Abstract
The composition of blackberry, raspberry, blueberry, redcurrant, and blackcurrant seed oil from the region of Asturias (Spain) was studied. The oil content of blueberry was 13.27 ± 0.11 g/100 g seed, blackberry 15.68 ± 0.59 g/100 g seed, raspberry 10.55 ± 0.82 g/100 g seed, redcurrant 9.11 ± 0.59 g/100 g seed, and blackcurrant 26.15 ± 0.76 g/100 g seed. Polyunsaturated fatty acid contents was also studied, the most notable findings being 67.96 ± 1.96 g/100 g oil of linoleic acid in blackberry, 14.91 ± 0.11 g/100 g oil of γ-linolenic acid in blackcurrant, and 4.48 ± 0.6 g/100 g oil stearidonic acid in redcurrant g/100 g oil. Sterol content was also studied, the most notable findings being 87.58 ± 1.11 g/100 g total sterols and 10.01 ± 0.08 g/100 g total sterols in ß-sitosterol and campesterol, respectively, in redcurrant. The most notable finding as regards tocopherols content was 109.17 ± 4.96 mg/100 g oil in raspberry, of which γ-tocopherol constitutes 58.19 ± 0.61 mg/100 g oil. As to tocotrienol, blackcurrant stood out with a content of 6.43 ± 0.36 mg/100 g oil. These seeds have a high nutritionalvalue added.
INTRODUCTION
Berries and currants are mainly used to produce juices, jams, and liqueurs as well as for fresh consumption. They are an exceptional source of vitamin C and have medicinal properties.[Citation1] Worldwide production of these berries has grown significantly in recent years. The production of blueberries was 123,000 tonnes in the USA, the major producer worldwide.[Citation2] Poland is the leading producer in the European Union (EU) at 15,000 tonnes, while Spain produces around 1200 tonnes.[Citation3] Russia is the main producer worldwide of raspberries at 175,000 tonnes, while Poland is the leading producer in the EU at 38,000 tonnes. Production in Spain is low. Russia is the main producer worldwide of currants at 402,000 tonnes, while Poland is the main EU producer at 108,000 tonnes. Mexico is the leading producer of blackberries at 46,000 tonnes, although some states in the USA, such as Oregon, produce considerable amounts.[Citation2] Plantations of these berries have been grown and developed in recent years in Asturias, Spain. Annual blueberry production was 60 tonnes, raspberry 20 tonnes, currants 10 tonnes, and blackberry 5 tonnes. All are for fresh consumption (export) or for making jams or juices.
The content and composition of the seed oil of these berries varies depending on climate and geographical situation.[Citation2,Citation4] These seeds contain substantial amounts of polyunsaturated fatty acid rich oils,[Citation5] tocopherols, tocotrienols,[Citation6,Citation7] and sterols[Citation8,Citation9] compared with other seeds.[Citation10,Citation11] The oils of these berry seeds have been shown to help prevent certain types of illnesses, such as atopic dermatitis and gastric ulcers,[Citation12] and have an influence on the lipid content in plasma[Citation13] and in the immune response.[Citation14]
The aim of this research work consisted in a study of the oils in selected seeds of commercial blackberry, raspberry, blueberry, redcurrant, and blackcurrant grown from the region of Asturias, Spain. The composition in fatty acids, sterols, tocopherols, and tocotrienols was characterised, as well as the total content in oil and moisture. The composition of this oil is rich in bioactive nutraceutical compounds, mainly for the high content in linoleic, linolenic fatty acids and for the remarkable high value for stearidonic fatty acid (SDA), which is an effective fatty acid for increasing tissue eicosapentanoic acid (20:5n-3) and docosahexanoic acid (20:5n-3). SDA-rich oils have a useful dietary resource for increasing tissue concentration of long chain n-3 fatty acids in humans and is a dietary alternative to fish and encapsulated fish oil supplements. Thus, those compounds may be used to assess the potential use of the oils from these seeds in foodstuffs products and the oil can also be incorporated in cosmetic preparations for the content in linoleic and linolenic acids.
MATERIALS AND METHODS
Materials
Seeds of the different berries and currants used in the present study were obtained from three plantations in the region of Asturias, Spain: El Malaín-Villaviciosa for blackberry (variety Tenac) and blackcurrant (variety Junifer); El Ayerán-Aller for raspberry (variety Meeker) and blueberry (variety Ivanhoe); and redcurrant (variety Smoothstem) in Mestre-Piloña. The seeds were collected during a period of suitable ripening and temporarily kept for 24 h in a refrigerated chamber. Once prepared at room temperature, the juice was extracted from representative samples of each berry employing a stainless steel hydraulic press equipped with 10 plates with a diameter of 12 cm, using a pressure of less than 50 kg cm−2. The solid matter was dried at room temperature and the skin and pulp were separated from the seeds using different sized sieves.
Solvent Oil Extraction and Water Content
Seeds were ground in a Fritsch Pulverisette (VWR, Spain) equipped with a stainless steel rotor and 1-mm sieve ring. Oil was extracted in a Soxhlet glass apparatus using hexane as a solvent.[Citation15] The extracted oil was separated and filtered at 40°C through filter paper and stored in darkness at 4°C until analysis. Moisture was determined by weight loss after heating in an oven at 105°C in accordance with the International Union of Pure and Applied Chemistry (IUPAC).[Citation16]
Fatty Acid Analysis
Fatty acid methyl esters (FAME) were analyzed by gas chromatography. FAME were extracted with n-heptane after cold methylation with 2N KOH in methanol.[Citation17] FAME analysis was performed on an HP-5890-II apparatus (Hewlett-Packard, Palo Alto, CA, USA) using a fused silica capillary SP-2380 column (60 m × 0.25 mm, 0.2 μm film thickness). The oven temperature was kept at 160 °C for 13 min and was then raised to 190 °C at a rate of 1.5 °C/min and held isothermally for 20 min. The injector temperature was kept at 225 °C, while the detector temperature was 250 °C. Hydrogen (19 psi inlet pressure) was used as a carrier gas, while the make-up gas was nitrogen. Standards of each fatty acid were used to identify the fatty acids. These were purchased from Sigma-Aldrich (St. Louis, MO, USA): palmitic (P = C16:0), palmitoleic (Po = C16:1), stearic (S = C18:0), oleic (O = C18:1 ω9 cis), linoleic (L = C18:2), linolenic (Lo = C18:3), alpha, gamma linolenic, and stearidonic (C18:4). Fatty acids were identified in the samples by comparing retention times for standards and samples. The area was expressed as percentages of areas of the total fatty acids.
Sterol Analysis
The unsaponifiable fraction was extracted as described.[Citation18] A 0.5 mL 5-α-cholestanol (Fluka, Buchs, Switzerland) solution in chloroform was added to 5 g of oil as an internal standard. The mixture was saponified for 0.5 h with 50 mL of 2 N ethanolic potassium hydroxide. The solution was then passed to a 500-mL decanting funnel, 100 mL of distilled water was added, and the mixture was extracted twice with three 80-mL portions of diethyl ether. The diethyl ether extracts were combined in another funnel and were washed several times with 100-mL portions of water, until the wash reached neutral pH. The diethyl ether solution was dried over anhydrous sodium sulphate and evaporated to dryness in a rotary evaporator at 30 °C under reduced pressure. After purification by thin-layer chromatography,[Citation19] the sterols fraction was analyzed and quantified by gas chromatography in an HP 5890-II apparatus equipped with a split-splitless injector and a flame ionization detector. An HP-5 fused silica capillary column (30 m × 0.32 mm i.d., 0.25 μm film thickness) was used, with hydrogen (7 psi inlet pressure) being the carrier gas and nitrogen the make-up gas. The oven temperature was maintained isothermally at 265 °C for 30 min. The injector temperature was 280 °C, while the detector was kept at 300 °C. Previously, the sterols fraction was derivatized as trimethylsilyl ethers (TMS) according to the method proposed by the European Communities.[Citation18] Sterols were identified by comparison of the mass spectral data with those of authentic reference compounds and by comparing their retention times with sterols from olive, sunflower, and soybean oils.[Citation19]
Tocopherol Analysis
Tocopherols were quantified by high performance liquid chromatography (HPLC). The HPLC system consisted of a low press quaternary pump HP-1050, a Rheodyne injection valve (20 μL loop), a thermostatic furnace, and a fluorescence detector RF-235 (Shimadzu, Kyoto, Japan). Separation was performed in a 250 × 4 mm particle size 5 μm Lichrospher Si-60 (Merck, Darmstadt, Germany) column. The column and detector were kept at a temperature of 40 °C. The mobile phase was n-hexane/2-propanol 99/1 (v/v). The flow rate was supported at 1 mL/min isocratic elution. Quantification was carried out by a calibration system based on standards.[Citation20]
RESULTS AND DISCUSSION
shows the content in oil, moisture, and seed yield in blackberry, raspberry, blueberry, blackcurrant, and redcurrant. The highest content in oil corresponded to blackcurrant at 26.15 ± 0.76 g/100 g seed, followed by blackberry and blueberry at 15.68 ± 0.59 g/100 g seed and 13.27 ± 0.11 g/100 g seed, respectively. The values found for blackcurrant are similar to those obtained by Barowska et al.[Citation9] in a study characterising the oil in Canadian blackcurrant seeds. The moisture contents ranged between 9.55 ± 0.10 g/100 g seed in blackberry and 16.66 ± 0.21 g/100 g seed in redcurrant.
Table 1 Overall composition of blackberry, raspberry, blueberry, blackcurrant, and redcurrant seeds, oil, and moisture
The fatty acid composition of seeds in blackberry, raspberry, blueberry, blackcurrant, and redcurrant is shown in . All the berry seeds had high amounts of polyunsaturated fatty acids, mainly linoleic acid and linolenic acid. Blackberry seed oil has the highest amount of linoleic acid at 67.96 ± 1.96 g/100 g oil, followed by raspberry at 54.27 ± 1.54 g/100 g oil. The lowest value corresponded to blueberry seed oil at 35.84 ± 1.13 g/100 g oil. These values were similar to those reported by Bushman et al.[Citation7] in a study on the antioxidant effect of berry seed oils. C18:3n3 is also found in all the berries at high percentages ranging from 23.34 ± 0.22 g/100 g oil in redcurrant to 6.08 ± 0.08% in blueberry. 18:3n6 acid is present in blackcurrant at 14.89 ± 0.08 g/100 g oil and at 9.16 ± 0.06 g/100 g oil in redcurrant. This fatty acid was detected at low levels in other seeds. Helbig et al.,[Citation21] in a study of berry seeds press residues, reported data for C18:3n6 of 12.55 g/100 g oil for blackcurrant seed oil. This value was slightly lower than that found in the present study at 14.89 ± 0.16 g/100 g oil. Worth highlighting is the presence of stearidonic acid (C18:4) in redcurrant seed oil at 4.48 ± 0.06 g/100 g oil and in blackcurrant seed oil at 3.89 ± 0.04 g/100 g oil. These values were somewhat higher than those found by Helbig et al.[Citation21] in blackcurrant seeds at 2.94 g/100 g oil and by Bakowska et al.[Citation9] at 3.5 g/100 g oil; similar results were found for those reported by Ruiz del Castillo et al.[Citation5,Citation22] in a study of genotypic variation in fatty acid of blackcurrant seeds (3.3–3.9 g/100 g oil). On the other hand, oleic acid is present in all the seeds at percentages ranging from 18.00 ± 0.22 g/100 g oil in blueberry to 7.50 ± 0.12 g/100 g oil in blackberry. Palmitic acid registered values ranging from 6.88 ± 0.06 g/100 g oil in redcurrant to 2.73 ± 0.06 g/100 g oil in raspberry. Worth noting among the remaining fatty acids is stearic acid with low amounts ranging from 2.10 ± 0.09 g/100 g oil in blackberry to 0.87 ± 0.02 g/100 g oil in raspberry. These values are within the same range as those found by Parry et al.[Citation23] in berry seed oils of this study.
shows the content in sterols in blackberry, raspberry, blueberry, blackcurrant, and redcurrant seed oils. β-Sitosterol was the majority sterol in all the seeds as percentages of total sterols. Redcurrant seed oil was the one with the highest content at 87.58 ± 1.11%, while blackberry seed oil was the one that presents the lowest values at 77.77 ± 1.12%. Likewise, redcurrant seeds had the highest content in campesterol at 10.01 ± 0.08% and raspberry the lowest at 4.51 ± 0.05%. The stigmasterol content in blackberry was worth noting at 4.87 ± 0.05%, while redcurrant presents a content of only 0.24 ± 0.01% (
Figure 1 Representative chromatogram of the sterol fraction of raspberry seed oil. Peak identification: (1) cholesterol; (2) 2,4-methilencholestenol; (3) campesterol; (4) Δ5,23-stigmastadienol; (5) stigmasterol; (6) Δ7-campesterol; (7) clerosterol; (8) β-sitosterol; (9) sitostanol; (10) Δ5-avenasterol; (11) Δ7-stigmastadienol; (12) Δ7-stigmasterol. I.S.: Internal standard, 5α-colestanol.
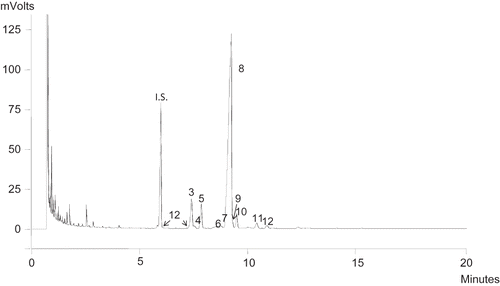
Table 2 Fatty acid composition (g/100 g oil) of blackberry, raspberry, blueberry, blackcurrant, and redcurrant seed oil samples
Table 3 Total sterol composition (%) of lipid fractions extracted from blackberry, raspberry, blueberry, blackcurrant, and redcurrant seed oil samples
Table 4 Tocopherol and tocotrienol contents (mg/100 g oil) of lipid fractions extracted from blackberry, raspberry, blueberry, blackcurrant, and redcurrant seed oil samples
shows the content in tocopherols and tocotrienols in the studied seed oils. Total tocopherols ranged from 109.17 mg/100 g oil in raspberry to 1.24 mg/100 g oil in blueberry seed oil. γ-Tocopherol was the most important tocopherols in all the berries ranging from 58.19 ± 0.61 mg/100 g oil in blackberry to raspberry, 0.96 ± 0.09 mg/100 g oil in blueberry. Van Hoed et al.[Citation1] reported data for total tocopherol in blackberry, raspberry, and blackcurrant higher (138.87 ± 14.8 mg/100 g oil; 211.25 ± 108.9 mg/100 g oil; and 37.52 ± 12.4 mg/100 g oil) than those presented in this study (109 ± 0.29 mg/100 g oil; 1.24 ± 0.01 mg/100 g oil; and 57.5 ± 0.12 mg/100 g oil) but somewhat higher than those reported by Helbig et al.[Citation22] in a variety of blackcurrant II (35.5 mg/100 g oil) but not in the variety of blackcurrant I (106.6 mg/100 g oil). As regards the content in tocotrienols, γ-tocotrienol was the greatest for blueberry seed oil presenting the highest content at 6.16 mg/100 g oil and β-tocotrienol the highest value for raspberry with 2.71 mg/100 g oil.
CONCLUSIONS
On the basis of the present study, it may be concluded that the nutritional properties of the studied berry seed oils possess a high added value. The composition in lipids of these seeds presents a high content in the polyunsaturated fatty acids C18:3n3 in some seeds, but C18:3n6, C18:4, sterols (β-sitosterol), and tocopherols (γ-tocopherol) is high in others. Extraction of these plant oils is thus proposed as an option to obtain high added value oils. The growth in production of these berries and currants in Asturias, Spain opens up an additional channel for their use.
REFERENCES
- Van Hoed , V. , Clercq , N. , Wchim , C. , Andejelkovic , M. , Leber , E. , Dewettinck , K. and Verhe , R. 2009 . A source of specialty oils high content of bioactives and nutricional value . Journal of Food Lipids , 16 ( 1 ) : 33 – 49 .
- FAOSTAT. Principales productores de Arándanos. 2005
- Commission , European . 2006 . Review of the sector of soft fruits and cherries intended for processing in the EU . Report on the situation of the sector of soft fruits and cherries intended for processing. Report No. SEC838. , : 1 – 17 .
- Jonhansson , A. , Laine , T. , Linna , M. and Kallio , H. 2000 . Variability in oil content and fatty acid composition in wild northern currants . European Food Research and Technology , 21 ( 4 ) : 277 – 283 .
- Ruiz del Castillo , M.D. , Dobson , G. , Brennan , R. and Gordon , S. 2002 . Genotypic variation in fatty acid content of blackcurrant seeds . Journal of Agricultural and Food Chemistry , 50 ( 2 ) : 332 – 335 .
- Kallio , H. , Yang , B. and Peippo , P. 2002 . Effects of different origings and harvesting time on vitamin C, tocopherols, and tocotrienols in sea buckhorn (Hippohae rannoides) berries . Journal of Agricultural and Food Chemistry , 50 ( 10 ) : 6136 – 6142 .
- Bushman , B.S. , Phillips , B. , Isbell , T. , Ou , B. , Crane , J.M. and Knapp , S.J. 2004 . Chemical composition of caneberry (Rubus spp.) seeds and oils and their antioxidant potential . Journal of Agricultural and Food Chemistry , 52 ( 26 ) : 7982 – 7987 .
- Yang , B. , Karlsson , R.M. , Oksman , P.H. and Kallio , H.P. 2001 . Phytosterols in sea buckthorn (Hippophae rhamnoides) berries: Identification and effects of different origins and harvesting times . Journal of Agricultural and Food Chemistry , 49 ( 11 ) : 5620 – 5629 .
- Barowska , A.M. , Schieber , A. and Kolodziejczyk , P. 2009 . Characterization of Canadian black currant (Ribes nigrum L.) seed oils and residues . Journal of Agricultural and Food Chemistry , 57 ( 24 ) : 11528 – 11536 .
- Rui , Y. , Wenya , W. , Rashid , F. and Qing , L. 2009 . Fatty acids composition of apple and pear seed oils . International Journal of Food Properties , 12 ( 4 ) : 774 – 779 .
- Vineet , K. , Anita , R. , Billore , S.D. and Chauhan , G.S. 2006 . Physico-chemical properties of immature pods of Japanese soybean cultivars . International Journal of Food Properties , 9 ( 1 ) : 51 – 59 .
- Xing , J. , Yang , B. , Dong , Y. , Wang , J. and Kallio , H.P. 2002 . Effect of sea buckthorn (Hippophae rhamnoides L.) seed and pulp oils on experimental models of gastric ulcer in rats . Fitotherapia , 73 ( 7–8 ) : 644 – 650 .
- Tahvonen , R.L. , Hietanen , A. , Sankelo , T. , Korteniemi , V. , Laaskso , P. and Kallo , H. 1998 . Blackcurrant seeds as a nutrient source in breakfast cereals produced by extrusion cooking . Zeitschrift für Lebensmittel-Untersuchung und Forschung , 206 ( 5 ) : 360 – 363 .
- Wu , X. , Meydani , M. , Leka , L.S. , Nightingale , Z. , Handelman , G. , Blumberd , J. and Maydani , S.N. 1999 . Effect of dietary supplementation with black currant seed oil on the immune response of healthy elderly subjects . American Journal of Clinical Nutrition , 70 ( 4 ) : 536 – 540 .
- International Union of Pure and Applied Chemistry (IUPAC) . 1987 . “ Method 1122. Determination of oil content (extraction method) ” . In Standard Methods for the Analysis of Oils, Fats and Derivatives , 7th , Edited by: Paquot , C. and Haufenne , A. 14 – 16 . Oxford , UK : Blackwell Scientific Publications .
- International Union of Pure and Applied Chemistry (IUPAC) . 1987 . “ Method 1121. Determination of moisture and volatiles matter content ” . In Standard Methods for the Analysis of Oils, Fats and Derivatives , 7th , Edited by: Paquot , C. and Haufenne , A. 13 – 16 . Oxford , UK : Blackwell Scientific Publications .
- International Union of Pure and Applied Chemistry (IUPAC) . 1987 . “ Methods 2301. Preparation of the fatty acid methyl esters ” . In Standard Methods for the Analysis of Oils, Fats and Derivatives , 7th , Edited by: Paquot , C. and Haufenne , A. 123 – 129 . Oxford , UK : Blackwell Scientific Publications .
- European Communities Commission. Regulations (EEC) No. 2568/91 on the characteristics of the olive and olive residue oil and on the relevant methods of analysis. Official Journal of the European Communities 1991, L248, 1
- León-Camacho , M. and Morales , M.T. 2000 . “ Gas and liquid chromatography: Methodology applied to olive oil ” . In Handbook of Olive Oil: Analysis and Properties , Edited by: Harwood , J.L. and Aparicio , R. 159 – 208 . Gaithersburg , MA : Aspen . 2000
- International Union of Pure and Applied Chemistry (IUPA) . 1987 . “ Method 2411. Identification and determination of tocopherols ” . In Standard Methods for the Analysis of Oils, Fats and Derivatives , 7th , Edited by: Paquot , C. and Haufenne , A. 174 – 219 . Oxford , UK : Blackwell Scientific Publications .
- Helbig , D. , Bohm , V. , Wagner , A. , Schubert , R. and Jahreis , G. 2008 . Berry seed press residues and their valuable ingredients with special regard to black currant seed press residues . Food Chemistry , 111 ( 4 ) : 1043 – 1049 .
- Ruiz del Castillo , M.L. , Gary , D. , Brennan , R. and Gordon , S. 2004 . Fatty acid content and juice characteristics in black currant (Ribes nigrum L.) genotypes . Journal of Agricultural and Food Chemistry , 52 ( 4 ) : 948 – 952 .
- Parry , J. , Su , L. , Luther , M. , Zhou , K. , Yurawecz , M.P. and Whittaker , P. 2005 . Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils . Journal of Agricultural and Food Chemistry , 53 ( 3 ) : 566 – 573 .
