Abstract
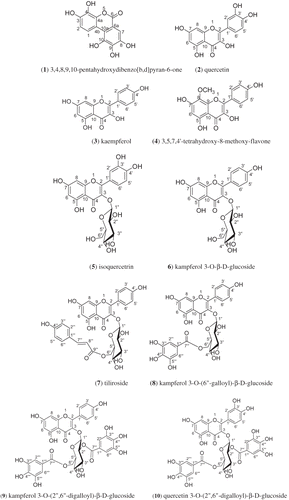
The objective of this study was to investigate the antioxidant activity, chemical constituents, and structure-activity relationship from the flowers of Rosa chinensis Jacq. The antioxidant activity of the different polar solvent extracts were assayed by a 1,1-diphenyl-2-picrylhydrazyl scavenging experiment. The extract with the strongest 1,1-diphenyl-2-picrylhydrazyl scavenging capacity was further isolated and purified by column chromatography. The structures of the isolated compounds were elucidated on the basis of spectral analysis and physiochemical properties. The antioxidant activity of these compounds was assayed by a 1,1-diphenyl-2-picrylhydrazyl scavenging experiment and ascorbic acid was chosen as the positive control. The ethyl acetate fraction had the strongest 1,1-diphenyl-2-picrylhydrazyl scavenging activity. Ten compounds were obtained, represented as: 3,4,8,9,10-pentahydroxydibenzo[b,d]pyran-6-one (1), quercetin (2), kaempferol (3), 3,5,7,4′-tetrahydroxy-8-methoxy-flavone (4), isoquercetrin (5), kampferol 3-O-β-D-glucoside (6), tiliroside (7), kampferol 3-O-(6′′-galloyl)-β-D-glucoside (8), kampferol 3-O-(2′′,6′′-digalloyl)-β-D-glucoside (9), quercetin 3-O-(2′′,6′′-digalloyl)-β-D-glucoside (10). The 1,1-diphenyl-2-picrylhydrazyl scavenging capacity for these compounds, in descending order, were as follows: 10 > 9 > 1 ≈ 8 > 5 > ascorbic acid ≈ 2 > 6 > 7 > 3 > 4. Compounds 1, 8, 9, and 10 have the stronger antioxidant activity. Our results suggested the antioxidant activities of these compounds might be influenced by the number and position of hydroxyl groups in their aromatic rings.
INTRODUCTION
In recent years, the effects of free radical-mediated oxidative damage show increasing correlation with disease etiology. Antioxidants are being increasingly relied upon as a part of modern food processing and in the prevention of chronic diseases. Because of the toxicity and side effects of some synthetic antioxidants, people are gradually turning to natural antioxidants of high performance and low toxicity.[Citation1–3 Citation Citation3 Rosa chinensis Jacq belongs to the Rosa genus of the Rosaceae family, a resource that is readily available in China due to its wide cultivation. Flos Rosae chinensis is the flower of Rosa chinensis Jacq. It is commonly used in traditional medicine and has some nutritional function and rich phytochemicals. These bioactive compounds have been shown to have a positive impact on health. The antioxidant activity of the extractive by different polarity solvent extraction has been reported,[Citation4–6 Citation Citation6 but these reports seldom touched on antioxidant activity of the chemical composition of Flos Rosae chinensis. Due to its potentially huge economic and social values, we should pay close attention to its systematic study and possible deep exploitation regarding its bioactive constituents. In this study, an activity-directed fractionation and purification process was used to isolate 1,1-diphenyl-2-picrylhydrazyl radical (DPPH·) scavenging components from Flos Rosae chinensis.
MATERIALS AND METHODS
Materials
The flower bud of the red big flower Flos Rosae chinensis comes from Nanyang, Henan, China. The Flos Rosae chinensis was identified by associate professor Chengxue Pan, Zhengzhou University, and he indicated that it was anthodium of China rose belonging to the Rosa genus of the Rosaceae family.
Chemicals and Equipments
The following chemicals were used: DPPH· (Sigma, St. Louis, MO, USA), ascorbic acid standard preparation (Sigma), normal phase silica gel filler (200–300 screen mesh, Qingdao Ocean Chemical Plant, Qingdao, China), polyamide filling-material (100–200 screen mesh, Zhejiang Luqiaosijia Biochemistry Plastics Plant, Hangzhou, China), antiphase ODS filler (grain size 50 μm, YMC, Kyoto, Japan), couple of Sephadex LH-20 (grain size 20–150 μm, GE, Fairfield, CI, USA), MCI filler (grain size 75–150 μm, Mitsubishi, Tokyo, Japan), thin-layer chromatography gel silica GF254 (Qingdao Ocean Chemical Plant), polyamide membrane (Zhejiang Luqiaosijia Biochemistry Plastics Plant), and 95% alcohol (medicine); the rest are analytically pure. The following equipment was used: DPX-400 type supraconduction nuclear magnetic resonance analyzer (TMS internal standard, Bruker, Fällanden, Switzerland) and UV1601 type ultraviolet spectrophotometer (Shimadzu, Tokyo, Japan).
Extraction and Separation
Dried and powdered Flos Rosae chinensis was extracted three times in 95% ethanol under reflux. This extract was then filtered through absorbent gauze, and the filtrate was concentrated under a vacuum to remove ethanol. The suspension was successively extracted with petroleum ether, chloroform, ethyl acetate, and n-butanol. The antioxidant activity of the extracts was assayed by a DPPH free radical scavenging experiment.[Citation7,Citation8] The extract with the strongest DPPH· scavenging capacity was further purified. Some compounds of the extract were separated and purified using column chromatography over a silica gel column, ODS RP column, Sephadex LH-20 column, polyamide column, and MCI column. Their structures were identified by spectroscopic methods. The antioxidant activity of these compounds was assayed by a DPPH free radical scavenging experiment.
Antioxidant Activity Assay
Preparation of test solutions
First, 0.2 mM DPPH· ethanol solution and pH 7.4 (25°C) Tris-HCl buffer were prepared. Each extraction of the petroleum ether, chloroform, ethyl acetate, n-butanol, and water was evaporated to near dryness under a vacuum using a rotary evaporator at 60°C. The samples were then porphyrizated and weighed. Each extraction and monomer compound was precisely weighed and dissolved in methanol and prepared series concentration. The antioxidant activity of these compounds was assayed by a DPPH free radical scavenging experiment.[Citation7,Citation8] The DPPH assay, which has widespread use in free radical-scavenging assessment, is based on a reaction between the free DPPH radical and molecules that can donate hydrogen atoms (such as most antioxidants).[Citation9] As a result, a stable non-radical form of the DPPH was obtained, with simultaneous change of the violet color to pale yellow due to the picryl group present in the solution.[Citation7] The disappearance of the DPPH radical absorption by the action of antioxidants is taken as a measure of antioxidant activity. The decrease in absorbance (A) was measured at 517 nm, and their IC50 value was calculated from a regression analysis of probability unit[Citation10] using SPSS 12.0 software (SPSS Inc., Chicago, IL, USA). The IC50 value was defined as the concentration at which the extract was required for 50% scavenging of DPPH; a smaller IC50 value corresponds to a higher antioxidant activity. Briefly, an application of samples are displayed in .
Table 1 Application of sample
Statistical Analysis
All results were based on three to four independent replicate samples for each extract. The results presented are the mean ± standard deviation. They were compared by analysis of variance (ANOVA) using SPSS 12.0 software. Pairwise multiple comparisons were done by SNK significant difference test with the family error rate held at 0.05.
RESULTS
DPPH· Scavenging Capacity of Extracts
Results for the IC50 values of each extract are presented in . The differences of the IC50 values among the groups were statistically significant (p < 0.001), and the IC50values showed that the DPPH· scavenging capacity of the extracts were as follows: ascorbic acid > ethyl acetate fraction > n-butanol fraction > water-soluble fraction ≈95% ethanol fraction > chloroform fraction > petroleum ether fraction. The ethyl acetate fraction had the strongest DPPH· scavenging activity.
Table 2 The DPPH scavenging activities of the extracts of the flowers of Rosa chinensis Jacq 
Separation and Purification of Ethyl Acetate Fraction
Ten purified compounds were obtained from the ethyl acetate fraction of Flos Rosae chinensis ().[Citation11–17 Citation Citation Citation Citation Citation Citation17 They were 3,4,8,9,10-pentahydroxydibenzo[b,d]pyran-6-one (1); quercetin (2); kaempferol (3); 3,5,7,4′′-tetrahydroxy-8-methoxy-flavone (4); isoquercetrin (5); kampferol 3-O-β-D-glucoside (6); tiliroside (7); kampferol 3-O-(6′′-galloyl)-β-D-glucoside (8); kampferol 3-O-(2′′,6′′-digalloyl)-β-D-glucoside (9); and quercetin 3-O-(2′′,6′′-digalloyl)-β-D-glucoside (10). Among them, compound 1 belonged to coumarin, and the other nine compounds belonged to flavonoids. Compounds 1, 4, 7, 8, 9, and 10 were the first to be found from Flos Rosae chinensis.
DPPH-Free Radical-Scavenging Activity
The IC50 values of these compounds obtained from ethyl acetate fraction of Flos Rosae chinensis are shown in . There were significant differences (p < 0.001) in antioxidant activities among the groups, but there was no significant difference (p > 0.05) in antioxidant activities between compound (1) and compound (8), compound (2), and ascorbic acid. The IC50 values showed that the DPPH-scavenging activity decreased in the following order: quercetin 3-O-(2′′,6′′-digalloyl)-β-D-glucoside (10) > kampferol 3-O-(2′′,6′′-digalloyl)-β-D-glucoside (9) > 3,4,8,9,10-pentahydroxydibenzo[b,d]pyran-6-one (1) ≈ kampferol 3-O-(6′′-galloyl)-β-D-glucoside (8) > isoquercetrin (5) > ascorbic acid ≈ quercetin (2) > kampferol 3-O-β-D-glucoside (6) > tiliroside (7) > kaempferol (3) > 3,5,7,4′-tetra-hydroxy-8-methoxy-flavone (4). With the exception of compounds 10, 9, 1, 8, and 5, they were better than vitamin C as DPPH free radical scavengers. Further, the IC50 values of all the five classes of compounds were lower than 0.020 mM, while the compounds 2, 3, 4, 6, and 7 had comparable or slightly higher IC50 values, indicating that these five classes of compounds 10, 9, 1, 8, and 5 were the most potent DPPH scavengers among the ten compounds.
Table 3 Free radical-scavenging activity (IC50) of the isolated ten compounds of the flowers of Rosa chinensis Jacq 
DISCUSSION
It is interesting to consider the correlation between antioxidant activity and the number and position of hydroxyl groups in their aromatic rings of these compounds, as the number and position contribute directly to antioxidant activity.[Citation2] In this study, we observed the correlation of the number of hydroxyl groups in the aromatic rings of each compound and the IC50 values, which indicated that the antioxidant activities of these compounds might be more influenced by the number of hydroxyl groups.[Citation18] The results showed that compounds 10, 9, 1, and 8 had significant DPPH· radical-scavenging capacities with IC50 values of 0.010, 0.013, 0.015, and 0.016 mM, respectively, all of which were much more active than ascorbic acid. Obviously, compound 10 with ten phenolic hydroxyl groups showed the highest antioxidant activities among these compounds, followed by compound 9 with nine phenolic hydroxyl groups and the antioxidant activity was higher; then antioxidant activities of compounds 1 and 8 with five or six phenolic hydroxyl groups were lower than compounds 10 and 9. All of the four compounds above were more active than those with three or four hydroxyls, such as compounds 5, 2, 6, 7, 3, and 4. These properties may come from their polyphenol structures, they contain one or two galloyl; therefore, they have more phenolic hydroxyl groups. Stable phenol-oxyradicals were formed in flavone and galloyl fraction and have stronger antioxidant activity. These observations were consistent with the results reported by Bian Xiaoli.[Citation19]
Among the ten compounds, four demonstrated higher DPPH· radical-scavenging activities than the positive control, ascorbic acid. In addition, this investigation of DPPH· radical-scavenging compounds, suggests that the potency of these compounds could provide a chemical basis for some of the health benefits attributed to Rosa chinensis Jacq. The results also indicated that the extracts and pure compounds from Rosa chinensis Jacq might be used as natural antioxidants and alternatives to synthetic antioxidants. Further studies are necessary to assess the chemical profile of the DPPH-active n-butanol fraction, which in this study had a DPPH· radical-scavenging capacity similar to the ethyl acetate fraction.
CONCLUSION
Flos Rosae chinensis is a good source of flavonoids. The extracts of Flos Rosae chinensis have strong antioxidant activities, especially the middle-polar fraction (ethyl acetate fraction). Ten compounds are obtained from the ethyl acetate fraction of Flos Rosae chinensis. Among them, compound 1 belongs to coumarin and the other nine compounds belong to flavonoids, and compounds 1, 4, 7, 8, 9, and 10 are the first time found from Flos Rosae chinensis. This suggests that the antioxidant activity of Flos Rosae chinensis mainly depended on its content of flavonoids. Compounds 1, 8, 9, and 10 showed the stronger antioxidant activity. The antioxidant activities of these compounds might be influenced by the number and position of hydroxyl groups in their aromatic rings.
REFERENCES
- Zhu , Y.Z. , Huang , S.H. , Tan , B.K. , Sun , J. , Whiteman , M. and Zhu , Y.C. 2004 . Antioxidants in Chinese herbal medicines: A biochemical perspective . Natural Product Reports , 21 : 478 – 489 .
- Zijia , Z. , Liping , L. , Moore , J. , Tao , W. and Zhengtao , W. 2009 . Antioxidant phenolic compounds from walnut kernels (Juglans regia L.) . Food Chemistry , 113 : 160 – 165 .
- Jie , H. , Xinchu , W. and Kaishun , B. 2008 . Antioxidants from a Chinese medicinal herb—Lithospermum erythrorhizon . Food Chemistry , 106 : 2 – 10 .
- Yingfen , H. , Bolu , H. , Jie , M. and Hu , H. 2000 . Study on the antioxidant action of Chinese rose . Science and Technology of Food Industry , 21 : 24 – 26 .
- Dexin , Z. 2008 . Research on the role of DPPH·free radical's clearance of Chinese herb . Tianjin Chemical Industry , 22 : 32 – 34 .
- Aiyun , Z. , Bolu , H. , Hu , H. and Peiyu , Y. 2000 . The primary study on antioxidative activities of some plants . Natural Product Resesrch and Development , 12 : 42 – 44 .
- Tohma , H.S. and Gulç , I. 2010 . Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza Glabra L.) . International Journal of Food Properties , 13 : 657 – 671 .
- Li , W. , Hua-neng , X. , Huiyuan , Y. and Hui , Z. 2011 . Phenolic composition and radical scavenging capacity of Vaccinium Bracteatum Thunb . leaves. International Journal of Food Properties , 14 : 721 – 725 .
- Szabo , M.R. , Radu , D. , Gavrilas , S. , Chambre , D. and Iditoiu , C. 2010 . Antioxidant and antimicrobial properties of selected spice extracts . International Journal of Food Properties , 13 : 535 – 545 .
- An , S.L. , Mo , Y.X. and Ou , C.Q. 2002 . Probit analysis with SPSS 10.0 software . Journal of First Military Medical University , 22 : 1019 – 1021 .
- Nawwar , M.A.M. and Souleman , A.M.A. 1984 . 3,4,8,9,10-Pentahydroxy-dibenzo[b,d] pyran-6-one from Tamarix nilotica . Phytochemistry , 23 : 2966 – 2967 .
- Xiang , C. , Meili , G. , Hui , S. and Yadan , C. 2010 . Study on chemical constituents of Celosia cristata seed . Journal of Jilin Agricultural University , 32 : 657 – 660 .
- Jijun , C. , Jinsu , C. , Siying , C. and Jun , Z. 1999 . The chemical constituents of Rhodiola atuntsuensis . Acta Botanica Yunnanica , 21 : 525 – 530 .
- Funayama , S. and Hikino , H. 1979 . Hypotensive principles of Diospyros Kaki leaves . Chemical & Pharmaceutical Bulletin , 27 : 2865
- Xiaofang , X. , Huijun , L. , Ping , L. , Xu , F. and Changqi , Y. 2006 . Chemical constituents in the buds of Lonicera macranthoides . Chinese Journal of Natural Medicines , 4 : 347 – 351 . 2006
- Haiyan , Z. and Pengfei , T. 2004 . Study on flavonoids from Lysimachia Clethroides . Chinese Journal of Natural Medicines , 2 : 59 – 61 .
- Yanze , L. , Yangjie , W. , Ke , Y. , Chunru , J. , Aijun , H. , Yoshida , T. and Okuda , T. 1997 . Astragalin 2″,6″-di-O-gallate from Loropetalum chinense . Phytochemistry , 46 : 389 – 391 .
- Sroka , Z. and W , Cisowski. 2003 . Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids . Food and Chemical Toxicology , 41 : 753 – 758 . 2003
- Xiaoli , B. , Qing , P. and Jun , D. 2003 . The antioxidativity of 2′-O-galloyl-3-β-galactosyloxy quercetin and the structure-activity relationships . Journal of Xian Jiao tong University (Medical Sciences) , 24 : 452 – 454 .