Abstract
Total phenolic, flavonoid, and tannin contents of 21 Ethiopian khat (Catha edulis Forsk) leaves and their related antioxidant activities were determined in the extracts of the young leaves, matured leaves, and tips of tender stem near the young shoots. A simplified, rapid, and robust method was also optimized for the analysis of total tannins using ovalbumin as a precipitating agent and Folin Denis reagent as the quantification technique. Among the solvents tested, aqueous mixtures of 70 and 80% acetone and 80% methanol provided higher phenolic compounds extraction efficiency than the corresponding pure solvents and other binary mixtures. Results of the analysis revealed that total phenols ranged from 129 to 274 mg tannic acid equivalent/g of dried young leaves and 89.3 to 175 mg tannic acid equivalent/g of dried tender stem tips. Total tannin content ranged between 70.2−153 mg tannic acid equivalent/g and 49.4−103 mg tannic acid equivalent/g of the dried young leaves and tips of tender stems, respectively. Similarly, total flavonoids concentration as catechin equivalent varied between 26 to 75 and 26 to 56 mg catechin equivalent/g of dried young leaves and tips of tender stems, respectively. Khat cultivars were found to pose a substantial antioxidative activity (as ascorbic acid equivalent) ranging between 173−290 and 118−211 mg ascorbic acid equivalent/g of dried young leaves and tips of tender stems near the young shoot, respectively. Matured leaves of khat accumulated a significantly lower concentration of secondary metabolites compared to the corresponding young leaves. This study reveals that khat leaves and tender stems accumulated a substantial amount of secondary metabolites, particularly tannins.
INTRODUCTION
Phenolic compounds are substances containing one or more aromatic rings with one or more hydroxyl groups constituting of a large group of about 8000 compounds with varied structures and chemical properties.[Citation1–5 Citation Citation Citation Citation5
Phenolic compounds have been demonstrated to exhibit numerous biological and pharmacological activities that are of interest in human and veterinary medicine, such as inhibition of lipid oxidation, mutagenicity of carcinogens, and tumor promotion. Simple phenolics, flavoinoids, and tannins have long been recognized to possess anti-inflammatory, antioxidant, antiallergic, hepatoprotective, antithrombotic, antiviral, and anticarcinogenic activities. Thus, they are collectively known as antioxidants.[Citation3,Citation6]
Apart from an extensive nutritional importance survey, numerous studies have also demonstrated the harmful effect of certain phenolic compounds like tannins on animals and humans.[Citation7] In many experiments, it has been shown clearly that the ability of tannins to form strong complexes with proteins causes negative effects on appetite and nutrient utilization, particularly of proteins in herbivores.[Citation8,Citation9] Tannic acid (hydrolysable tannin) had shown hepatic necrosis in humans and grazing animals. Ingestion of high levels of tannins can cause gastroenteritis and congestion of the intestinal wall in rats.[Citation10] Tannins can affect the utilization of vitamins and minerals and inhibition of digestive enzymes.[Citation9,Citation11–14 Citation Citation Citation14
Khat (Catha edulis Forsk) is a natural stimulant plant of an evergreen shrub of the plant family Celastraceae, which is cultivated as a bush or small tree reaching 7 m in height. Khat grows in countries bordering the Red Sea and along the east coast of Africa. The people of these countries have chewed khat for centuries.[Citation15,Citation16] Its cultivation extends from Southern Africa to the Arabian Peninsula, more specifically in Yemen, Ethiopia, Kenya, Madagascar, Somalia, Tanzania, and others as well.[Citation17,Citation18] The most favored part of the plant is the leaves, particularly the young shoots near the top of the plant. However, leaves and stems at the middle and lower sections are also used. Khat is chewed for its stimulating property. This is due to the presence of the phenylalkylamines in the plant.[Citation17] In Ethiopia, khat is grown in most parts of the country and marketed under different names. There is an ever-growing demand both for domestic consumption and for the export market.[Citation19–21 Citation Citation21
Few reports have been made on antioxidant activity of khat extract.[Citation22,Citation23] Recently, Dudai et al.[Citation23] from Yemen reported that khat has significant antioxidant activities and was found to contain up to 205 mg chlorogenic acid equivalent/g of dry khat leaves. Parallel to this, they have reported that total phenolic compounds in oven-dried leaves of 13 khat varieties were about 0.1 mg TAE/g of dry leaves, which is less then 0.01% of the total dry mass. This result is far below the optimum phenolic compounds reported in any green plants having such high antioxidant activity. Hence, the data looks contradictory.
A study on rats, through intragastric administration of khat flavonoids fractions, resulted in antioxidant effect by increasing the activities of GST, catalase, and uric acid levels. However, the authors followed tedious extraction (about a week) and isolation steps, where a significant loss might occur during a prolonged extraction period.[Citation24] Thus, an efficient extraction method for khat secondary metabolites is crucial for further studies.
The long-term health impact of khat chewing is not well established. Scattered reports from Yemen and elsewhere indicate that chronic khat consumption may be a leading cause of cancer, cellular toxicity, and other metabolic disorders.[Citation25–27 Citation Citation27 Most authors tried to link the effects with the alkaloid fractions.[Citation28,Citation29]
Among several authors, there is a claim that khat polyphenols like tannins, which are mutagenic and carcinogenic in laboratory animals,[Citation30–32 Citation Citation32 might account for the effects of khat. It has been speculated that tannins in khat cause thickening of the mucosa of the esophagus and esophagopharynx[Citation29,Citation33] and that these changes might lead to malignancy.[Citation34] Furthermore, chronic chewers often complained of symptoms suggestive of stomatitis, oesophagitis, gastritis, and constipation. Though no systematic work has been done, there seems to be an agreement among researchers as well as khat users themselves concerning the gastrointestinal problems brought on by khat chewing. These are probably due to the astringency of khat tannins ingested (direct effect) and the sympathomimetic action of khat amines (indirect effect).[Citation29,Citation33–36 Citation Citation Citation36 Thus, it is important to quantitatively investigate tannin contents in different khat cultivars for further study on this aspect and to generate supportive evidence for further clinical studies.
Apart from mentioning about the existence of khat tannins, no systematic work has been reported. Al-Hebshi et al.[Citation26] reported concentration of 18 mg of tannins per gram of leophilized total solid obtained after extraction with water. The result is not quantitative, i.e., it has to be reported in terms of dry mass or fresh weight basis of the plant. Furthermore, water was used as an extracting solvent, which is less likely to be efficient for tannins extraction from plant material. Al-Motarreb et al.[Citation17] reported tannic acid concentration of 39 different khat cultivars grown in Yemen with concentration of 3–10 g/mg. The authors did not mention about the method of analysis and whether the data are for total tannins or particular tannic acid concentration. In addition, they did not mention whether it is in dry weight basis or fresh weight basis and the unit they reported is g/mg, which is odd to understand.
A number of papers have been reported for total tannin determination in plant extracts. Currently existing analytical methods for tannin analysis have been reviewed by Herderich and Smith.[Citation37] In summary, the methods can be broadly categorized into measurements based on colorimetric methods, gravimetric methods, and chromatographic analysis.
Colorimetric methods are most frequently used, whereby tannins are first removed from the plant extract via precipitating with proteins (gelatin, BSA, collagen fiber, etc.) and polymeric adsorbents (polyvinylpolypyrrolidone [PVPP], polyethylene glycol, methyl cellulose, etc.).[Citation37–41 Citation Citation Citation Citation41 Then direct or indirect methods are used for the analysis. In the case of direct method, the tannin-protein precipitated is dissolved in alkaline media like sodium dodecyl sulfate solution and reacting with ferric chloride reagent and absorbance of the red solution is measured at 510 nm.[Citation39] In this case, after precipitation and centrifugation, the supernatant is removed and then precipitate should be washed two to three times to remove the carry over non-tannin phenolics on the surface of the precipitate and on the wall of the container. In doing so, it takes some time during centrifugation at each washing stage and, hence, it is difficult for routine analysis. Furthermore, if the tannin content is small, then some of the precipitate may be lost during washing unless serious precaution is taken.
Other direct methods of analysis reported so far involve direct measurement of absorbance at about 280 nm of the original sample and non-tannin solution after removal with protein or PVPP and a difference is taken.[Citation37,Citation42] In such a case, all phenolic compounds may not have exactly the same maximum absorbance at 280 nm. Further, protein also absorbs at that particular wavelength.[Citation37]
In addition, Bajaj and Devsharma[Citation38] reported indirect (by difference) method of tannin analysis in tea infusion after removal of tannins with gelatin solution. They treated the original tea infusion and non-tannin filtrate with Folin Denis reagent and absorbance was measured at 725 nm. The authors did not mention the ratio of gelatin to tannin for effective precipitation. Secondly, effect of solution pH and extraction time was not mentioned. However, a number of other reports have been made on wine tannins or astringency analysis using gelatin. In most of the cases, three days of extraction time has been reported.[Citation43]
Llaudy et al.[Citation43] proposed ovalbumin for astringency evaluation in red wine. They used a direct method of analysis, i.e., absorbance of the wine samples was measured at 280 nm before and after removal. The authors also claimed that the protein solution interfered with wine phenolics absorbance. Secondly, they did not propose optimum tannin to protein ratio as well as optimum pH of the buffer solution for efficient precipitation since tannin-protein precipitation is pH dependent.[Citation39] Furthermore, unlike plant tannins, most tannin in red wine exists in highly polymeric form due to the fact that during wine aging most tannins polymerize with each other and with other phenolics compounds. Thus, optimization of a specific method for each type of tannin source is of particular interest since tannin/protein interaction is specific for different tannins as well as different proteins.[Citation44]
Thus, this study proposes a reproducible method using ovalbumin as a precipitation agent that makes it possible to determine plant tannins alternatively. The study also compared the efficiency of tannin precipitation with that used in the previous report by Bajaj and Devsharma.[Citation38] Furthermore, Folin Denis reagent and ferric chloride reagents have been compared for efficient quantification of tannins in the khat plant extract.
Thus, in view of the importance of plant secondary metabolites and the large number of health-related problems reported due to khat chewing, the present study was undertaken to investigate major khat secondary metabolites (tannins, flavonoids, and total phenolics) including their antioxidant activities so as to create room for further in vivo and in vitro studies of khat secondary metabolites. The present study tried to propose plausible sample preparation and quantification method for the major khat polyphenols.
MATERIALS AND METHODS
Instrument
A T60 UV-Vis spectrophotometer (Oasis Scientific, Inc., USA) equipped with 1-cm pathlength quartz cells was used for the absorbance measurements.
Chemicals and Reagents
All of the reagents were of high purity. Gelatin, ferric chloride, anhydrous AlCl3, and Na2NO2 (Fluka, Switzerland); sulfuric acid, NaCl, ovalbumin, sodium carbonate (×10 H2O), and ethanol (Research Lab Fine Chem, Mumbai, India); tannic acid, sodium acetate, NaOH, and ascorbic acid (BDH, England); D-catechin, HCl, 1,1-diphenyl-2-picryldrazyl (DPPH), glacial acetic acid, methanol, and acetone (Sigma Aldrich); sodium tungstate (Na2WO4.2H2O) and phosphomolybdic acid (Scharlau Chememia S.A.) were used as received. Folin Denis reagent: to 75 mL of water, 10 g of sodium tungstate (Na2WO4.2H2O), 2 g of phosphomolybdic acid, and 5 mL of concentrated phosphoric acid were mixed and refluxed at 100°C for 2 h and then diluted to 100 mL. The golden yellow solution was kept in a brown bottle in a refrigerator. Fresh solution of the reagent was prepared every week. Gelatin solution: 0.25 g of gelatin was soaked in a saturated sodium chloride solution for 1 h, warmed until the gelatin dissolves, then it was diluted to 100 mL with saturated sodium chloride. Acid sodium chloride solution: 25 mL of concentrated sulfuric acid was added to 375 mL of saturated sodium chloride solution. Sodium dodecyl sulfate solution (SDS) (1% w/v): 1 g of SDS was dissolved in 100 mL of deionized water. SDS-triethanolamine (TEA) (1% SDS (w/v) and 7% (v/v) triethanolamine in distilled water) solution: 1 g SDS was dissolved in 7 mL of triethanolamine and 93 mL distilled water. Ferric chloride reagent (0.01 mol/L ferric chloride in 0.1 mol/L HCl).
Study Site Description
Khat samples were collected from different regions of the country, namely, Oromiya region, Southern Nation Nationality Peoples' Region (SNNPR), and Amhara region. These regions are best known in khat cultivation for local consumption and export market to the capital city, Addis Ababa, Ethiopia. Some of the khat cultivars are also exported to the neighboring countries. Most of the studied cultivars were collected from the farm land while a few of them were collected from khat markets of Addis Ababa. Specific areas of the sampling site with respective trade names of khat cultivars analyzed are given in .
Table 1 Sampling area, sampling province, and name of khat cultivar
For those samples collected from the particular farm land, three to five nearby farming areas were selected. From each farm land, samples were collected and ramped with banana leaf and brought to the lab. While for those samples collected from the market, six kiosks were randomly selected and a minimum quantity available in the kiosk was bought and brought to the laboratory.
Sample Preparation
Upon arrival at the laboratory, different parts of the plant (chewable parts or young leaves, tips of tender stem near to the young shoot and older leaves) were isolated from each sample. Similar cultivars were mixed and one representative bulk sample was taken for each cultivar and a total of 21 bulk samples were obtained for each plant part. Then stems were dried in the oven at 40°C while parts of the young leaves and older leaves samples were freeze-dried, while part of it was oven dried under the same conditions as the stem parts for comparison purposes. After drying, the dried plant materials were ground to a fine powder (0.5 mm) with a ceramic mortar and pestle. Ground powders were packed in polyethylene bags and stored at room temperature in the dark until use.
Chemical Analysis of Samples
Total phenolic analysis
In order to select an optimum procedure for extraction of phenolic compounds from different parts of the khat plant, various solvents were evaluated for their extraction efficiency. Pure solvents (water, acetone, methanol, and ethanol) and their binary mixtures with water were evaluated. Each analysis was made in triplicate.
Dried (finely ground) plant material (100 mg) was taken in an Erlenmeyer flask of approximately 50 mL capacity. Ten mL of extracting solvent was added and the flask was kept on magnetic stirrer and stirred continuously for the prescribed period of time. The contents of the flask was then transferred to centrifuge tubes and subjected to centrifugation for 10 min. The supernatant (10 mg/mL) was then collected and kept in the refrigerator until analysis. A portion of the centrifugate was taken and diluted with the extracting solvent ten times. The diluted samples were taken and total phenolic contents were determined by Folin Denis reagent reported by Padda and Picha with slight modification.[Citation45] Namely, 100 μL of sample was taken and the volume was adjusted to 8 mL with de-ionized water. Then 1 mL of freshly prepared Folin Denis reagent was added followed by 1 mL of 20% Na2CO3 solution. A few minutes later, absorbance was measured at 760 nm using UV-Vis spectroscopy against reagent blank. Finally, 70% acetone was selected for optimal extraction of phenolic compounds.
After an optimum solvent was selected, extraction time was evaluated following the above procedure. Finally, 2 h was selected as the optimum extraction time. The effect of drying (oven drying at 40°C) and freeze drying method was compared for young leaves of Sebeta khat cultivar. Freeze drying gave a relatively better result than oven drying.
Following the optimized procedure, 100 mg of powdered sample (young leaves and tender stems near to the young leaves) was taken and extracted with 10 mL of 70% (v/v) acetone for 2 h. After a set period of time, the extract was centrifuged and the supernatant was taken for analysis as above. Tannic acid was used as a standard solution for calibration curve and results were interpreted as tannic acid equivalent (TAE). The completeness of the extraction was checked by re-extracting the residue left in the centrifuge tube with 5 mL of the solvent. Recovery of total phenols in the second supernatant was <5% of that in the first supernatant. Therefore, the second extraction step was omitted.
In order to compare leaf maturity with phenolic compounds distribution, two khat cultivars, Sebeta and Awadai type khat, were analyzed following the same procedure as above.
Determination of total tannin
In this study total tannins were determined using a gelatin method reported by Bajaj and Devsharma[Citation38] and by the ovalbumin method after its optimization. For ovalbumin method parameters, including ratio of tannin to protein (ovalbumin), precipitation time and pH of buffer solution have been optimized using standard tannic acid solution before applying to a real sample. Furthermore, the efficiency of both Folin Denis reagent and ferric chloride reagents were evaluated using standard tannic acid solution for determination of protein precipitable phenolics and non-protein precipitable phenolics.
Tannin determination using gelatin
Total tannins in four different khat cultivars were determined using the methodology described by Bajaj and Devsharma[Citation38] with slight modification. Following the above procedure, samples were extracted with 80% methanol to get 2 mg/mL. Then 1 mL extract was taken and mixed with 0.2 g kaolin in an Erlenmeyer flask. Next, 2 mL of gelatin and 2 mL of acid sodium chloride solutions were added and the content was stirred continuously for 2 h and then was kept at room temperature for 3 days. After that, it was centrifuged at 3000 rpm for 10 min. Then, 200 μL and 100 μL of the supernatant and the original solutions were individually mixed with 7.8 mL and 7.9 mL of water, respectively, to have 8 mL of solution. Finally, absorbance of the original solution and the supernatant was measured at 760 nm after addition of 1 mL of Folin Denis reagent and 1 mL of 20% aqueous sodium carbonate solution. Total tannins were determined by the difference between total phenolics and non-protein precipitable phenolics.
Tannin determination using ovalbumin method
Acetate buffer (0.1 mol/L) at a particular pH was prepared and 1 g NaCl was added per 100 mL of buffer solution. Then, 0.25 g of ovalbumin was taken and suspended in 50 mL of acetate buffer and kept for 2 h without stirring and shaking until it dissolved completely (concentration = 5 mg/mL).
Next, 5 mg/mL of tannic acid was prepared in de-ionized water. The following ratio of tannin to protein was taken to select optimum concentration of ovalbumin for efficient removal of protein precipitable tannins: 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, and 1:10 ratio of 2 mL of each of tannic acid and protein were taken by diluting the tannic acid solution with water to the desired concentration. Then the mixture was shaken for 5 min and kept for 40 min and centrifuged. Finally, 0.5 mL of the supernatant was analyzed for non-precipitated tannins following the above procedure (Folin Denis reagent).
Between 20 min to 72 h have been reported for precipitation of tannins with different protein.[Citation43] Thus, in this work, the time for complete precipitation of tannins was evaluated. A 1:5 ratio of tannin to protein was mixed and shaken for 5 min and kept for 10, 20, 30, and 40 min and centrifuged for 10 min. Then the absorbance of the supernatant was measured as above. Results of the analysis (not shown) revealed that precipitation was completed within 10 min and no change in absorbance was observed for prolonged extraction time. Then 15 min was selected as the optimum time for all samples analyzed.
Acetate buffer was prepared at different pH levels (2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, and 6.5) as above. Then, 4 mg/mL of ovalbumin was prepared with each buffer solution. Next, 0.8 mg/mL of tannic acid was prepared in water, and 2 mL each of tannic acid and ovalbumin were mixed and kept for 15 min after shaking for 5 min. Later, it was centrifuged for 10 min and 0.5 mL of the supernatant was adjusted to 8 mL with water. An amount of 1 mL each of Folin Denis reagent and carbonate solution was added and the absorbance was read at 760 nm.
Following the optimized procedure, the efficiency of ferric chloride and Folin Denis reagents were compared. Namely, 20 mg TA in 25 mL of water (0.8 mg/mL) and 4 mg/mL ovalbumin in acetate buffer (0.1 mol/L, pH, 4.5) were prepared. Then, 2 mL of each of the reagents were mixed for 5 min and kept for 15 min, centrifuged for 10 min, and filtered off and kept for analysis. The precipitate was carefully washed two times with 2 mL of the buffer solution and centrifuged for 10 min each. Finally, the precipitate was dissolved in 3 mL of sodium dodecyl sulfate solution (SDS) (1% w/v). Then, 0.5 mL of it was taken and diluted to 1 mL with SDS solution followed by 3 mL SDS-triethanolamine solution and 1 mL of Fe3+ reagent and kept for 30 min. Finally, absorbance was taken at 510 nm. Similarly, 0.5 mL of the supernatant and 0.1 mL of the stock solution was taken and analyzed as previous using Folin Denis reagent. It should be noted that the amount of precipitate should not be large enough to minimize volume error. Thus, diluted extract is advisable to use.
After selecting optimum conditions, total tannin concentration of methanolic extract of each khat cultivar was determined using Folin Denis reagent in triplicate. Results are interpreted as tannic acid equivalent (TAE). Matured leaves of Sebeta khat were also analyzed for comparison.
Freeze drying and oven drying methods were compared for Bahir Dar type khat and the following results were obtained: freeze dried sample (139 ± 4 mg TAE/g of dried leaves) and oven dried sample (134 ± 7 mg TAE/g of dried leaves).
Total flavonoids analysis
Total flavonoid contents in different khat varieties were analyzed using colorimetric aluminium chloride methods proposed by Zhishen et al.[Citation46] and results of the analysis are expressed in mg catechin equivalent (CE)/g of dry matter.
Total flavonoids in young and matured leaves of Bahir Dar type khat was determined and found to be 70.1 ± 6.8 and 61.7 ± 4.0 mg CE/g of dry leaves, respectively.
Antioxidant activity of khat
Antioxidant activity was measured by a radical scavenging assay, using 1,1-diphenyl-2-picryldrazyl (DPPH).[Citation47] The scavenging activity of khat cultivars was measured by monitoring the reduction of DPPH in the presence of khat leaves and stem extract. A 400 μmol/L DPPH solution was prepared by adding 7.84 mg of DPPH to a 50 mL volumetric flask and diluting to volume with methanol. Then, 20 mg of ascorbic acid was dissolved in 100 mL of deionized water. The sample of freeze-dried phenolic extract was prepared (2 mg/mL) in 70% acetone as mentioned above.
An aliquot of 1 mL of ascorbic acid at different concentrations (2, 4, 6, 8, 16, and 20 μg/mL) was mixed with 4 mL of methanol and 0.8 mL of 0.4 μmol/L DPPH (dissolved in methanol). The mixture was vigorously shaken and left to stand at room temperature for 60 min in a dark room. Similarly, an aliquot of 1 mL of diluted khat extract at different concentrations (20, 40, 60, and 80 μg/mL) was mixed with 4 mL of methanol and 0.8 mL of 0.4 μmol/L DPPH. The control contained only 0.8 mL of 0.4 μmol/L DPPH solution and 5 mL of methanol instead of sample, while pure methanol was used as the blank. Absorbance was read at 517 nm by using an UV-Vis spectrophotometer. The scavenging effect was calculated using the following equation:
Calibration curve was constructed for the standard ascorbic acid as % scavenging activity versus mass of standard ascorbic acid.
RESULTS AND DISCUSSION
Effect of Variable Parameters on Extraction of Total Phenolic Compounds
Pure water, pure methanol, pure ethanol, pure acetone, and their binary mixture of various combinations with water are commonly used to extract phenolic compounds from samples.[Citation3] The ability of different solvents in extracting phenolic compounds was compared by performing Folin Denis assay method. The results were expressed as tannic acid equivalents (mg TAE/g of dry leaves). shows that all the solvents were capable of extracting phenolics but aqueous acetone (70%) was a more effective solvent than the remaining solvent combinations for extracting phenolic compounds from khat leaves samples. Acetone (70%) gave the highest total phenolic content (TPC) (mg TAE/g of dry leaves), which is 206 followed by acetone (80%) 196 and methanol (80%) 191 mg/g of dry leaves, whereas pure water and other combinations of aqueous binary solvents showed lower extraction efficiency. Except for 70 and 80% acetone and 80% methanol, the different types of solvent have a significant effect (p < 0.05) on TPC.
Table 2 Effect of different solvents on extraction of total phenolic compounds
Acetone is a more effective solvent for extraction of condensed tannins as tannins have relatively high molecular weight compounds.[Citation3,Citation48] It is strongly believed that the higher the molecular weight of the solvent, the lower the polarity, which allows other substances of about the same molecular weight to be easily extracted. This can be correlated to “like dissolve like” or “polarity versus polarity” principle as both acetone and tannins are of high molecular weight. Solubility of phenolics is affected by the polarity of solvents used, which is why it is very difficult to develop an extraction procedure suitable for extraction of all plant phenolics.[Citation3,Citation49]
Mixtures of acetone and methanol with water have revealed to be more efficient in extracting phenolic constituents than compared to mono-component solvent system. Addition of a small quantity of water to organic solvent usually creates a more polar medium, which facilitates the extraction of polyphenols as suggested by Spigno et al.[Citation50] By increasing the proportion of water to acetone, the polarity of the solvent also increases. When this is achieved, the solvent system is able to extract phenolic substances from both ends of the polarity (highest polarity substances and low polarity substances), as well as those of moderate polarity.[Citation51] Generally, 70 and 80% acetone and 80% methanol exhibited comparable extraction efficiency. Therefore, in the case where acetone is not preferable for a particular analysis like protein precipitation assay of tannins as acetone interferes with this assay,[Citation52] 80% methanol will be the best choice of interest. Thus, acetone (70%) was chosen for the determination of total phenolic compounds and total flavonoids extraction while 80% methanol was used for extraction of protein perceptible tannins.
As can be seen from , extent of extraction of TPC increases with time till 90 min and no further increment has been observed for a prolonged extraction time. Thus, 120 min was selected as an optimum extraction time for all the khat cultivars, including leaves and stems.
Table 3 Optimization of extraction time for total phenolic compounds in khat
Effect of drying on quantitative extraction of phenolic compounds was compared for young leaves of Sebeta khat cultivar. Yield of the extract was high in the freeze dried leaves (172 ± 4 mg TAE/g of dry leaves) followed by oven-dried samples at 40°C (164 ± 4 mg TAE/g of dry leaves). The slight decrease in total phenolic content in the oven-dried sample might be due to instability of some phenolic compounds at relatively higher temperatures.[Citation3]
TPC Content in Different Khat Cultivars
In this work, the TPC of 21 samples of khat cultivars grown in Ethiopia, belonging to 21 different brands, was analyzed. For each cultivar, both the young leaves and the tender stem tips near to the young shoot have been analyzed.
Results are presented in . TPC were expressed in terms of tannic acid (TA) equivalents per gram of the dry plant part used. As shown in , the total phenolic content in khat plants significantly varied among most of the analyzed khat cultivars, both in young leaves and stems. The mean concentration of phenolics ranged from 129 to 274 mg TAE/g of young leaves and 89.3 to 175 mg TAE/g of tender stem tips.
Table 4 Average concentration (X ± SD, mg TAE/g of dry matter), (n = 3) of total phenolic compounds and total tannins in different khat cultivars in dry weight basis
Khat leaves from Guragie, Bahir Dar, Sike, Hirna, Suke, Gelemso, Awadai, Mokonisa, Chengie, and Belechie showed the higher levels of total phenolics (274, 263, 242, 239, 237, 220, 219, 213, 203, 201 mg TAE/g of dry leaves, respectively), while Berdaye, Gebeli, and Gerbicho (145, 129, and 148 mg TAE/g of dry leaves, respectively) showed lower levels of total phenolic compounds in the edible portion of young leaves. However, the remaining khat leaves cultivars contained between 150 and 200 mg TAE/g of dry leaves.
The tender stem tips of khat accumulated a relatively higher concentration of phenolic compounds but for most of the cultivars it is 1.5- to 2-fold less than the corresponding phenolics in leaves. These results are in agreement with previous works reporting high leaf/stem polyphenol proportions in other plants.[Citation53] The results reveal that khat leaves are rich in phenolic content comparable to unprocessed fresh tea plants.[Citation54]
The large difference of khat cultivars in terms of total phenolics in the khat plant could be due to variation in seasonal, genetic, and agronomic factors. Furthermore, variations in age of harvested khat cultivars also have a profound effect on phenolic compounds accumulation. Even chewers prefer khat leaves from old trees rather than those collected from recently cultivated ones. This might be related not only to the alkaloids content but also with phenolic compounds in the leaves.
Erturk et al.[Citation54] reported that harvesting season has a significance influence on tea leaves' phenolic content. They noticed that those harvested in the sunny season had accumulated two-fold phenolic content than those harvested in the rainy season. The same might be true in khat leaves since khat growing regions in Ethiopia have significant variation in climatic conditions throughout the year.
Fortunately, those khat cultivars widely and commonly consumed in Ethiopia and exported to different countries accumulated more concentration of phenolic compounds. Thus, it is possible to say that phenolic concentration in the leaves could be one of the biomarkers for consumer preference.
Effect of leaf maturity was compared for phenolic distribution in two khat cultivars. Results are given in . Looking at the table; young leaves accumulated more concentration of phenolic compounds than the corresponding older leaves. This result also corroborated with other reports.[Citation55]
Table 5 Total phenolic compounds in young and matured leaves of selected khat cultivars, results are given as (X ± SD, mg TAE/g of dry leaves), (n = 3)
Compared with results reported by Dudai et al.[Citation23] about 1000 times higher concentration of TPC in Ethiopian khat have been noticed. This could be due to variation in various environmental factors listed above and some possible problems mentioned under the introduction section.
Total Tannin Analysis
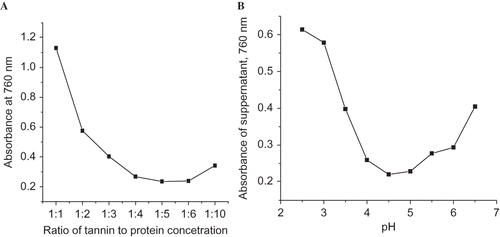
In this report, ovalbumin was evaluated for successful precipitation of tannin from khat cultivars. Before applying for real sample analysis, various parameters have been evaluated, such as ratio of tannin to protein (); pH of the buffer solution () and extraction time (not shown) have been optimized. Once tannin is removed, the non-tannin supernatant is reacted with Folin Denis reagent in the presence of carbonate solution.
Looking at , binding ability of ovalbumin increases with increasing pH of the buffer solution and reaches its maximum binding affinity at a pH value of approximately 4.5, i.e., the absorbance of the supernatant at pH = 4.5 was minimal due to the fact that most of tannic acids have been precipitated out from the solution. At this pH value, tannin to protein ratio of 1:5 for a precipitation time of 15 min gave maximum precipitation efficiency and was recorded as the optimum ratio for real sample analysis ().
After precipitation, analysis of total tannins was evaluated using Folin Denis reagent and ferric chloride reagent (). In both cases, more or less comparable protein precipitable tannic acid was recovered. Hence, the authors recommend that Folin Denis method will be the method of choice as it will be applicable for lower concentration of tannins, minimizing analysis time and analyte loss that may arise in the case of ferric chloride method, which is accompanied by multiple washing steps after filtration.
Table 6 Comparison of Folin Denis reagent and ferric chloride reagents for tannic acid analysis for the stock solution of 20 mg TA in 25 mL of solution
Following the optimized procedure, total tannin content in the khat cultivars were assayed using ovalbumin precipitation and Folin Denis method. Results are presented in . For comparative purposes, the gelatin method was used for selected khat cultivars. Results are presented in on dry weight basis as tannic acid equivalent (TAE).
Table 7 Total tannin concentration of selected khat cultivars, mg TAE/g of dry leaves (X ± SD) using gelatin method
The study has shown that young leaves of khat () possess high tannins content ranging from 70.2–153 mg TAE/g of dry matter. The high content of tannins in khat leaves correlates with its bitterness of the leaves. For instance, chewers are claiming that Suke type khat is highly astringent and less likely be used by them frequently unless shortage of khat occurs in the market. Similarly, tips of tender stems accumulated lower concentration of tannin ranging from 49.4–103 mg TAE/g of dry matter, which is evident that the stem is less bitter than the leaves.
Similar to total phenolic compounds, significant difference (p < 0.05) has been noticed for tannin content in matured leaves and young leaves. Tannins accumulation in young leaves and matured leaves in Sebeta type khat was found to be 91.0 ± 5.6 and 74.6 ± 3.5 mg TAE/g of dry matter, respectively.
Compared with the gelatin method (), less tannins concentrations were measured than with ovalbumin. This might be due to the fact that increased precipitation time and/or increased equilibrium gelatin/tannin concentration ratio resulted in soluble tannin-protein complex instead of the insoluble ones.[Citation56] Thus, ovalbumin will be the best choice for khat tannins analysis. Thus, results showed that appropriate pH, precipitation time, and gelatin/tannin concentration ratio should be optimized for plant phenolic analysis if gelatin is used as a precipitating agent.
The occurrence of high tannin content in khat cultivars may lead us to support the earlier hypothesis, i.e., association of gastrointestinal problems and cellular toxicities mentioned so far with such high concentration of tannins in the plant. Habitual users try to attenuate the gastrointestinal problem by food adaptation, notably by eating a meal with high fat content prior to the khat session in order to facilitate intestinal transit,[Citation57] and they prefer to spend the chewing session accompanied by excessive water and more preferably with milk. But still some chewers dislike incorporating milk in the chewing session while chewing because they believe that they feel less stimulation effect of khat if milk is simultaneously used.
Most khat chewers are suffering from mouth (lips) drying, which could be due to the fact that proline-rich proteins in the mouth might easily be removed via precipitation with the high tannin content in the leaves during chewing.
Results of the present study reveal that one should take care about the high concentration of khat tannins, which are responsible for precipitation of proteins, starch, digestive enzymes, and essential mineral nutrients from the diet[Citation10,Citation11] and prevent their bioavailability and digestibility. Thus, chewers should take their meal a few hours before and after chewing.
On the other hand, many health benefits of polyphenols (tannin and flavonoids) have been reported. A review by Chung et al.[Citation9] showed that in vivo and in vitro studies directly and indirectly support the preventive polyphenol effect against oral cancer and are responsible for its positive antioxidant, anti-inflammatory, and antimicrobial effects. Concentration and types of tannins may matter in the importance and/or health impact of khat tannins. Therefore, further studies with respect to individual members' identification as well as toxicological effects should be required. Generally, our finding is an input for further study to clearly understand the effect of tannins on health.
Total Flavonoids Content
In this work, total flavonoids content in 21 khat cultivars grown in different parts of Ethiopia have been investigated and results are given in on dry weight basis as catechin equivalent (CE).
Table 8 Total flavonoid contents and antioxidant activity in different khat cultivars (X ± SD mg/g, n = 3, dry weight basis of triplicate analysis)
Flavonoid constitutes one of the most important groups of phenolics in plants. In this respect, a significant correlation (r = 0.70; p < 0.05) was observed between flavonoids and total phenolics content of khat leaves. Flavonoids contents of the different khat leaves cultivars evaluated varied ranging from 26 to 75 mg CE/g of dry matter (), while in the tender stem tips, the concentration of flavonoids varied between 26–56 mg CE/g of dry stem. ANOVA results revealed that, for some of the cultivars, there is a wide variation in flavonoids contents (p < 0.05) within the leaves and stems. While in some of them, the difference is not that much pronounced (p ≥ 0.05). Presence of at least one similar letter within a column of indicates absence of significant difference for pairwise ANOVA test and otherwise the data are significantly different. As for total phenolic compounds and tannins, flavonoids in leaves are significantly higher than the corresponding flavonoids in the stem.
Apart from mentioning the antioxidant, anti-inflammatory, and antimicrobial activity of khat, to the best of our knowledge, literature is scarce on the quantitative reporting of flavonoids of khat cultivars. Thus, this report will be an input for further study on the health benefits of khat flavonoids.
Total flavonoids in young and matured leaves of Bahir Dar type khat was determined and found to be 70.1 ± 6.8 and 61.7 ± 4.0 mg CE/g of dry matter, respectively. Higher concentration of flavonoids in the young leaves is also evident from the fact that most young shoots of khat leaves are either dark green or brownish and/or reddish, which is a consequence of the presence of flavonoids,[Citation5] unlike the older leaves with most of the cases being a light green color.
Antioxidant Activity Analysis
Most reports on khat were on its health impact. However, its health benefit to the chewers has gotten little attention. As in other phenolic rich foods, Catha edulis cultivars were found to pose a substantial antioxidative activity.
Antioxidant activity of fresh leaves and tips of tender stem in 21 khat cultivars is given in . In a comparison between 21 Catha edulis varieties grown in most parts of Ethiopia, all of the samples had a substantial antioxidative activity, ranging 173–290 mg AAE/g of dry matter in the young leaves and 118–211 mg AAE/g of dry matter in stems (). Looking at the table, there is a significant variation (p < 0.05) among some of the cultivars investigated, while no significant variations have been observed (p ≥ 0.05) for the remaining cultivars for both the leaves and stems. The same letter(s) within a particular column indicate those varieties that have no significant variation in antioxidant activity among the cultivars, while lack of at least one similar letter within a given column indicates there is a significant variation in antioxidant activity.
Thus, the Catha edulis varieties featuring the top antioxidative activity might be considered as promising candidates for further evaluation of their antioxidative properties.
The results for antioxidant activity clearly outline that khat shoots could be one of the richest sources among plants in terms of antioxidant activity. The significant difference of khat shoots for antioxidant activity across varieties is supposed to be the effect of change of ecological parameters, such as climatic conditions, seasonal variation, age of harvested cultivars, as well as physiochemical nature of the soil, which are responsible factors besides cultivar variation. Traditionally, khat infusion, which was known as Abyssinia tea, was widely used for treating certain diseases.[Citation58,Citation59] To date, khat leaves infusion, particularly the matured leaves infusion, is also used among some chewers. The infusion is locally known as “Hawuza.”
The effect of leaf maturity on the antioxidative activity was also determined. Dried young leaves and matured leaves of Bahir Dar khat were found to be 281 and 209 mg AAE/g of dry matter, respectively. Young leaves had higher activity than mature leaves, which is also correlated with tannins, flavonoids, and total phenolic compounds. The high antioxidant activity of young leaves is in agreement with previous reports.[Citation23] However, for some of the cultivars, significantly higher antioxidant activity has been obtained in this study.
In general, high antioxidant activity in khat extract and increased case study reports on cancer consequence among khat chewers is an indication of the health impact of khat chewing.
Correlation Between the Antioxidant Capacity and Secondary Metabolites Content
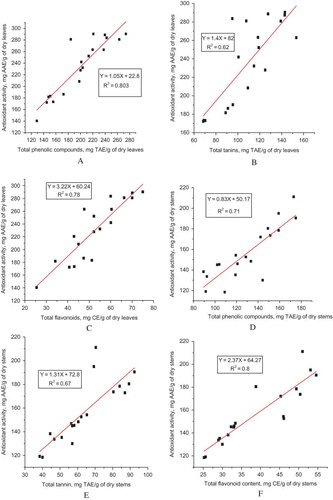
Correlation between total phenolic, tannin, and flavonoid contents in young leaves and tips of tender stems and radical scavenging activity of 21 khat extracts were analyzed. The correlation curves are depicted in – with correlation coefficient (r 2) of 0.62–0.80. In general, extracts with a high radical scavenging activity showed a high phenolic, flavonoid, and tannin content as well, but good correlations could not be found among them (Tables ). A direct correlation between radical scavenging activity and phenolic, tannin, and flavonoid content of the samples failed to demonstrate by linear regression analysis. This lack of relationship is in agreement with other literatures.[Citation23,Citation53] It is known that only phenolic compounds with a certain structure and particular hydroxyl position in the molecule can act as proton donating and show radical scavenging activity.[Citation60]
CONCLUSION
This study holds the documentation of khat total phenolic compounds, flavonoids, tannins, and the antioxidative properties of the different cultivars grown in the country as well as method of extraction procedure and quantification technique for future in vivo and in vitro studies.
The extraction of khat phenolic compounds depends largely on drying technique, type of solvent used, and extraction time. Among the methods tested to extract the total phenolic compounds of khat cultivars, the aqueous acetone and aqueous methanol extraction is recommended for being the most suitable method for this type of samples regarding the distribution of its phenolic compounds. In addition, a plausible tannin assay method was proposed using ovalbumin as a precipitating agent and Folin Denis reagent for quantification. The results indicate that the ovalbumin method gave significantly better efficiency than the gelatin method. Furthermore, Folin Denis reagent and ferric chloride-based assays yield comparable protein perceptible tannin concentration.
Ethiopian khat accumulated a substantially high concentration of phenolic compounds, tannins, and flavonoids having wide variation across the cultivars. The authors' results confirmed that the frequent complaint about gastric problems among chronic khat chewers might be due to the high concentration of khat tannins. The khat extracts analyzed in this study presented high antioxidant activity in terms of free radical scavenging, thus indicating possible benefits to human health.
ACKNOWLEDGMENTS
The authors express their gratitude to the Department of Chemistry, Addis Ababa University, Ethiopia, for providing the laboratory facilities. Minaleshewa Atlabachew is thankful to the Department of Chemistry, Bahir Dar University, Ethiopia, for sponsoring his study. Staff members of Ethiopian Health and Nutrition Research Institute (EHNRI) are gratefully acknowledged for their generous provision of important chemicals.
REFERENCES
- Vermerris , W. and Nicholson , R. 2006 . Phenolic Compound Biochemistry , 285 Springer : Dordrecht, the Netherlands .
- Sudjaroen , Y. 2009 . Plant-derived phenolic antioxidants and cancer prevention . Thai Cancer Journal , 29 : 126 – 134 .
- Dai , J. and Mumper , R.J. 2010 . Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties . Molecules , 15 : 7313 – 7352 .
- Grotewold , E. 2006 . The Science of Flavonoids , 274 Springer : New York .
- Crozier , A. , Jaganath , I.B. and Clifford , M.N. 2006 . “ Phenols, polyphenols and tannins ” . In Plant Secondary Metabolites Occurrence, Structure and Role in the Human Diet , Edited by: CroZier , A. , Clifford , M.N. and Ashihara , H. 1 – 21 . Victoria , , Australia : Blackwell .
- Khanbabaee , K. and Ree , T.V. 2001 . Tannins: Classification and definition . Natural Product Reports , 18 : 641 – 649 .
- Lua , Y. and Bennick , A. 1998 . Interaction of tannin with human salivary proline-rich proteins . Archives of Oral Biology , 43 : 717 – 728 .
- Silanikove , S. , Perevolotsky , A. and Provenza , F.D. 2001 . Use of tannin-binding chemicals to assay for tannin and their negative postingestive effects in ruminants . Animal Feed Science and Technology , 91 : 69 – 81 .
- Chung , K.T. , Wong , T.Y. , Wei , C.I. , Huang , Y.W. and Lin , Y. 1998 . Tannins and human health: A review . Critical Reviews in Food Science and Nutrition , 38 : 421 – 464 .
- Chung , K.T. , Wei , C.I. and Johnson , M.G. 1998 . Are tannins a double-edged sword in biology and health? . Trends in Food Science and Toxicology , 9 : 168 – 175 .
- Mitjavila , S. , Lacombe , C. , Carrera , G. and Derache , R. 1977 . Tannic acid and oxidized tannic acid on the functional state of rat intestinal epithelium . Journal of Nutrition , 107 : 2113 – 2121 .
- Ahmed , A.E. , Smithard , R. and Ellis , M. 1991 . Activities of the enzymes of the pancreas, and the lumen and mucosa of the small intestine in growing broiler cockerels fed on tannin-containing diet . British Journal of Nutrition , 65 : 189 – 197 .
- Santos-Buelga , C. and Scalbert , A. 2002 . Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health . Journal of the Science of Food and Agriculture , 80 : 1094 – 1117 .
- Frutos , P. , Hervás , G. , Giráldez , F.J. and Mantecón , A.R. 2004 . Review . Tannins and ruminant nutrition. Spanish Journal of Agricultural Research , 2 : 191 – 202 .
- Abdulsalam , A. , Jian-Kai , Z. and Xue-Feng , Y. 2002 . Solid phase microextraction of flavor analysis in Harari khat . Journal of Zhejiang University Science , 5 : 428 – 431 .
- Al-hebshi , N. , Al-haroni , M. and Skaug , N. 2006 . In vitro antimicrobial and resistance-modifying activities of aqueous crude khat extracts against oral microorganisms . Archives of Oral Biology , 51 : 183 – 188 .
- Al-Motarreb , A. , Baker , K. and Broadley , K.J. 2002 . Khat: Pharmacological and medical aspects and its social use in Yemen . Phytotherapy Research , 16 : 403 – 413 .
- Atlabachew , M. , Chandravanshi , B.S. and Redi , M. 2011 . Profile of major, minor and toxic metals in soil and khat (Catha edulis Forsk) cultivars in Ethiopia . Trends in Applied Science Research , 6 : 640 – 655 .
- Atlabachew , M. , Chandravanshi , B.S. , Zewge , F. and Redi , M. 2011 . Fluoride content of Ethiopian khat . Toxicological and Environmental Chemistry , 93 : 32 – 43 .
- Karlsson , S. 2006 . Reducing farming household vulnerability in connection to khat cultivation , 67 Oppsala , , Sweden : M.Sc. Thesis, Swedish University of Agricultural Sciences, Department of Urban and Rural Development Environmental Communication .
- Belew , M. , Kebede , D. , Kassaye , M. and Enquoselassie , F. 2000 . The magnitude of khat use and its association with health, nutrition and socio-economic status . Ethiopian Medical Journal , 38 : 11 – 26 .
- Al-Zubairi , A. , Ismail , P. , Pei , C.P. , Wahab , S.I.A. and Rahmat , A. 2008 . Biochemical effect of sub-chronic administration of Catha edulis (khat) crude extract in rat. Research Journal of Pharmacology . 2007 , 1 : 84 – 90 .
- Dudai , N. , Fischer , R. , Segev , D. , Chaimovitish , D. , Rosenzweigh , N. and Shimoni , M. 2008 . Antioxidant activity of khat (Catha edulis Forsk) . Acta Horticulturae , 778 : 85 – 92 .
- Al-Qirim , T.M. , Shahwan , M. , Zaidi , K.R. , Uddin , Q. and Banu , N. 2002 . Effect of khat, its constituents and restraint stress on free radical metabolism of rats . Journal of Ethnopharmacology , 83 : 245 – 250 .
- Kassie , F. , Darroudi , F. , Kundi , M. , Schulte-Hermann , R. and Knasmuller , S. 2001 . Khat (Catha edulis) consumption causes genotoxic effects . International Journal of Cancer , 92 : 329 – 332 .
- Al-Hebshi , N.N. , Nielsen , O. and Skaug , N. 2005 . In vitro effects of crude khat extracts on the growth, colonization, and glucosyltransferases of Streptococcus mutans . Acta Odontologica Scandinavica , 63 : 136 – 142 .
- Al-Ahdal , M.N. , McGarry , T.J. and Hannan , M.A. 1988 . Cytotoxicity of khat (Catha edulis) extract on cultured mammalian cells: Effects on macromolecule biosynthesis . Mutation Research , 204 : 317 – 322 .
- Feyissa , A.M. and Kelly , J.P. 2008 . A review of the neuropharmacological properties of khat . Progress in Neuro-Psychopharmacology & Biological Psychiatry , 32 : 1147 – 1166 .
- Karunamoorthi , K. , Udeani , G. , Babu , S.M. and Mossie , A. 2010 . Psychopharmacosocial aspects of Catha edulis Forsk (Fam. Celastraceae) . African Journal of Pharmaceutical Sciences and Pharmacy , 1 : 112 – 129 .
- Korpassy , B. and Mosony , M. 1950 . The carcinogenic activity of tannic acid. Liver tumors induced in rats by prolonged subcutaneous administration of tannic acid solution . British Journal of Cancer , 4 : 411 – 420 .
- Kirby , K.S. 1960 . Induction of tumours by tannin extracts . British Journal of Cancer , 14 : 147 – 150 .
- Bichel , J. and Batch , A. 1968 . Investigations on the toxicity of small chronic doses of tannic acid with special reference to possible carcinogenicity . Acta Pharmacologica et Toxicologica , 26 : 41 – 45 .
- Kennedy , J.G. , Teague , J. , Rokaw , W. and Cooney , E. 1983 . A medical evaluation of the use of qat in North Yemen . Social Science and Medicine , 17 : 783 – 793 .
- Craddock , V.M. 1993 . Cancer of the Oesophagus: Approaches to the Etiology , 284 Cambridge : Cambridge University Press .
- Halbach , H. 1972 . Medical aspect of chewing khat . Bulletin of the World Health Organization , 47 : 21 – 29 .
- Hassan , N.A.G.M. , Gunaid , A.A. , El Khally , F.M.Y. and Murray-Lyon , I.M. 2002 . The subjective effects of chewing qat leaves in human volunteers . Annals of Saudi Medicine , 22 : 34 – 37 .
- Herderich , M.J. and Smith , P.A. 2005 . Analysis of grape and wine tannins: Methods, applications and challenges . Australian Journal of Grape and Wine Research , 11 : 205 – 214 .
- Bajaj , L.L. and Devsharma , A.K. 1977 . A colorometric method for the determination of tannins in tea . Mikrochimica Acta [Wein] , 2 : 149 – 253 .
- Hagerman , A.E. and Butler , L.G. 1978 . Protein precipitation method for the quantitative determination of tannins . Journal of Agricultural and Food Chemistry , 26 : 809 – 812 .
- Liao , X. and Shi , B. 2995 . Selective removal of tannins from medicinal plant extracts using a collagen fiber adsorbent . Journal of the Science of Food and Agriculture , 85 : 1285 – 1291 .
- Sarneckis , C.J. , Dambergs , R.G. , Jones , P. , Mercurio , M. , Herderich , M.J. and Smith , P.A. 2006 . Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis . Australian Journal of Grape and Wine Research , 12 : 39 – 49 .
- Antoine , L.M. , Simon , C. and Pizzi , A. 2004 . UV spectrophotometric method for polyphenolic tannin analysis . Journal of Applied Polymer Science , 91 : 2729 – 2732 .
- Llaudy , M.C. , Canals , R. , Canals , J. , Rozeas , N. , Arola , L. and Zamora , F. 2004 . New method for evaluating astringency in red wine . Journal of Agriculture and Food Chemistry , 52 : 742 – 746 .
- Asquith , T.N. and Butler , L.G. 1986 . Interactions of condensed tannins with selected proteins . Phytochemistry , 25 : 1591 – 1593 .
- Padda , M.S. and Picha , D.H. 2007 . Methodology optimization for quantification of total phenolics and individual phenolic acids in sweetpotato (Ipomoea batatas L.) roots . Journal of Food Science , 72 : C412 – C416 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoids contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 . 1999
- Molyneux , P. 2004 . The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for the antioxidant activity . Songklanakarin Journal of Science and Technology , 26 : 211 – 219 .
- Alasalvar , C. , Karamac , M. , Amarowicz , R. and Shahidi , F. 2006 . Antioxidant and antiradical activities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnut green leafy cover . Journal of Agriculture and Food Chemistry , 54 : 4826 – 4832 .
- Naczk , M. and Shahidi , F. 2004 . Extraction and analysis of phenolics in food . Journal of Chromatography A , 1054 : 95 – 111 .
- Spigno , G. , Tramelli , L. and De Faveri , D.M. 2007 . Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics . Journal of Food Engineering , 81 : 200 – 208 .
- Zhang , Z-S. , Li , D. , Wang , L.J. , Ozkan , N. , Chen , X.D. , Mao , Z-H. and Yang , H-Z. 2007 . Optimization of ethanol-water extraction of lignans from flaxseed . Journal of Separation and Purification Technology , 57 : 17 – 24 .
- FAO/IAEA . “ Quantification of tannins in tree foliage ” . In FAO/IAEA working document , 26 Vienna , , Austria; 2000 : IAEA .
- Lizcano , L.J. , Bakkali , F. , Ruiz-Larrea , B. and Ruiz-Sanz , J. 2010 . Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use . Food Chemistry , 119 : 1566 – 1570 .
- Erturk , Y. , Ercisli , S. , Sengul , M. , Eser , Z. , Haznedar , A. and Turan , M. 2010 . Seasonal variation of total phenolic, antioxidant activity and minerals in fresh tea shoots (Camellia Sinensis) . Pakistan Journal of Pharmaceutical Science , 23 : 69 – 74 .
- Chutichudet , B. , Chutichudet , P. and Kaewsit , S. 2010 . Influence of developmental stage on activities of polyphenol oxidase, internal characteristics and colour of lettuce cv . grand rapids. American Journal of Food Technology , 6 : 215 – 225 .
- Hagerman , A.E. and Robbins , C.T. 1987 . Implications of soluble tannin-protein complexes for tannin analysis and plant defense mechanisms . Journal of Chemical Ecology , 13 : 1243 – 1259 .
- Hassan , N.A.G.M. , Gunaid , A.A. and Murray-Lyon , I.M. 2007 . Khat (Catha edulis): Health aspects of khat chewing . Eastern Medeteranian Health Journal , 13 : 706 – 718 .
- Getahun , A. and Krikorian , A.D. 1973 . Chat: Coffee's rival from Harar, Ethiopia. I . Botany, cultivation and use. Economic Botany , 27 : 353 – 377 .
- Paris , M.R. and Moyse , H. 1958 . Abyssinian tea (Catha edulis Forsk., Celastraceae) . Bulletin on Narcotics , 10 : 29 – 34 .
- Rice-Evans , C.A. , Miller , N.J. and Paganga , G. 1996 . Structure–antioxidant activity relationships of flavonoids and phenolic acids . Free Radical Biology and Medicine , 20 : 933 – 956 .