polyphenols form salts and chelates with iron, copper, and zinc.Citation[6] Some wine cations, such as iron, copper, and magnesium, can form complexes with those anthocyanins having an ortho-diphenyl group bound to their aromatic ring (delphinidin, petunidin, and cyanidin), originating a batochromic effect.Citation[1] Tannins can also experiment a similar reaction. In both cases, it is visually perceived as an increase in the blue color.Citation[7, Citation8]
The content of metals in wines depends on several factors, including the grape variety, soil composition, climate, and winemaking technique.Citation[9– Citation12] Taking into account that metals in the wine come mainly from the solid parts of the grape berry, their content in the dissolved phase increases during the maceration step. Therefore, technological features, chemical factors, and physical parameters could also affect the metal extraction.Citation[2] In the degradation of tissue cell walls, different enzymatic reactions are involved, which are responsible for the extraction and solubilization of the vacuolar constituents. Therefore, the addition of pectolytic enzymes may result in a change in the mineral profile of a wine. The prefermentative maceration, based on the must cooling, permits to extend the contact of the must with the solid parts of grape berries. Several punch-down and pump-over techniques have been also compared in relation to their effectiveness on the extraction of the vacuolar constituents. Ganimede autovinificator aims to improve the must maceration with skins, through a combination of prefermentative maceration and automatic pump-over techniques. On the other hand, long macerations at excessively high temperatures probably cause a thorough extraction of metals from the grape berry.
The assessment of the grape variety used and the geographical origin of red wines according to the metal profile is difficult because the winemaking process can cause alterations in the metal composition that would hinder the differentiation. Taking into account that the maceration step influences the metal extraction from the solid parts of grape berries into the dissolved phase and the lack of works published on the effect of the winemaking technique on the metal profile of a certain wine, the aim of the present work was to evaluate the influence of six winemaking techniques usually used in a winery (Ganimede autowinemaker, prefermentative maceration, addition of pectolytic enzymes, procyanidin tannins and ellagitannins, addition of oak chips, delestage, and conventional procedure) on the metal content (copper, iron, manganese, zinc, calcium, magnesium, and potassium) in red wines. The grape variety used was Mencía, one of the most important Galician cultivars (northwest of Spain). Furthermore, the use of the metal content as a useful tool in differentiating the red wines elaborated using different maceration techniques was evaluated. The phenolic composition, anthocyanin profile, and chromatic characteristics were also correlated with the metal content.
MATERIALS AND METHODS
Reagents and Standards
All solutions were prepared in deionized water and the chemicals used were of analytical-reagent grade, supplied by Merck (Darmstadt, Germany) or Panreac (Barcelona, Spain). Standards for phenolic compounds were purchased from Sigma-Aldrich (St. Louis, MO, USA). All of the stock standard solutions were stored at −20°C away from light before use. The metal stock standard solutions (1000 mg/l) were purchased from Panreac. Working standard solutions were prepared daily by appropriately diluting the stock standard solutions.
Winemaking
Mencía wines were elaborated in the experimental winery of the Estación de Viticultura e Enoloxía de Galicia. Approximately 6000 kg of grape berries from Denomination of Origin Monterrei (northwest Spain) were desteemed and crushed. The harvest mass, previously homogenized in order to reduce differences in solid/liquid ratios, was distributed in 10 stainless steel maceration tanks (250 l) in order to realize the fermentation/maceration experiments in duplicate and in a Ganimede autowinemaker (2500 l). Sodium metabisulphite was added (5 g/hl) to the grape must, which was then inoculated with Saccharomyces cerevisiae (LSA) commercial yeast (20 g/hl) except for prefermentative maceration and Ganimede autowinemaker. The maceration time selected was 12 days, independently on the technique used, in order to avoid the effect of time variable.
The functioning of Ganimede autowinemaker was as follows: Temperature was maintained at 16–17°C for 7 days before inoculating. The carbon dioxide produced by the fermentation process constantly agitated the mass of marc and kept wet all of the skins being dispersed. When the by-pass valve was opened, the large gas volume trapped was released into the cap and a vigorous mixing action with must took place. The whole process was repeated even before fermentation by the addition of carbon dioxide from an external source. In the cold treatment performed in two maceration tanks, temperature was maintained at 10°C using solid carbon dioxide for 4 days before inoculating. In the following two maceration tanks, 6 g of pectolytic enzymes (Vinozym Vintage FCE; Lamothe Abiet, ZA, France) were added when starting fermentation, 25 g of procyanidin tannins extracted from white grape seeds (Tanéthyl; AEB, Brescia, Italy) and 25 g of ellagitannins derived from oak wood (Ellagitan Chêne; AEB) were added during fermentation, and 25 g of Tanéthyl and 12.5 g of Ellagitan Chêne were added when alcoholic fermentation was completed. In two other maceration tanks, 750 g of oak chips without toasting (Ellagitan Chips; AEB) were added. The following two maceration tanks were submitted to delestage procedure during the first four maceration days. Once a day, the solid part of the grape berries was maintained separate from the must/wine for 6 h. In the last two maceration tanks, a conventional procedure was performed.
All maceration experiments were carried out by duplicate. After alcoholic fermentation, the wines obtained after soft pressing and simple draining were racked. Malolactic fermentation occurred spontaneously. Finally, wines were bottled after adjusting their free sulphur dioxide content to 25 mg/l. Two bottles were analyzed for each maceration tank.
Spectrophotometric Analysis
Metal determination
Potassium determination required the addition to standards and samples of 5% cesium chloride as an ionization suppressor and a reference solution containing 10% ethanol, 7 g/l citric acid, 3 g/l sacharose, 10 g/l glycerol, 0.1 g/l calcium chloride anhydrous, and 0.1 g/l magnesium chloride anhydrous. On the other hand, calcium determination required the addition of 50 g/l lanthanum chloride as an ionization suppressor. A Perkin-Elmer (Norwalk, CT, USA) Model 2380 atomic absorption spectrometer equipped with an acetylene-air flame was used for element determination (copper, iron, manganese, zinc, calcium, magnesium, and potassium). Cathodeon hollow-cathode lamps were used as the radiation source. The instrumental parameters used were those recommended by the manufacturer.
Phenol determination
The methods used for the phenolic compounds determination in the red wines obtained were previously described.Citation[13] The chromatic characteristics were measured by using a Perkin-Elmer ultraviolet-visible spectrophotometer Model Lambda 20 (Norwalk, CT, USA). This was equipped with the CIELab software version 1.0 for determining CIELab parameters. All spectrophotometric analyses were done by duplicate.
HPLC Analysis
The anthocyanin compounds present in Mencía wines were separated and identified by high performance liquid chromatography using a diode array detector (HPLC-DAD). For this, the wines were previously submitted to solid phase extraction in Oasis HLB 200 mg cartridges from Waters Corp. (Milford, MA, USA).Citation[13, Citation14] The eluents used were, first, 5% acetic acid in a mixture of acetonitrile and water (10:90) and, second, 2% ammonium hydroxide in a mixture of acetonitrile and water (10:90). Both fractions were combined. The experiments were done by duplicate.
The chromatographic system consisted of an Agilent 1100 Series instrument (Milford, MA, USA), equipped with a SunfireTM C18 analytical column (5 μm, 4.6 × 250 mm) from Waters Corp. (Milford, MA, USA) that was heated at 40°C. The chromatographic separation required the use of three mobile phases: 50 mmol/l ammonium dihydrogen phosphate at pH 2.6 (mobile phase A), acetonitrile (mobile phase B), and 0.2 mol/l phosphoric acid at pH 1.5 (mobile phase C). The elution gradient profile was 100% A at 0 min, 92% A and 8% B at 8 min, 0% A and 14% B at 20 min, 1.5% A and 16.5% B at 25 min, 0% A and 21.5% B at 35 min, 0% A and 50% B at 70 min, and 30% A and 70% B at 80 min. The mobile phase was returned to its initial condition at 5 min. All of the solvents were filtered through a 0.20-μm filter and the mobile phase flow-rate selected was 0.5 ml/min. The determination of anthocyanins was carried out at 520 nm. All analyses were done by duplicate. All of these compounds were identified by comparing retention time and UV spectra to those of standards. The acylated forms of anthocyanins were identified by comparing the retention time of each chromatographic peak with available data in the literature. Anthocyanin amount was determined by comparing the area of an individual peak with the total peak area and the results were expressed as percentages.
Statistical Analysis
SPSS 11.5 for Windows (SPSS Inc., Chicago, IL, USA) was used in order to establish statistical differences in the mean values of the metal content among winemaking techniques by one-way analysis of variance (ANOVA). For this purpose, Tukey test for p < 0.05 was used. Linear discriminant analysis was also used to try the characterization of the red wines elaborated by several winemaking techniques based on the metal content. The criterion used for the selection of the principal function was higher variance, the confidence level being 95%. All results were evaluated using discriminant analysis in order to permit an evaluation of the importance of the contribution made by individual metals to the discrimination among groups. Therefore, it was used to investigate the minimum number of metals needed to separate the six winemaking techniques. In addition, it was used to ascertain whether the data allowed the separation of red wines by their chromatic quality. Pearson correlation coefficients were calculated to determine significant correlations between parameters, which were used to establish relationships between metals and phenolic composition.
RESULTS AND DISCUSSION
Influence of Winemaking Techniques on Metal Content
The concentrations of the seven metals determined in all wine samples are shown in . No wine presented contents higher than the maximum limits of 1 mg/l for copper and 5 mg/l for zinc, established by the OIV. Therefore, any contamination introduced during the winemaking process should be of no concern. All wines had iron contents below 7 mg/l, considered as the minimum concentration necessary to form ferric casse. Furthermore, the contents obtained were similar to those found by other authors in wine samples,Citation[15] with the exception of manganese and zinc for whose concentrations were higher and lower, respectively, in this study. They were also similar in red wine samples of different denominations of origin from Galicia,Citation[16] with the exception of manganese showing again higher concentrations in this study. However, the manganese contents determined in French red wines ranged from 0.435 to 7.84 mg/l, the high manganese content in wines being explained by the high content of this element in soils.Citation[17]
Table 1 Concentration (mg/l) of metals in red wines elaborated using different winemaking techniques
The contents of metals in wines were influenced by the winemaking technique used. It was found that wines from prefermentative maceration have higher contents of copper and zinc, but lower contents of iron, manganese, calcium, and magnesium. However, the addition of enzymes and tannins involved greater concentrations of iron, calcium, and magnesium. The lowest values obtained for the potassium amount corresponded to the addition of oak chips during alcoholic fermentation. On the other hand, the delestage technique reported higher contents of manganese and magnesium, but lower contents of zinc. Finally, Ganimede autowinemaker permitted to obtain lower concentrations of copper, iron, and calcium, whereas the potassium contents resulted to be higher. Therefore, it can be observed that zinc and magnesium were the most robust elements for the assessment of the geographical origin of Galician red wines. However, some others, such as manganese and potassium, seem to be the most dependent elements on the winemaking technique as important changes in their concentration occurred during the winemaking process.
Metal Content in Differentiating Winemaking Techniques
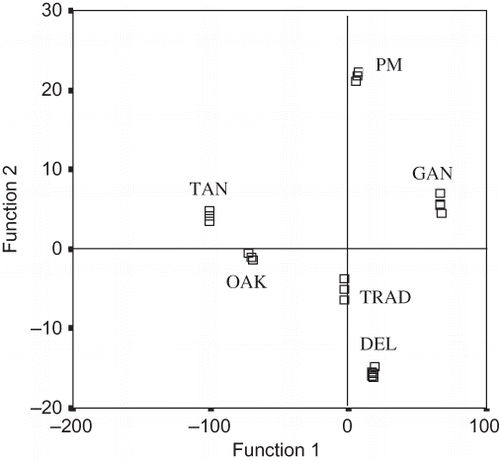
Figure 1 shows the classification of Mencía red wines according to the winemaking technique used. A total of 24 wines were analyzed with observations on 7 metals. In all cases, the samples associated with a designated group were well separated from the other groups. From the coefficients standardized of the canonical discriminant functions (), iron was the dominant variable in the first one that represented 95.1% of the total variability, while manganese and calcium dominated in the second one that represented 4.1% of the total variability. Thus, most of the separation of the six red wine groups was achieved on the basis of just three elements: iron, manganese, and calcium. These functions produced a classification success rate of 100% for all the red wines studied.
Table 2 Coefficients of canonic discriminant functions
In , it is evident that Ganimede autowinemaker and prefermentative maceration with the addition of dry ice were separated from other winemaking techniques, but they were nearer between themselves. Both methodologies included a step of prefermentative maceration (refrigeration or dry ice) and were characterized by the highest and most positive values for function 1 (Ganimede autowinemaker) or function 2 (prefermentative maceration), respectively. On the other hand, the techniques based on the addition of tannins from oak chips or commercial seed procyanidins and ellagic tannins, during alcoholic fermentation, were located nearer between themselves than the others. Furthermore, the delestage technique was close to the conventional technique, possibly due to the absence of additions or a prefermentative step. Hence, the addition of tannins can influence the metal concentration in wine as a consequence of the association of metal-tannins, whereas prefermentative maceration facilitates the extraction and solubilization of the metals from the grape solid parts into the must/wine.
Correlation Study Between Phenolic Composition and Chromatic Characteristics with Metal Content
In red wines, color depends mainly on the phenolic composition, anthocyanins being the compounds responsible for their red color. On the other hand, some metals intervene in multiple processes, such as complex formation, polymerization, flocculation, and precipitation.Citation[18] So, the complexing capacity of anthocyanins and tannins on metals has been provedCitation[8] resulting in small changes in the wine color.Citation[7] Taking into account the influence of the interactions between phenolic compounds and metals on the wine color, the phenolic composition and chromatic characteristics were also determined in the red wines studied. The results obtained were recently published.Citation[13] The anthocyanin profile is shown in . The variation of phenolic compounds among different winemaking techniques was comparable to that published for red wines from Spanish cultivars,Citation[19, Citation20] but significant differences in the amounts of these compounds were found among the different winemaking techniques studied.
Table 3 Anthocyanin profile determined in red wines elaborated using different winemaking techniques
Table 4 Pearson correlations among metals (mg/l) and phenolic compounds in red wine samples
Correlations between the contents of elements and phenolic compounds in red wines were also investigated, and several statistically significant dependencies were found as shown in . Correlation coefficients higher than 0.5 at p < 0.01 are only discussed. Iron, calcium, and potassium had a very strong correlation, which was higher than 0.77 with total polyphenols, tannins, and flavanols reactive to dimethylaminecinnemaldehyde. A similar relationship was also found between potassium and ionization index, and between calcium or potassium and flavanols reactive to vanillin. In addition, lesser correlations were also found between manganese or zinc and polyvinylpolypirrolidone index (0.68–0.75), and between iron or magnesium and flavanols reactive to vanillin (0.64–0.68). The absence of associations between copper and phenolic compounds can be due to the low copper concentrations in the red wines analyzed. Thus, some authors recommend maintaining the copper concentrations below 0.3–0.5 mg/l in order to minimize the appearance of precipitates of condensed phenolic compounds in the bottled wine.Citation[2] Furthermore, other authors indicate that the interaction between copper and the wine components is weak.Citation[21]
The correlation coefficients between the concentrations of metals and anthocyanin compounds in red wines are given in . It was found that iron shows a statistically significant dependence on delphinidin-3-glucoside, petunidin-3-glucoside, peonidin-3-glucoside, malvidin-3-glucoside, malvidin-3-acetylglucoside, and malvidin-3-caffeoylglucoside (0.63–0.80). Zinc was well correlated with cyanidin-3-glucoside and peonidin-3-glucoside (0.63–0.66). Furthermore, calcium was strongly correlated with cyanidin-3-glucoside, peonidin-3-glucoside, malvidin-3-glucoside, peonidin-3-acetylglucoside, malvidin-3-acetylglucoside, malvidin-3-caffeoylglucoside, petunidin-3-cumaroylglucoside, and peonidin-3-cumaroylglucoside (0.64–0.90). Finally, significant relationships were found between potassium and delphinidin-3-glucoside, cyanidin-3-glucoside, peonidin-3-glucoside, malvidin-3-glucoside, peonidin-3-acetylglucoside, malvidin-3-acetylglucoside, and malvidin-3-caffeoylglucoside (0.70–0.92).
Table 5 Pearson correlations among metals (mg/l) and anthocyanin compounds (%) in red wine samples
Other authors reported that 20–30% of iron is complexed with polyphenols (tannins and anthocyanins) and proteins,Citation[6, Citation21, Citation22] as well as that less than 15% of zinc is complexed with polyphenols, which is in concordance with the relationships obtained in this study.Citation[6] The fact that some elements are significantly correlated with phenolic compounds seems to indicate a certain ability to modify chromatic characteristics as shown in . The elements can be divided into several groups on the basis of statistically significant correlations. Copper, manganese, and magnesium were not correlated with chromatic characteristics. Iron and calcium had a positive correlation with chromatic intensity and percentage of red color, but a negative one with tonality and percentages of yellow and blue color. On the other hand, potassium had a negative correlation with chromatic intensity and percentage of red color, but a positive one with tonality and percentages of yellow and blue color. Nevertheless, the highest correlation coefficients corresponded to potassium. Zinc showed only correlation coefficients comprised between 0.50 and 0.60 at p < 0.05. It implies that higher concentrations of iron and calcium, but lesser ones of potassium, permit to obtain red wines with greater chromatic intensity and percentage of red color, whereas a lesser content of iron and calcium, but a higher one of potassium, involves red wines with greater tonality and percentages of yellow and blue color. This behavior can be due to the three elements that are strongly correlated themselves (correlation coefficients = 0.78–0.88 at p < 0.01). Iron and calcium are positively correlated, whereas the two elements show a negative correlation with potassium.
Table 6 Pearson correlations among metals (mg/l) and chromatic characteristics in red wine samples
CONCLUSIONS
The suitability of the metal concentrations as a chemical tool for the differentiation of the red wines elaborated using different winemaking techniques has been confirmed, since the last ones influenced the elemental composition of the red wines obtained. Satisfactory classification was achieved on the basis of three elements: iron, manganese, and calcium. Significant correlations (p < 0.01) between the metal concentration and phenolic composition, including anthocyanin profile, as well as between the metal content and chromatic characteristics, were found. The red wines produced by Ganimede autowinemaker or prefermentative maceration experimented a bathochromic effect with an increase in the blue color, whereas those ones elaborated with the addition of pectolytic enzymes and tannins showed a hyperchromic effect with an increase in the red color. This reinforces the importance of studying both metal profile of the wine and its correlation with the phenolic composition to establish reliable relationships. The management of the winemaking technique on the basis, not only of phenolic compounds but also of their association with metals, would permit to improve the color stability during the wine shelf-life.
ACKNOWLEDGMENTS
A contract for doctors in the INIA-CCAA system, supplied by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria and co-financed with European Social Fund, is gratefully acknowledged by Susana Río Segade.
REFERENCES
- Ribéreau-Gayon , P. , Glories , Y. , Maujean , A. and Dubourdieu , D. 2003 . Tratado de Enología (Tomo II) Química del vino, estabilización y tratamiento , Buenos Aires . Hemisferio Sur (Ed.)
- Pyrzynska , K. 2004 . Analytical methods for the determination of trace metals in wine . Critical Reviews in Analytical Chemistry , 34 : 69 – 83 .
- Peña-Neira , A. , Hernández , T. , García Vallejo , C. , Estrella , I. and Suárez , J.A. 2000 . A survey of phenolic compounds in Spanish wines of different geographical origin . European Food Research and Technology , 210 : 445 – 448 .
- Raczkowska , J. , Mielcarz , G. , Howard , A. and Raczkowski , M. 2011 . Uplc and spectrophotometric analysis of polyphenols in wines available in the Polish market . International Journal of Food Properties , 14 ( 3 ) : 514 – 522 .
- Río Segade , S. , Orriols , I. , Giacosa , S. and Rolle , L. 2011 . Instrumental texture analysis parameters as winegrapes varietal markers and ripeness predictors . International Journal of Food Properties , 14 ( 6 ) : 1318 – 1329 .
- Karadjova , I. , Izgi , B. and Gucer , S. 2002 . Fractionation and speciation of Cu, Zn and Fe in wine samples by atomic absorption spectrometry . Spectrochimica Acta Part B , 57 : 581 – 590 .
- Usseglio-Tomasset , L. 1998 . Química Enológica , Mundi-Prensa : Barcelona .
- Hidalgo Togores , J. 2003 . Tratado de Enología , Mundi-Prensa : Barcelona .
- Almeida , C.M.R. and Vasconselos , M.T.S.D. 2003 . Multielement composition of wines and their precursors including provenance soil and their potentialities as fingerprints of wine origin . Journal of Agricultural and Food Chemistry , 51 : 4788 – 4798 .
- Castiñeira Gomez , M.M. , Brant , R. , Jakubowski , N. and Anderson , J. 2004 . Changes of the metal composition in German white wines through the winemaking process. A study of 63 elements by inductively coupled plasma-mass spectrometry . Journal of Agricultural and Food Chemistry , 52 : 2953 – 2961 .
- Kment , P. , Mihaljevic , M. , Ettler , V. , Sebek , O. , Strnard , L. and Rohlová , L. 2005 . Differentiation of Czech wines using multielement composition—A comparison with vineyard soil . Food Chemistry , 91 : 157 – 165 .
- Fabani , M.P. , Arrúa , R.C. , Vázquez , F. , Diaz , M.P. , Baroni , M.V. and Wunderlin , D.A. 2010 . Evaluation of elemental profile coupled to chemometrics to assess the geographical origin of Argentinean wines . Food Chemistry , 119 : 372 – 379 .
- Soto Vázquez , E. , Río Segade , S. and Orriols Fernández , I. 2010 . Effect of the winemaking technique on phenolic composition and chromatic characteristics in young red wines . European Food Research and Technology , 231 : 789 – 802 .
- Del Álamo Sanza , M. , Nevares Domínguez , I. , Cárcel Cárcel , L.M. and Navas Gracia , L. 2004 . Analysis for low molecular weight phenolic compounds in a red wine aged in oak chips . Analytica Chimica Acta , 513 : 229 – 237 .
- Greenough , J.D. , Longerich , H.P. and Jackson , S.E. 1997 . Element fingerprinting of Okanagan Valley wines using ICP-MS: Relationships between wine composition, vineyard and wine colour . Australian Journal of Grape and Wine Research , 3 : 75 – 83 .
- Rebolo , S. , Peña , R.M. , Latorre , M.J. , García , S. , Botana , A.M. and Herrero , C. 2000 . Characterisation of Galician (NW Spain) Ribeira Sacra wines using pattern recognition analysis . Analytica Chimica Acta , 417 : 211 – 220 .
- Cabrera-Vique , C. , Teissedre , P.L. , Cabanis , M.T. and Cabanis , J.C. 2000 . Manganese determination in grapes and wines from different regions of France . American Journal of Enology and Viticulture , 51 : 103 – 107 .
- Esparza , I. , Santamaría , C. , García-Mina , J.M. and Fernández , J.M. 2007 . Complexing capacity profiles of naturally occurring ligands in Tempranillo wines for Cu and Zn. An electrochemical approach for cupric casse . Analytica Chimica Acta , 599 : 67 – 75 .
- Álvarez , I. , Aleixandre , J.L. , García , M.J. and Lizama , V. 2006 . Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines . Analytica Chimica Acta , 563 : 109 – 115 .
- Pérez Lamela , C. , García Falcón , M.S. , Simal Gándara , J. and Orriols Fernández , I. 2007 . Influence of grape variety, vine system and enological treatments on the colour stability of young red wines . Food Chemistry , 101 : 601 – 606 .
- McKinnon , A.J. and Scollary , G.R. 1997 . Size fractionation of metals in wine using ultrafiltration . Talanta , 44 : 1649 – 1658 .
- Paleologos , E.K. , Giokas , D.L. , Tzouwara-Karayanni , S.M. and Karayannis , M.I. 2002 . Micelle mediated methodology for the determination of free and bound iron in wines by flame atomic absorption spectrometry . Analytica Chimica Acta , 458 : 241 – 248 .