Abstract
In this study, “orange flavored soft drink” samples sweetened with 100% aspartame (0.5 g/L) were stored in three different pH values and temperatures. The samples with the pH values of 2.75, 3.25, and 4.57 were kept in 20, 30, and 40°C temperatures over a period of 5 months. The remaining aspartame was determined at the 1st, 2nd, 3rd, 4th, and 5th months by HPLC. In the same pH groups, it was clearly observed that stability of aspartame decreased with increasing storage temperature. It was also determined in each of the same pH groups that aspartame stability increased as the pH value was raised from 2.75 to 4.57. Aspartame was the least stable at pH 2.75 when stored in 40°C, whereas it was the most stable at pH 4.57 and 20°C. At the end of 5th month in 40°C, stability tests could not be performed and remaining aspartame could not be determined.
INTRODUCTION
Records from the earliest civilizations show that man has always valued foods that taste sweet. Historically, sugar has been used to sweeten most of the foods and drinks that we eat and drink in the last 100 years. Some health problems have been appearing due to the high consumption of sugar and sugar-made foods.Citation[1, Citation2] It is determined that sugar is one of the main reasons of tooth decay, obesity, increasing blood sugar level and serum triglycerides, and also it is dangerous for people who have diabetes.Citation[3]
Aspartame is a dipeptide composed of two amino acids: L-aspartic acid and L-phenylalanine. Phenylalanine is an essential amino acid. These constituents exist in many foods and are metabolized exactly by the same way, as they would come from meat, cheese, fish, vegetables, fruit juice, or milk. Aspartame taste profile is the closest to sugar taste among all other artificial sweeteners and it is approximately 200 times sweeter than sugar.Citation[4]
The stability of aspartame varies depending on time, temperature, pH, and water activity.Citation[5– Citation8] Aspartame is less stable in liquid systems and the stability is primarily of a function of pH, temperature, and time. The aspartame molecule slowly hydrolyzes at low pH to produce a tasteless molecule aspartyl-phenylalanine and methanol. An alternative route at pH 5 and above is that aspartame may cyclize to form its diketo-piperazine with the elimination of methanol. These conversion products can be subsequently hydrolyzed to its individual amino acids—aspartic acid and phenylalanine. Stability during storage of beverages containing aspartame follows first-order kinetics and, to an extent, is predictable if parameters of pH and temperature are known. Expected results can, however, be confounded by order interaction with ingredients that can have a positive or negative impact on stability and also perceived sweetness of beverages changes over time.Citation[9]
Aspartame is progressively degraded to diketo-piperazine when stored at temperatures ranging from 30 to 80°C in liquid solution. It is, therefore, not suggested to be used in high temperature treated foods (cooking, sterilization, etc.). Similarly, at elevated temperatures, aspartame has been shown to react with reducing sugars via the Maillard reaction.Citation[10] Aspartame stability is good at room temperature at pH 3.0–4.0 and maximum stability is observed at pH 4.2. The dipeptide structure is hydrolyzed below pH 3.4 and diketo-piperazine formation and crystallization occur above pH 5.0. In both cases, a transformation takes place, which results in sweetness loss.Citation[7, Citation8] Buffer salts catalyze the degradation of aspartame, with greater loss at higher buffer concentrations. In low to intermediate moisture systems, aspartame degradation increases as water activity increases.Citation[10]
While temperature is the major contributor to aspartame degradation, pH and reactive solutes also influence its stability. Aspartame is not recommended for use in liquid with a pH level higher than 7.0. It is important to choose appropriate pH levels and restricted storage conditions to obtain best aspartame stability in liquid preparations. Ultra high temperature (UHT), high temperature short time (HTST), and hot filling systems are used effectively. Shelf life is affected by acidity of medium. Products including phosphoric acid have the shortest shelf life. Aspartame loss is less than 3% in the long time low temperature pasteurization method and even below 1% in the HTST pasteurization method. The aspartame loss is at negligible levels in the UHT sterilization method.Citation[6] Storage time, temperature, and pH do not only affect the sweetness potency of aspartame, but also makes the shelf life of the product shorter. So, stability of aspartame and its degradation products are significantly important in order to make a sufficient quality control of diet and diabetic foodstuffs.Citation[11]
The aim of this study is to determine the effects of different storage time and conditions on aspartame stability and sweetness of food products. The results of the present study will help the technological problem faced during production and storage of foods containing aspartame and will contribute to the development of new products for people who have a restriction for natural sugar intake and will lead to further scientific research studies.
MATERIALS AND METHODS
Materials
Orange flavored soft drinks with different pH values and sweetened with 100% aspartame (Ajinomoto, Switzerland) (0.5 g/L drinks) stored at different temperature conditions and the stability of samples were studied during 5 months. The samples were prepared in three different formulations, which belong to a private company. shows the general formulation of orange flavored soft drinks.
Table 1 Orange flavored soft drink recipe used in the study
Preparation of Samples
First, the acidity regulators, citric acid (4.05 g/L), ascorbic acid (0.05 g/L), and trisodium citrate (0.1 g/L), were dissolved with aspartame (0.5 g/L). Sodium benzoate (0.1 g/L) and potassium sorbate (0.2 g/L) were dissolved in another beaker and added into the acidulant and aspartame mixture. Finally, a clouding agent (1 g/L), coloring agents (Sunset Yellow and Tartrazine, 0.09 and 0.04 g/L, respectively), and orange flavor (0.5 g/L) were added to the first mixture and stirred until all ingredients were dissolved and the final mixture completed up to 1 liter.
Fifteen orange flavored soft drink samples with three different pH degrees (2.75, 3.26, and 4.57) were stored in incubators whose temperatures were fixed to 20, 30, and 40°C for 5 months of storage. Fifteen samples with their reference samples and totally 90 samples were stored in 30-mL bottles. The pH of the samples was measured with a Testo Model 230 pH-meter (Testo, Sparta, USA).
Analysis of Aspartame
During the storage period, analysis was done on the 1st, 2nd, 3rd, 4th, and 5th months by the HPLC analyzing method on remaining aspartame % basis. Aspartame analyses were conducted according to the method belonging to Ajinomoto Switzerland AG Company (Zug, Switzerland).Citation[12] The manufacturer of the HPLC system was Waters Co. (Pump: Waters 515; Autosampler: Waters 717 Plus; Detectors: Waters 2487, Waters Co., Milford, USA).
Chromatographic Conditions
In this study, a Bondapak C18 column (Waters Co., Milford, USA) was used and a 210-nm wavelength was preferred in UV detection. Orange flavored soft drink samples containing 500 mg/L aspartame, were filtered through a 0.45-μm filter. Aspartame-containing samples were dissolved in 10% acetonitril solution and 90% buffer solution and aspartame was separated from the soft drink samples. Aspartame was eluted isocratically with water–acetonitrile (90:10) including 0.00125 M Sodium-dihydrogen-phosphate, and its pH was fixed to 3.5 with phosphoric acid. The flow rate was fixed to 1.5 mL/min at room temperature. One mL orange flavored soft drink was diluted in 6.5 mL of solvents. Injection volume was used as 20 μL in this study. The mobile phase was degassed prior to analysis. The samples were identified and quantified through comparison of areas and retention times established with standards. The chromatograms presented limits of quantification and limits of detection of 2 and 5 μg/mL for aspartame, respectively. Recovery of aspartame solution after sample preparation was >95%.
Statistical Analysis
All the experiments were done in triplicate. The data were analyzed by the SAS (Statistical Analysis Systems Institute, Inc., Cary, USA) statistical package program, and differences in the treatment effects on aspartame stability were compared using Duncan's Multiple Range test.
RESULTS AND DISCUSSION
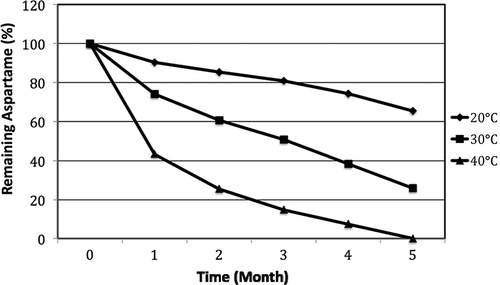
Results of the soft drinks with pH 2.75, which were stored at 40°C during 148 days, could not be read. As shown in , the samples at the same pH (2.75) and stored at 20°C were analyzed at the end of the 1st, 2nd, 3rd, 4th, and 5th months. The remaining aspartame as percent of initial amounts were determined to be 90.3, 85.3, 80.9, 74.2, and 64.5%, respectively. Concerning the samples at the same pH (2.75) and stored at the 30°C, the remaining aspartame amount decreased significantly on the 34th day. Results at the end of the 1st, 2nd, 3rd, 4th, and 5th months showed the remaining aspartame as 74, 60.60, 50.70, 38.30, and 28.84%, respectively (p < 0.01). The aspartame amount of samples stored at 40°C after 1 month was 43.30% and decreased to 25.40, 14.80, and 7.40% at the 2nd, 3rd, and 4th months, respectively. The remaining aspartame amount could not be determined as it was in a trace amount at the end of the total storage of 5 months.
Figure 1 Stability of aspartame of orange flavored soft drink of pH 2.75 during storage at different temperatures.
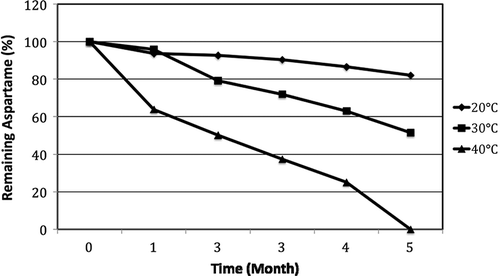
The second group of the samples was sweetened with 100% aspartame and had a pH 3.25 and was stored at 20, 30, and 40°C during 5 months. The remaining aspartame amounts were determined to be 93.70, 92.60, 90.40, 86.60, and 82.10%, respectively. The remaining aspartame samples with a pH of 2.75 and stored at 30°C, decreased significantly on the 1st and 2nd months. Analysis results at the end of the 1st, 2nd, 3rd, 4th, and 5th months showed the remaining aspartame as 95.90, 79.20, 72, 63.10, and 51.50%, respectively (p < 0.01). When the temperature was 40°C, the remaining aspartame values were 63.90, 50.10, 37.40, and 25.10% after the 1st, 2nd, 3rd, and 4th months, respectively. The remaining aspartame amount could not be determined after 5 months of storage as it was in a trace amount (p < 0.01) ().
Figure 2 Stability of aspartame of orange flavored soft drink of pH 3.25 during storage at different temperatures.
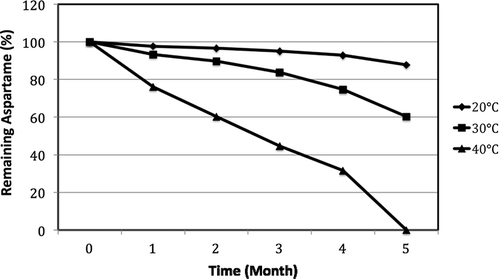
The third group of samples sweetened with 100% aspartame having a pH level of 4.57 have been stored at 20, 30, and 40°C during the 5th month (). The remaining aspartame amounts were 97.96, 96.60, 95.00, 92.80, and 87.80% after the 1st, 2nd, 3rd, 4th, and 5th months, respectively (p < 0.01). When the temperature was increased to 30°C, the remaining aspartame amounts at the end of the first, 2nd, 3rd, 4th, and 5th months were determined to be 93.30, 89.70, 83.30, 74.60, and 60.30%, respectively (p < 0.01). The analytical results were not readable on the 14th day for orange flavored samples with a pH level of 4.57, which were stored at 40°C (p < 0.01).
Figure 3 Stability of aspartame of orange flavored soft drink of pH 4.57 during storage at different temperatures.
In this study, the lowest aspartame was observed for the samples with a pH 2.75 and stored at 40°C; the highest stability was observed for the samples having a pH of 4.57 and stored at 20°C. Woo and ChangCitation[13] investigated the effects of temperature and pH on thermal stability of aspartame within the range of 60–100°C temperature and pH 3.0–7.0 and they reported that the lowest stability was observed at pH 7.0 and the highest aspartame stability at pH 4.0.
Bell and LabuzaCitation[14] evaluated the stability of aspartame in commercially sterilized skim milk beverages that contained different buffer salts, buffer concentrations, and flavor. The effects of pH and temperature on aspartame stability in these dairy beverages were also studied. The half-lives were 1 to 4 days at 30°C and 24 to 58 days at 4°C, and decreasing the pH from 6.7 to 6.4 doubled the stability of aspartame. In the present study, results indicated that increasing the pH from 2.75 to 3.25 resulted in an increase in the remaining aspartame from 25.84 to 51.50% after 5 months of storage and enhanced the stability at 99.30% in the samples stored at 30°C. If the pH increases up to 4.57, the stability of aspartame increases 17% more than pH 3.25 and 133% more than pH 2.75.
Fellows et al.Citation[15] investigated the stability of aspartame in three different fruit preparations at constant pH and stored at three different temperatures during 6 months. They reported the remaining aspartame to be around 60% at 21°C after 6 months. According to their results, estimated shelf life of aspartame was 2 months at 32.20°C and 4–6 months at 4.4°C. In this study, at 20°C after 5 months the remaining aspartame amounts were 65.4% at pH 2.75, 82.10% at pH 3.25, and 87.80% at pH 4.57. The remaining aspartame amounts at 30°C after 2 months were 60.60% at pH 2.75, 79.20% at pH 3.25, and 89.70% at pH 4.57. In another study, Fellows et al.Citation[16] researched the stability of aspartame during the fermentation of milk and reported that the remaining aspartame in yoghurts was 95% after incubation at 43°C for 6 h and 90% for samples incubated at 32.2°C for 13 h.
Rei et al.Citation[17] investigated the stability of aspartame in cakes, which were packed in polyethylene bags and stored at 25, 4, or −18°C during 2 weeks. Subsequently, the recovery of aspartame after baking and storage was determined, pH of the samples ranged from 6.7 to 7.0. Recovery was found to be 24 and 41% for powder and encapsulated aspartame, respectively.
Wetzel and BellCitation[10] studied the chemical stability of encapsulated aspartame in sugarless cakes and determined the remaining aspartame amount in various cake recipes. The stability of encapsulated aspartame in cakes varied between 22 and 58% depending on the recipe. Between the range of pH 7 and pH 5, aspartame stability was increased 8–16 times for each decreasing level of pH. In this study, the highest aspartame stability was determined for the samples having a pH of 4.57.
Hence, the results of the study on the stability of aspartame in orange flavored soft drinks, the effects of storage conditions, storage time, and pH level, were found to be significant on the stability of aspartame (p < 0.01). Increasing temperature decreased the stability of aspartame whereas increasing pH value between the ranges of pH 2.75–4.57 increased its stability. Also, the interactions between storage temperature time, pH and time, and storage temperature and pH on aspartame loss were found to be significant (p < 0.01). Increasing storage time and temperature together decreased the remaining aspartame amount. Remaining aspartame decreased quantitatively with the extended storage time and increasing pH level between 2.75 and 4.57 increased the stability of aspartame. With the increasing storage temperature and decreasing pH between 2.75–4.57 stability of aspartame decreased as well. In the orange flavored soft drinks the triangular effect of storage temperature, pH value, and storage time on aspartame stability was found to be significant (p < 0.01).
CONCLUSION
In this research, orange flavored soft drinks that were prepared to be analyzed for stability of aspartame with a pH 2.75, 3.25, and 4.57 were stored at 20, 30, and 40°C. In this study, the remaining aspartame amount decreased with increasing storage temperature. Optimum aspartame and, thus, sweetness stability in fruit flavored soft drinks can be maintained by controlling the storage conditions, temperature, and pH.
REFERENCES
- Pietrzyk , S. , Fortuna , T. , Dyrek , K. , Labanowska , M. , Bidzinska , E. and Orawska , J. 2010 . Effects of saccharose substitutes on physicochemical properties and free radical generation in oxidized potato starch . International Journal of Food Properties , 14 : 1255 – 1263 .
- Hiatt , A.N. , Taylor , L.S. and Mauer , L.J. 2010 . Effects of co-formulation of amorphous maltodextrin and deliquescent sodium ascorbate on moisture sorption and stability . International Journal of Food Properties , 14 : 726 – 740 .
- Homler , B. 1984 . Properties and stability of aspartame . Food Technology , 38 : 50 – 55 .
- Anon . 2004a . Ajinomoto Aspartame Technical Information Ajinomoto Switzerland AG , , Switzerland
- Şeftalioglu , F. 1989 . Soğutulmuş sütlü tatlılarda kullanılan aspartamın farklı depolama koşullarındaki stabilitesi , Ankara , , Turkey : Master Thesis, Hacettepe University, Institute of Natural and Applied Science .
- Dogan , M. and Boroglu , E. 2000 . “ Süt Endüstrisinde Aspartame E951, Süt Mikrobiyolojisi ve Katkı Maddeleri VI ” . In Süt ve Süt Ürünleri Sempozyumu 479 – 486 . Tekirdağ , , Turkey
- Maisons, A. French Food Safety Agency. Assessment Report. 2002 http://www.greenfacts.org/aspartame/aspartamegb.pdf (http://www.greenfacts.org/aspartame/aspartamegb.pdf)
- Anon. What is aspartame? 2004 http://www.greenfacts.org/es/aspartameo/n-3/aspartame-1.html (http://www.greenfacts.org/es/aspartameo/n-3/aspartame-1.html)
- O'Donnell , K. 2006 . “ Aspartame and neotame ” . In Sweeteners and Sugar Alternatives in Food Technology , Edited by: Mitchell , H. 86 – 100 . Oxford, UK, Blackwell Publishing Ltd .
- Wetzel , C. and Bell , L. 1997 . Chemical stability of encapsulated aspartame in cakes without added sugar , Auburn , AL, USA : Nutrition and Food Science, Auburn University .
- Demiralay , E. and Özkan , G. “ Optimization strategy for isocratic separation of α-Aspartame and its breakdown products by reversed phase liquid chromatography ” . In Department of Chemistry, Faculty of Science and Literature, Suleyman Demirel University Isparta , , Turkey 2004
- Anon . 1996 . HPLC analysis of aspartame in beverages/food products. R-S-RF /602, Ajinomoto Ag, Zug, Switzerland ,
- Woo , J.K. and Chang , N.C. 1996 . Effect of temperature and pH on thermal stability of aspartame . Korean Journal of Food Science , 28 : 311 – 315 .
- Bell , L.N. and Labuza , T.P. 1994 . Aspartame stability in commercially sterilized flavored dairy beverages . Journal of Dairy Science , 77 : 34 – 38 .
- Fellows , J.W. , Chang , S.W. and Shazer , W.H. 1991 . Stability of aspartame in fruit preparations used in yogurt . Journal of Food Science , 56 : 689 – 691 .
- Fellows , J.W. , Chang , S.W. and Keller , S.E. 2002 . Effect of sundae-style yogurt fermentation on the aspartame stability in fruit preparation . The Nutrasweet Company , 74 : 863 – 870 .
- Rei , M.H. , Su , C.Y. and Chen , Y.L. 2000 . Stability of aspartame in low-sugar chiffon cake . Taiwanese Journal of Agricultural Chemistry and Food Science , 38 : 470 – 475 .