Abstract
Nutritive value of mature figs (Ficus carica L.) was investigated in five Tunisian cultivars, ‘Bouhouli’ (BHL) and ‘Zidi’ (ZD) (dark skin figs); ‘Thgagli’ (THG), ‘Bidhi’ (BD), and ‘Khedri’ (KHD) (yellow-green skin figs). Sugars, organic acids, fibres, and polyphenols were analysed in representative fruit samples from two distinct regions known to develop fig crops. Tunisian figs were characterized by predominance of glucose (6.30 g/100 g fresh weight) and fructose (5.10 g/100 g fresh weight). Citric acid (0.35 g/100 g fresh weight) was the major organic acid in all cultivars, almost three times higher than malic acid (0.13 g/100 g fresh weight). Average content of alcohol insoluble solids was 3.3 g/100 g FW. Four main polyphenols could be identified: two anthocyanins (cyanidin-3-glucoside; cyanidin-3-rutinoside), one flavonol (rutin), and one hydroxycinnamic acid (5-cafeoylquinic acid), revealed only in ‘BD’ samples. Cyanidin-3-rutinoside was the most abundant compound among all cultivars. Compared to common fruit, figs are among high sugar leveled fruit with significant dietary fibre content. Dark skin ‘ZD’ fruit were the most interesting figs with the highest concentration of sugars, organic acids, and polyphenols, especially cyanidin-3-rutinoside. This cultivar could be better advised for fresh consumption. However, the three lighter cultivars are more suitable for drying.
INTRODUCTION
Ficus carica is thought to have originated in western Asia and from there slowly spread through the Mediterranean region.[Citation1] It is one of the early domesticated fruit species[Citation2] and currently is an important crop worldwide. Total world fig production is over 1 million tonnes. Turkey, Egypt, Algeria, Iran, Morocco, Spain, and the USA yielded about 80% of total production.[Citation3] The world fig exchanges are more important as dried (77%). Turkey, USA, and Spain are the main exporters of dried figs.[Citation3, Citation4]
Figs (Ficus carica L.) are an important constituent of a Mediterranean diet. The fruit is highly nutritious and is consumed fresh or dried around the world.[Citation5] The fig is a syconium. It is a hollow, fleshy receptacle, enclosing numerous flowers, which never see the light and develop into drupelets within the receptacle after being pollinated. During the last stage of fruit development, there are significant changes in fruit colour, size, and texture.[Citation6] The fruits are highly perishable, climacteric, and breakdown rapidly after harvest.[Citation7]
Figs are appreciated for their size, lucid colour, and sweetness. When ripe, figs soften drastically offering to the fruit its fine texture. They are an excellent source of minerals, vitamins, and fibre. Like other fruit, figs contain sugars and organic acids that influence their quality. They have high amounts of crude fibre (5.5%, w/w) and polyphenols,[Citation8, Citation9] which are good for human health. Figs (Ficus carica L.) are adapted to arid and semi-arid environments and Tunisia is an important growing area. There are numerous local cultivars with the fruit eaten fresh and dried.[Citation10] The groves are located in sites with contrasting climates and soils, such as the plains, seacoasts, oases, and moist areas as high altitude.[Citation11] Figs represent the principal component of several agro-ecosystems in the southern areas, such as at the ‘Jessours’ region and constitute the second fruit crop in the Tunisian oases.[Citation12]
Previous work in Tunisia examined the genetic diversity of fig and established a reliable method identifying and preserving them.[Citation13] However, there is little information on the biochemistry of figs of the cultivars grown in this region. In this work, we report on the quality of five different fig cultivars grown in Tunisia. Information was collected on the levels of sugars, organic acids, fibres, and polyphenols. Recommendations are provided on the best cultivars to grow in this region and which cultivars are suitable.
MATERIAL AND METHODS
Plant Material
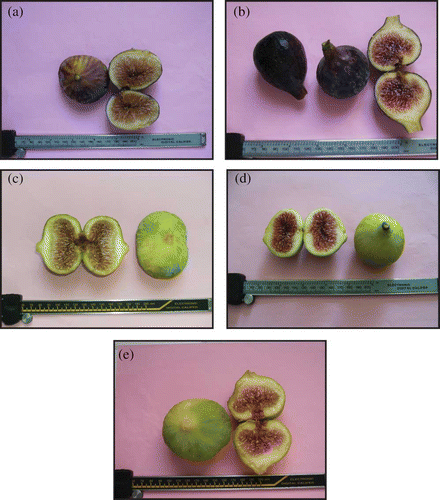
Fruit from ‘Bouhouli’ (BHL), ‘Zidi’ (ZD) (dark skin figs), and ‘Thgagli’ (THG), ‘Bidhi’ (BD) and ‘Khedri’ (KHD) (yellow green skin figs) were selected for this study (). Fruit were gathered from two main zones of commercial fig production: ‘Beja’ representing the North West where ‘Bouhouli’ represents 85% of plantings and ‘Monastir’ representing the Central East of Tunisia where ‘Bidhi’ represents 25% of plantings.[Citation10] resumes morphological aspects related to fruit of the five cultivars. Twenty fruits per tree and three trees per cultivar were harvested in late August 2009 and 2010 to form a sample of 60 ripe fruits from which 30 fruits (3 replicates of 10 fruits each) were randomly picked to compose the final fig samples for each cultivar. The samples were immediately stored at −20°C until used. They were then ground in liquid nitrogen using an IKA®A11 basic analytical mill (Ika Labortechnik, Staufen, Germany) and the powder was stored at −80°C.
Table 1 Morphological aspect of the five Tunisian fig cultivars: ‘BHL’ (Bouhouli), ‘ZD’ (Zidi), ‘THG’ (Thgagli), ‘BD’ (Bidhi), and ‘KDH’ (Khedri)
Determination of Sugars and Organic Acids
From each sample, 5 g of frozen powder are mixed with 20 mL ultra pure water. Samples were ground with an Ultraturrax T25 equipment (Ika Labortechnik, Staufen, Germany) to obtain a slurry. The mixture was homogenized and then centrifuged for 5 min at 4°C (9000 rpm). Samples were then filtered and the supernatant recovered. The extracts were kept at −20°C until analysis. Glucose, fructose, and sucrose, and malic and citric acids were quantified using enzymatic methods with kits for food analysis (Boehringer Mannheim Co., Mannhein, Germany) and expressed in g/100 g1 of fresh weight. These measurements were performed with an automatic analyser BM-704 (Hitachi, Tokyo, Japan).
Determination of Alcohol Insoluble Solids
Alcohol insoluble solids (AIS) or dietary fibre[Citation14] was prepared according to Renard.[Citation15] Approximately 10 g of powder was suspended in 35 ml of 96% boiling ethanol for 20 min. Samples were shaken vigorously to eliminate lumps and centrifuged at 9000 rpm for 10 min (15°C), then the supernatant was removed and another portion of 70% ethanol added. The washings were continued until absence of sugars as shown by negative reaction in a phenol sulphuric test.[Citation16] Samples were then washed three times with acetone/water (v/v 60:40), then once with acetone/water (v/v 80:20), and finally with pure acetone until discolouration of the supernatant. The residue was dried at 40°C for at least 48 h and after that weighed. Data were expressed as g/100 g1 of fresh weight (FW).
Determination of Polyphenols
Polyphenols were measured by high-performance liquid chromatography (HPLC)/diode array detection (DAD). They were extracted by suspension of the powder (200 mg) in 1200 μL acidic methanol (1% acetic acid, v/v), and 15 min sonication in a melting ice bath as described by Guyot et al.,[Citation17] followed by filtration (polytetrafluoroethylene [PTFE], 0.45 μm). HPLC/DAD analyses were performed using an Ultra Fast Liquid Chromatography Shimadzu Prominence system (Kyoto, Japan), including two pumps LC-20AD Prominence liquid chromatograph UFLC, a DGU-20A5 Prominence degasser, a SIL-20ACHT Prominence autosampler, a CTO-20AC Prominence column oven, a SPD-M20A Prominence diode array detector, a CBM-20A Prominence communication bus module, and controlled by LC Solution software.
Chromatographic separation was carried out using a 4 μm, 150 mm × 4.6 mm i.d. Phenomenex SF C18 column at 30°C. The mobile phase was composed of water/formic acid (98/2, v/v) (solvent A) and acetonitrile/water/formic acid (80/18/2, v/v) (solvent B) at a flow rate of 0.8 mL min−1. The following gradient was used: 0 min, 100% A; 30 min, 80% A and 20% B; 45 min, 40% A and 60% B; 50 min, 100% B; and 70 min, 100% A. Absorbance spectra were measured over wavelengths of 280 to 520 nm. Phenolics were identified by their UV-Vis spectra and elution times by comparison with standards. They were quantified by integration of their absorbance at 520 nm for anthocyanins, 320 nm for hydroxycinnamic acids, and 350 nm for flavonols in HPLC-DAD against calibration curves. Results were expressed in mg per g of fresh weight. The standard of 5-cafeoylquinic acid came from Sigma-Aldrich (Deisenhofen, Germany), while standards of rutin, cyanidin-3-glucoside, and cyanidin-3-rutinoside came from Extrasynthese (Lyon, France).
Data Analysis
The determination of metabolites (sugars, organic acids, AIS, and polyphenols) content in fig extracts were carried out in triplicate from samples harvested over 2 years. Data were subject to one-way analysis of variance (ANOVA). Significant differences were assessed with a Duncan test (p ≤ 0.05) and cultivars from homogeneous subsets were displayed. The final data results represent means of analysis over the 2 years. Statistics were performed using PC software package SPSS (Version 13.0; SPSS Inc.).
RESULTS AND DISCUSSION
Sugars
Glucose was the main sugar, followed by fructose, and then sucrose (). ‘ZD’ had the highest amount of sugars with ‘BHL’, ‘THG’, and ‘KHD’ being slightly lower. This response was generally reflected in the differences on individual sugars. Aljane et al.,[Citation18] working on figs from southern Tunisia, found a mean of 3.5 g/100 g FW for glucose and 2.5 g/100 g FW for fructose. For ‘ZD’, they found concentrations of 3.8 and 3.2 g/100 g FW glucose and fructose, respectively. Figs are rich in sugars[Citation8] with glucose and fructose being the most important.[Citation19, Citation20] Melgarejo et al.[Citation19] reported glucose and fructose as major sugars in the first (breba) and second (main) crops of four cultivars in Spain.
Table 2 Fruit chemical and biochemical composition of the five Tunisian fig cultivars: ‘BHL’ (Bouhouli), ‘ZD’ (Zidi), ‘THG’ (Thgagli), ‘BD’ (Bidhi), and ‘KHD ’ (Khedri)
Organic Acids
Citric acid was the major organic acid in the fruit, with lower concentrations of malic acid (). ‘ZD’, ‘THG’, ‘BD’, and ‘KHD’ had higher concentrations of organic acids, and ‘BHL’ exhibited lower amounts. This pattern reflected the differences in the concentration of citric acid, whereas there is no clear response for malic acid (0.11 to 0.17 g/100 g FW). Overall, ‘ZD’ could be listed as having relatively high sugar and acid concentrations, with ‘BHL’ having relatively high sugar rates but low acid concentrations. Organic acids are important constituents in figs and along with sugars contribute to fruit quality. Melgarejo et al.[Citation19] described malic, oxalic, and citric as the main organic acids in figs with concentration values ranging from 0.081 to 0.094%. The concentrations of malic and citric acids were higher in ‘Gobernador’ than in ‘Colar’, ‘Florancha’, or ‘Tio Antonio’ cultivars originated from the Alicante area in Spain. Hasnaoui et al.[Citation21] identified six organic acids in Tunisian pomegranate juice with malate and citrate representing about 50 and 23% of the total, respectively.
Alcohol Insoluble Solids
There were only small differences in the concentration of alcohol insoluble solids among the five cultivars (2.9 to 3.6 g/100 g FW) (). Our results agree with those of Owino et al.,[Citation7] who reported values of about 3.4% in fully ripe fruit of ‘Houraichi’ cultivar in Japan. Renard and Giniès[Citation22] reported values of 1.0 to 2.0 g/100 g FW in the flesh of five commercial plum cultivars and more than 4.5 g/100 g FW in the skin. Nunes et al.[Citation23] reported a mean value of 3.3 g/100 g FW in whole plum fruit.
Polyphenols
Four phenolic compounds were identified in the figs. Two universal components belong to anthocyanins: cyanidin-3-glucoside and cyanidin-3-rutinoside, and one universal compound belongs to flavonols: rutin. A hydroxycinnamic acid, such as 5-cafeoylquinic acid, was found only in ‘BD’. Cyanidin-3-rutinoside was the most abundant of the universal phenolics (), with its concentration 3 times higher than rutin and 15 times higher than cyanidin-3-glucoside. Cafeoylquinic acid was found only in ‘BD’ (yellow-green skin) at a low concentration. This suggests that pink flesh contains a high concentration of polyphenols. ‘ZD’ (dark skin) had the highest concentration of total polyphenols and then ‘BHL’ (dark skin). In ‘THG’, ‘BD’, and ‘KHD’ (yellow-green skin), anthocyanins are limited to the internal flower remnant. The concentration of cyanidin-3-rutinoside, the major polyphenol, was highest in ‘ZD’, followed by ‘BHL’ and then ‘KHD’, ‘BD’, and ‘THG’. Anthocyanins, hydroxycinnamic acids, and flavonols were described by HPLC as major groups in strawberries[Citation24] with anthocyanins being the most predominant phenolic compound. The antioxidant capacities of red fruits were relatively much higher than those of the other fruits.[Citation25]
Polyphenols contribute to taste, colour, and health benefits of figs. The three phenolic classes described above were reported by Piga et al.[Citation26] in black ‘Mattalona’ and white ‘San Pietro’ figs. Duenas et al.[Citation27] found cyanidin-3-rutinoside as the dominant polyphenol in five cultivars. The authors described other anthocyanins in figs like pelargonidin-3-glucoside, peonidin-3-rutinoside and acyl derivatives like cyan-3-malonylglucoside. Other compounds described were assigned to condensed pigments containing C–C linked anthocyanin (Cy or Pg) and catechin residues were not revealed in Tunisian figs. Moreover, the authors demonstrate significant differences in concentration of anthocyanins between pulp and skin ranging between 32 and 97 μg/g FW in the skin and from 1.5 to 14 μg/g FW in the pulp.
Using TLC and densitometry tests, Puech et al.[Citation28] reported that cyanidin-3-rhamnoglucoside was the dominant pigment in ripe skins of ‘Mission’, with cyanidin 3,5-diglucoside and pelargonidin 3-rhamnoglucoside. Solomon et al.[Citation5] found cyanidin-3-rhamnoglucoside to be the major pigment in the skin of six cultivars. Cyanidin-3-glucoside was a minor component, and anthocyanins concentrations were the lowest in lighter cultivars ‘Brunswick’ and ‘Kadota’ as described in Tunisian ‘BD’, ‘KHD’, and ‘THG’ cultivars, and again, skins had higher polyphenols than pulps. Veberic et al.[Citation29] found compounds like gallic acid, chlorogenic acid, syringic acid, (-)-epicatechin, (+)-catechin, and rutin in fruit from the northern Mediterranean region. This last compound was present at the highest concentration (up to 28.7 mg/100 g FW), followed by (+)-catechin (up to 4.03 mg/100 g FW). In our case, the dark fleshed ‘ZD’ and ‘BHL’ had higher concentrations of polyphenols than the white fleshed cultivars. The low concentrations were recorded in ‘THG’. Compared to cultivars described above, Tunisian figs had nearly the same concentrations of polyphenols.
Overview of Fruit Quality
‘ZD’ had higher concentrations of sugars than the other cultivars (16.4 g/100 g versus 11.8–14.3 g/100 g FW) and high concentrations of total polyphenols (15.7 mg/100 g versus 5.1–9.3 mg/100 g). The concentration of cyan-3-rutinoside was also higher in the coloured ‘BHL’ and ‘ZD’ (7.6–13.9 mg/100 g versus 3.5–4.2 mg/100 g). A different response was recorded for the concentration of organic acids, which were low in ‘BHL’ (0.36 g/100 g versus 0.45–0.60 g/100 g). The cultivars had almost similar concentrations of alcohol insoluble solids ranged from 2.9 to 3.6 g/100 g FW with ‘KHD’ being richer on AIS compared to the other cultivars. High sugar rates associated to prominent anthocyanin concentration seem to be the most important criterion of quality in figs.
CONCLUSION
Despite the different results obtained concerning quality traits of the five Tunisian cultivars, ‘ZD’ and ‘BHL’ seem to be more suitable for fresh consumption. The fruit can reach an important size with high sugar rates and polyphenols content. Whereas, the three yellow-green skin, ‘BD’, ‘KHD’, and ‘THG’, are rather appropriate for process since the fruit offers a light skin colour and a rounded shape suitable for drying.
ACKNOWLEDGMENTS
The authors wish to thank Mr. C. Giniès, Mz. Line Tichit, and M. Bogé for their excellent technical assistance. This work was supported by grants from the Ministry of Higher Education and Scientific Research–Tunisia (UR03AGR04) and the Agence Universitaire de la Francophonie–AUF (Grant G3-110/1649).
REFERENCES
- Stover , E. , Aradhya , M. , Ferguson , L. and Crisosto , C. 2007 . The fig: Overview of an ancient fruit . HortScience , 42 : 1083 – 1087 .
- Zohary , D. and Hopf , M. 2000 . Domestication of Plants in the Old World , Third , Oxford : University Press .
- FAOSTAT. © FAO Statistics Division. 2011 http//www.fao.org (http://http//www.fao.org) (Accessed: 1 April 2011 ).
- Sadhu , M.K. Fig. 1990 . In: Fruits: Tropical and Subtropical , Edited by: Kose , T.K. and Mitra , S.K. 650 – 663 . Calcutta , India : Naya Prokash .
- Solomon , A. , Golubowicz , S. , Yablowicz , Z. , Grossman , S. , Bergman , M. , Gottlieb , H.E. , Altman , A. , Kerem , Z. and Fleishman , M.A. 2006 . Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.) . Journal of Agricultural and Food Chemistry , 54 : 7717 – 7723 .
- Chessa , I. Fig. 1997 . Postharvest Physiology and Storage of Tropical and Subtropical Fruits , Edited by: Mitra , S. 245 – 268 . Oxon , UK : CAB International, Wallingford .
- Owino , W.O. , Nakano , R. , Kubo , Y. and Inaba , A. 2004 . Alterations in cell wall polysaccharides during ripening in distinct anatomical tissue regions of the fig (Ficus carica L.) fruit . Postharvest Biology and Technology , 32 : 67 – 77 .
- Vinson , J.A. 1999 . The functional food properties of figs . American Association of Cereal Chemists , 44 ( 2 ) : 82 – 87 .
- Vinson , J.A. , Zubik , L. , Bose , P. , Samman , N. and Proch , J. 2005 . Dried fruits: Excellent in vitro and in vivo antioxidants . Journal of the American College of Nutrition , 24 : 44 – 50 .
- Mars , M. , Gaaliche , B. , Ouerfelli , I. and Chouat , S. 2009 . Cropping systems and genetic resources of fig (Ficus carica L.) in Djebba and Kesra, two mountainous villages in North-West Tunisia . Revue des Régions Arides , 22 : 33 – 45 .
- Salhi-Hannachi , A. , Chatti , K. , Saddoud , O. , Mars , M. , Rhouma , A. , Marrakchi , M. and Trifi , M. 2006 . Genetic diversity of different Tunisian fig (Ficus carica L.) collections revealed by RAPD fingerprints . Hereditas , 143 : 15 – 22 .
- Mars , M. 2003 . “ Conservation of fig (Ficus carica L.) and pomegranate (Punica granatum L.) varieties in Tunisia ” . In Conserving Biodiversity in Arid Regions , Edited by: Lemons , J. , Victor , R. and Schaffer , D. 433 – 442 . The Netherlands : Kluwer Academic Publishers .
- Baraket , G. , Saddoud , O. , Chatti , K. , Mars , M. , Marrakchi , M. , Trifi , M. and Salhi-Hannachi , A. 2009 . Chloroplast DNA analysis in Tunisian fig cultivars (Ficus carica L.): Sequence variations of the TrnL-trnF intergenic spacer . Biochemical Systematics and Ecology , 36 : 828 – 835 .
- Englyst , H.N. and Hudson , G.J. 1987 . Colorimetric method for routine measurement of dietary fibre as non-starch polysaccharides. A comparison with gas-liquid chromatography . Food Chemistry , 24 : 63 – 76 .
- Renard , C.M.G.C. 2005 . Variability in cell wall preparations: Quantification and comparison of common methods . Carbohydrates Polymers , 60 : 515 – 522 .
- Dubois , M. , Gilles , K.A. , Hamilton , J.K. , Rebers , P.A. and Smith , F. 1956 . Colorimetric method for determination of sugars and related substances . Analytical Chemistry , 28 : 350 – 356 .
- Guyot , S. , Marnet , N. , Sanoner , P. and Drilleau , J.F. 2001 . Direct thiolysis on crude apple materials for high-performance liquid chromatography characterization and quantification of polyphenols in cider apple tissues and juices . Methods of Enzymology , 335 : 57 – 70 .
- Aljane , F. , Toumi , I. and Ferchichi , A. 2006 . HPLC determination of sugars and atomic absorption analysis of mineral salts in fresh figs of Tunisian cultivars . African Journal of Biotechnology , 6 : 599 – 602 .
- Melgarejo , P. , Hernandez , F.C.A. , Martinez , J.J. , Sanchez , M.J. and Salazar , D.M. 2003 . Organic acids and sugars from first and second crop fig juices . Acta Horticulturae , 605 : 237 – 239 .
- Genna , A. , Vecchi , P. , De Bruno , M. and Maestrelli , A. 2005 . Quality of Cosenza dried fig: A sensory and physicochemical approach . Acta Horticulturae , 798 : 72 – 77 .
- Hasnaoui , N. , Jbir , R. , Mars , M. , Trifi , M. , Kamal-Eldin , A. , Melgarejo , P. and Hernandez , F. 2011 . Organic acids . sugars, and anthocyanins contents in juices of Tunisian pomegranate fruits. International Journal of Food Properties , 14 : 741 – 757 .
- Renard , C.M.G.C. and Giniès , C. 2009 . Comparison of the cell wall composition for flesh and skin from five different plums. Food Chemistry , 114 : 1042 – 1049 .
- Nunes , C. , Saraiva , J.A. and Coimbra , M.A. 2008 . Effect of candying on cell wall polysaccharides of plums (Prunus domestica L.) and influence of cell wall enzymes . Food Chemistry , 111 : 538 – 548 .
- Wang , Q. , Tury , E. , Rekika , D. , Charles , M.T. , Tsao , R. , Hao , Y.J. , Dubé , C. and Khanizadeh , S. 2010 . Agronomic characteristics and chemical composition of newly developed day-neutral strawberry lines by agriculture and agri-food Canada . International Journal of Food Properties , 13 : 1234 – 1243 .
- Herken , E.N. and Guzel , S. 2010 . Total antioxidant capacity and total phenol contents of selected commercial fruit juices in Turkey . International Journal of Food Properties , 13 : 1373 – 1379 .
- Piga , A. , Del Caro , A. , Milella , G. , Pinna , I. , Vacca , V. and Schirru , S. 2008 . HPLC analysis of polyphenols in peel and pulp of fresh figs . Acta Horticulturae , 798 : 301 – 306 .
- Duenas , M. , Perez-Alonso , J.J. , Santos-Buelga , C. and Escribano-Bailon , T. 2008 . Anthocyanin composition in fig (Ficus carica L.) . Journal of Food Composition and Analysis , 21 : 107 – 115 .
- Puech , A.A. , Rebeiz , C.A. , Catlin , P.B. and Crane , J.C. 1975 . Characterization of anthocyanins in fig (Ficus carica L.) fruits . Journal of Food Science , 40 : 775 – 780 .
- Veberic , R. , Colaric , M. and Stampar , F. 2008 . Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region . Food Chemistry , 106 : 153 – 157 .
- IPGRI and CIHEAM . 2003 . “ L.]. Future Harvest Centre Support ” . In Descriptors for fig [Ficus carica ISBN 92-9043-598-4.