Abstract
This article investigated chemical characteristics of Mirabilis jalapa seed proteins. Proteins were extracted from the deoiled seeds of Mirabilis jalapa L. in aqueous solutions at various pH values and also by using different concentrations of NaCl, Na2SO4, CaCl2, and MgSO4 at pH 7.0. The nitrogen content (1.77 ± 0.23 g/100 seed) of the seeds and deoiled seeds showed high protein content (11.0 ± 0.75 g/100 seed). Amino acid analyses of the total protein isolates confirmed the presence of 17 amino acids of which 9 are essential. The molecular weight of total protein isolates was determined by gel filtration and sodium dodecyl sulphate-poly acrylamide gel electrophoresis method. Studies on surface structure of the total protein isolates and seed flour by scanning electron microscopy were also performed.
INTRODUCTION
Food is the basic need of human beings. As a result of population explosion, scarcity of food has become a serious problem in man's struggle for existence. More over, the acute shortage and high prices of conventional food proteins has resulted in the search for alternative and cheaper sources of proteins for the poverty stricken people of India. The vegetable proteins play a significant role in developing countries owing to their comparatively low prices. The paucity of cultivated land poses a problem towards the cultivation of conventional legumes. These factors inspired the authors to search for non-conventional protein sources from non-conventional plant resources in our country. These types of plants, which are found in abundance, have not been utilized. Such seeds can be used to meet the increasing demands for edible proteins. Nowadays protein isolates from plants are utilized in various foods to improve nutritional quality and functionality.[ Citation 1, Citation 2 ]
With this objective of finding non-conventional protein sources, work on the seeds of Mirabilis jalapa L. (Family: Nyctaginaceae) was undertaken. This plant grows abundantly in India especially in West Bengal.[ Citation 3 ] This plant is also known to possess antifungal, antimicrobial, antiviral, antispasmodic, antibacterial, diuretic, alternative carminative, catharic, hydragogue, purgative, stomatic tonic and vermifuge properties.[ Citation 4 − Citation 8 ] Many seeds of this plant are discarded every year in spite of their high protein content. To investigate the extent to which the seed protein of M. jalapa can be utilized, this communication deals with solubility studies, amino acid analysis, mineral content, molecular weight determination by gel filtration and sodium dodecyl sulphate-poly acrylamide gel electrophoresis (SDS-PAGE) method and surface morphology by scanning electron microscopy (SEM) of the seeds of Mirabilis jalapa L. (MJS).
MATERIALS AND METHODS
Materials
Dry seeds of M. jalapa L. were collected from the Forest Department of Government of West Bengal, India. A specimen voucher MJ1 has been preserved in the herbarium of the Botany Department, University of Calcutta. All chemicals used were of analytical grade and purchased from Sigma Chemical and Aldrich Co. St. Louis, MO, USA. Experiments where temperatures have not been specified were performed at temperatures ranging from 10–12°C and humidity 30–32%.
Sample Preparation
Dried seeds were powdered to increase the surface area and defatted with n-hexane (40–60°C) for 48 h. The hexane was removed and the powdered seeds were air-dried (in complete absence of sunlight) to remove the trace amount of n-hexane. The seed flour was then washed thrice with chloroform: methanol (3:1), air dried and stored in air tight sample bottles in a refrigerator (4°C) until used.
Protein Content
The nitrogen content of the defatted seeds were estimated by the micro-Kjeldahl method and the protein contents were determined (N × 6.25), following methods of AOAC.[ Citation 9 ]
Protein Solubility
The protein solubility profile of the de-oiled seed was determined under two different conditions. In one procedure the extraction was carried out with an electrical stirrer at room temperature for 10 min using de-ionized distilled water. The de-oiled seed-flour mixture (10:1 v/w) was extracted separately at pH 1–10. The pH of extraction was adjusted from 1–6, adding 0.5 M HCl and from 8–10 by addition of 0.5M NaOH. The respective pHs of the extractions were maintained throughout the experiments. The mixture was stirred for half an hour and then centrifuged using 10,000 rpm in a cooling C-24 centrifuge (manufactured by Remi Instrument, Bombay, India) for 10 min at 0°C. The pH of the supernatant was adjusted to pH 4.0 using 1 N trichloroacetic acid (TCA) and kept overnight in the refrigerator. Then it was again centrifuged at 5000 rpm in a cooling centrifuge for 10 min at 0°C and the supernatant was discarded. The residues were washed several times with distilled water by centrifugation and then freeze dried.[ Citation 10 ] The nitrogen content of each defatted seed protein was estimated by the micro-Kjeldahl method and the protein contents were determined by multiplying the nitrogen content by a factor of 6.25.[ Citation 9 ] In the second procedure the same protocol was followed for determining the solubility of protein isolated at a fixed pH 7.0 in presence of NaCl, Na2SO4, CaCl2, and MgSO4 at different molar concentrations (0.1 M to 1.0 M).[ Citation 11 ]
Preparation of Total Protein Isolates (TPI)
The total protein isolate was prepared from de-oiled seed flour by water extraction at pH 7.0.[ Citation 12 ]
Fractionation of Seed Protein
Defatted M. jalapa seed flour (25 g) was stirred in 250 ml of distilled water with a electrical stirrer for 30 min at room temperature (20°C). The mixture was stirred for half an hour and then centrifuged using 10,000 rpm in a cooling C-24 centrifuge for 10 min at 0°C. The pH of the supernatant was adjusted to pH 4.0 using 1N trichloroacetic acid (TCA) and kept over-night in the refrigerator. Then it was again centrifuged at 5000 rpm in a cooling centrifuge for 10 min at 0°C and the supernatant was discarded. The residues were washed several times with distilled water by centrifugation and then freeze dried.[ Citation 10 ] These freeze dried fractions were used for determination of amino acid composition, mineral content using SDS-PAGE electrophoresis and scanning electron microscopy (SEM) analysis.
Ash and Amino Acid Content
Ash contents were determined by the Association of Official Analytical Chemists method.[ Citation 9 ] Trace elements were also determined using a Varian Spectra AA-220 FS atomic absorption spectrometer. Amino acid composition of TPI was performed in a L.K.B. alpha plus amino acid analyzer (UK) taking precaution for cysteine, methionine, tyrosine by using proper protecting reagents.[ Citation 13 ] A ratio of essential to total amino acid was reported as E/T (%).
Gel Filtration
The method of Whitaker was used with slight modification for gel filtration chromatography.[ Citation 14 ] The protein extract at pH 7.0 was dialyzed using distilled water for 72 h at 4°C. It was then freeze-dried. The proteins obtained were again dissolved in 0.01 M phosphate buffer (pH 7.0) containing 0.2 M NaCl, resulting in a protein sample of 4 mg/ml concentration. The experiment was carried out at 20°C in a 2.0 cm i.d × 45 cm Sephadex G-200 column. The void volume of the column was measured using Blue Dextran. A protein sample (2 ml) was then applied. The eluting buffer was prepared using 0.01 M phosphate buffer (pH 7.0) containing 0.2 M NaCl. Fractions of 2 ml were collected at a rate of 0.4 ml/min and monitored at 280 nm with a Beckman DU spectrophotometer. Calibration was carried out with reference protein standards (BSA, ovalbumin, pepsin, and lysozyme). The molecular weight of each of the components (A–D) was also calculated by the following equation as described by Leach and O'Shea:[ Citation 15 ]
Gel Electrophoresis
Gel electrophoresis studies were performed with the TPI.[ Citation 16 ] SDS-PAGE was performed using 10% (w/v) vertical polyacrylamide non gradient gel at pH 6.8, using tris-glycine buffer containing 0.5% bromophenol blue (as marker), and 1.25% β mercaptoethanol. Protein samples (55 micro grams) were loaded onto each well and electrophoresis was carried out at a constant current of 3 mamp per lane. The samples were boiled in the water bath for 3 min prior to loading on to the gel.
The molecular weight markers (Sigma Chemicals Co., USA) used were bovine serum albumin (66 kDa), ovalbumin (4.5 kDa), pepsin (3.47 kDa), and lysozyme (14.34 kDa). Following electrophoresis, the gels were stained with Coomassie Brilliant Blue R-250 (1.25 g) in acetic acid:water:ethanol (227 ml:46 ml:227 ml) and de-stained in a mixture of methanol:acetic acid:water (7 ml:7 ml:86 ml) until the desired background color was obtained from the gel documentation system (G S 700 imaging densitometer biorad, USA).
Scanning Electron Microscopy
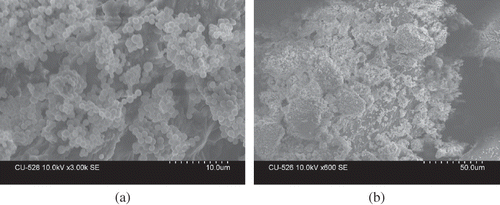
Structural morphology of the protein fractions was studied by scanning electron microscopy. Lyophilized TPI, along with seed flour, were mounted on circular aluminium stubs with double sticky tape and coated with 20 nm of gold using an IB2 ion coater. The samples were examined and photographed with a Hitachi scanning electron microscope (Hitachi S-3100N, Hitachi Ltd., Tokyo, Japan) at an accelerating potential of 20 kV.
Table 1 Protein solubility of M. jalappa seed in aqueous solution at various pH and in various concentration of different salt solutions at pH 7.0Footnote a
RESULTS AND DISCUSSION
Nitrogen and protein contents of the seeds were found to be lower than those of deoiled seeds (). The ash content of seeds was 3.47 g/100 g protein and that of the deoiled seed 2.91 g/100 g protein. The moisture content of seeds and de-oiled seeds were 6.43 g/100 g protein and 4.22 g/100 g protein, respectively. Solubility profiles of protein in water at different pH values and in the presence of different salts at different concentrations have been shown in and . Highest solubility was observed for Mirabilis jalapa protein under high alkaline condition (pH 10) and minimum solubility at pH 4. Solubility of M. jalapa protein was almost constant at a pH of 7–8. This solubility was observed to increase under both extreme acidic and alkaline pH as was also the case of Seasame cultivar according to Khalid et al.[ Citation 17 ] The difference in the solubility behavior of this seed protein may be due to the mechanism of peptide bond cleavage, presence of hydrogen bonding, electrostatic bonding, or hydrophobic bonding. Like all other seed proteins, MJS proteins were found to be more soluble in alkaline pH.[ Citation 18 ] Zwitter ion behavior of the amino acids forming the peptide chain would result in low protein solubility in acidic pH with respect to alkaline pH. Alkaline peptidization may also be responsible for an increase in solubility at higher pH. At the iso-electric region, the protein molecule possessed a minimum net charge. Thus, there was no electrostatic repulsion between the molecules and as a result solubility was minimal. Since TPI is a mixture of proteins, it cannot have a definite pH value. However, it showed a minimum solubility at pH 4 and maximum solubility at pH 10. The increase in the net negative charge on the protein dissociation helped the protein to aggregate and as a result solubility increased with higher pH of the solution.[ Citation 19 ]
Figure 1 Solubility profile of Mirabilis jalapa L. seed proteins at different pH. (Color figure available online.)
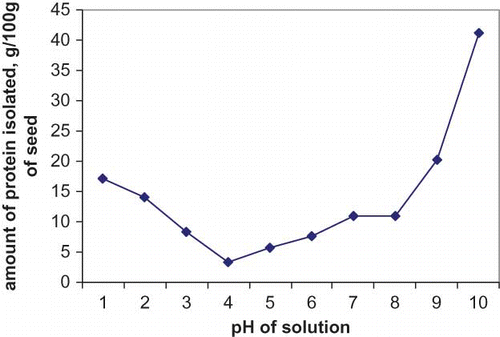
Figure 2 Solubility profile of Mirabilis jalapa L. seed proteins at different salt concentration. (Color figure available online.)
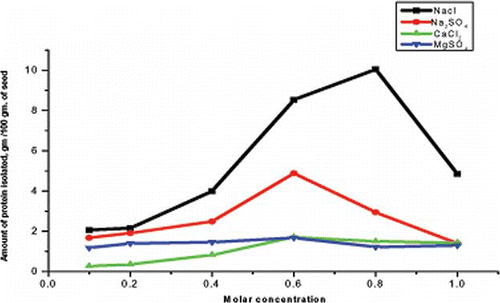
It is a well known fact that proteins can be extracted better in inorganic salt solutions than under salt free conditions. Ionic strengths have a profound effect on solubility of seed proteins.[ Citation 20 ] Salt related changes in water interactions may be produced by competitive binding of water and salt molecules by the amino acid side groups.[ Citation 21 ] Melander et al. analyzed salt dependence protein solubility based on dual effect of salt, i.e., electrostatic and hydrophobic interactions.[ Citation 22 ] The increase in solubility at low salt concentration depended on electrostatic and hydrophobic interactions. This is due to the “salting in” effect of electrostatic interactions while the precipitation of proteins at higher salt concentration is due to the “salting out” effect due to hydrophobic interactions.[ Citation 23, Citation 24 ]
Table 2 Comparative study of amino acid analysis in rice protein and M. jalapa L. seed protein
shows the effects of concentration of different monovalent and bivalent salts on protein solubility at pH 7. Maximum amount of MJS proteins were isolated at 0.8 M NaCl and minimum amount of proteins at 0.1 M CaCl2 at pH 7.0. revealed that NaCl is best for the purpose of extracting the proteins. Smaller size of sodium ion facilitates its approach towards the protein cavity resulting in a strong encapsulation and subsequent agglomeration. The marked effect of NaCl on solubility profile may be due to greater binding capacity of chloride ions. Chloride ions bind to the positively charge protein groups and thus increases net negative charge of protein. Due to this increase in net negative charge of proteins, solubility is enhanced by accentuating electrostatic repulsion.[ Citation 16 ] However, bivalent cations have affinity for carboxyl group of the proteins resulting in a reduced negative charge and hence insolubility.[ Citation 25 ] The salt-water interaction would also cause protein insolubility.[ Citation 26 ]
Amino acid composition of TPI showed 17 amino acid of which 9 are essential ().[ Citation 27 ] When compared with a conventional cereal viz. Oryza sativa rice, TPI of M. jalapa showed higher amounts of phenylalanine, cysteine, valine, histidine, glycine, aspartic acid, and glutamic acid. Thus, the amino acid composition indicated that seeds of M. jalapa L. are richer sources of several essential and sulfur containing amino acids. The amino acid composition and E/T (%) of TPI is almost comparable with that of rice proteins. Gel filtration of M. jalapa seed protein revealed four fractions (A–D) (). Two different methods used for the molecular weight determination of the four components showed that M. jalapa seed protein is a mixture of four polypeptides.
Table 3 Determination of molecular weights of M. jalapa L. seed proteins by gel filtration procedure
Table 4 Determination of molecular weights of M. jalapa L. seed protein by SDS-PAGE
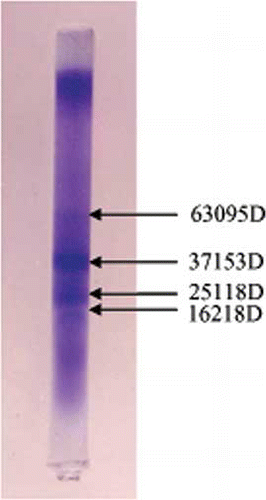
SDS PAGE patterns of TPI are shown in (). Most of the polypeptides were seen to bind SDS in a constant ratio such that they had essentially the same charge densities and migrated in the polyacrylamide gel according to their molecular weights. The whole seed protein isolate was characterized by the predominance of four subunits with molecular weights of 63095D, 37135D, 25118D, and 16218D (). The molecular weight distribution of total protein isolate in this method was close to the molecular weight determined from gel filtration study (). The results also showed that the proteins were simple in structure and showed the presence of several low molecular weight proteins. This is important as low molecular weight proteins are edible.
The mineral analysis () indicates the high concentration of potassium, zinc, calcium, and phosphorous in these seed proteins. Calcium:phosphorous is 1:2.5 and is more than the recommended 1:1.0. The content of lead and cadmium is low, which is beneficial to health. Structural morphology of TPI and seed-flour was studied with the aid of SEM. SEM pictures of seed flour and total protein isolate () showed distinct surface structures. The seed-flour showed granule like structure whereas TPI appeared as small flake like structures.
Table 5 Content of certain minerals in M. jalapa L. seeds protein (μg/g)
CONCLUSION
From the above investigations, it is evident that the protein (TPI) isolated from the seeds of M. jalapa contains most of the essential amino acids including appreciable amounts of sulfur containing amino acids. The seed protein showed high solubility in aqueous solution. The proteins can be easily extracted from the seeds. There are also plenty of low molecular weight proteins present in the seed. Considering all these factors, it appears that the M. jalapa seed protein would prove to be a good source of edible protein after proper toxicological screening and would serve as an important source of unexploited protein from legume. As M. jalapa seeds contain high amount of proteins and grows profusely in India, it can be used as an un-conventional and cheap source of protein.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to the Late Professor (Mrs.) Asima Chatterjee for encouraging them to begin work with this plant. The authors also wish to thank the Council of Scientific and Industrial Research, New Delhi, India (SRF (Ext) to AN) for financial assistance.
REFERENCES
- Adebowale , Y.A. , Schwarzenbolz , U. and Henle , T. 2011 . Protein isolates from Bambara groundnut (Voandzeia subterranean L.): Chemical characterization and functional properties . International Journal of Food Properties , 14 : 758 – 775 .
- Conforti , P.A. and Lupano , C.E. 2011 . Selected properties of Araucaria angustifolia and Arancaria araucana seed protein . International Journal of Food Properties , 14 : 84 – 91 .
- Chatterjee , A. and Pakrashi , S. 1982 . The Treatise of Indian Medicinal Plants , Vol. 1 , 77 New Delhi , India, : National Institute of Science Communication .
- Chopra , R.N. , Nayar , S.L. and Chopra , I.C. 1956 . Glossary of Indian Medicinal Plants , 168 New Delhi , India : Publication & Information Directorate Hillside Road CSIR .
- Cammue , B.P. , De Bolle , M.F. , Terras , F.R. , Proost , P. , Van Damme , J. , Rees , S.B. , Vanderleyden , J. and Broekaert , W.F. 1992 . Isolation and characterization of a novel class of plant antimicrobial peptides form Mirabilis jalapa L. seeds . The Journal of Biological Chemistry , 267 : 2228 – 2233 .
- Chifundera , K. , Kizungu , B. and Wa Mpoyi , M. 1991 . Antibacterial activity of Mirabilis jalapa seed powder . Journal of Ethnopharmacology , 35 : 197 – 199 .
- Kazuko , A. , Alma , R.C. , María del , C.R. , Martín , G.-H. and Francisco , J.L.-M. 2008 . Pharmacological study of antispasmodic activity of Mirabilis jalapa Linn flowers . Journal of Ethnopharmacology , 116 : 96 – 101 .
- Cristiani , I.B. , Walker , G.T. , Mateus , F.R. , Carina , F. , Maria , E.P. , Juliano , F. and Melânia , P.M. 2008 . Antinociceptive activity of Mirabilis jalapa in mice . Journal of Ethnopharmacology , 120 : 169 – 175 .
- AOAC . 1990 . “ AOAC Official Methods of Analysis ” . In Association of Official Analytical Chemists , 15th Washington , DC
- Prakash , V. and Nandi , P.K. 1978 . Isolation and characterization of α-globulin of Sesame seed (Sesamum indicum L.) . Journal of Agricultural and Food Chemistry , 26 : 320 – 323 .
- Basak , B. , Bhattacharyya , U.K. , Sinababu , A. and Laskar , S. 1994 . Extraction and chemical investigation of Kulthi (Macrotylona uniflorus Lam.) seed protein . Applied Biochemistry and Biotechnology , 49 : 281 – 290 .
- Dutta Samanta , T. and Laskar , S. 2009 . Chemical investigation of Erythrina variegata Linn. seed proteins . Food Chemistry , 114 : 212 – 216 .
- Gupta , M. , Sarkar , K. , Baral , R. and Laskar , S. 2010 . Some chemical investigations of Amoora rohituka seed proteins . Food Chemistry , 119 : 1057 – 1062 .
- Whitaker , J.R. 1963 . Determination of molecular weights of proteins by gel filtration on Sephadex . Analytical Chemistry , 35 : 1950 – 1953 .
- Leach , A.A. and O'Shea , P.C. 1965 . The determination of protein molecular weights of up to 225,000 by gel filtration on a single column of Sephadex G-200 at 25°C and 40°C . Journal of Chromatography , 17 : 245 – 251 .
- Weber , K. and Osbrone , M. 1969 . The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis . The Journal of Biological Chemistry , 244 : 4406 – 4412 .
- Khalid , E.K. , Babiker , E.E. and Tinary , A.H. 2003 . Solubility and functional properties of sesame seed proteins as influenced by pH and/or salt concentration . Food Chemistry , 82 : 361 – 366 .
- Laskar , S. , Sinhababu , A. , Thakur , S. and Basak , B. 1998 . Extraction and chemical investigation of Lagerstroemia speciosa seed proteins . Journal of American Laboratory , 30 : 22 – 24 .
- Prakash , V. 1986 . Effect of sodium chloride on the extractability of proteins from sesame seed (Sesamum indicum L.) . Journal of Agricultural and Food Chemistry , 34 : 256 – 259 .
- Tomotake , H. , Shimaoka , I. , Kayashita , J. , Nakajoh , M. and Kato , N. 2002 . Physicochemical and functional properties of buckwheat protein product . Journal of Agricultural and Food Chemistry , 50 : 2125 – 2129 .
- Chou , D.H. and Morr , C.V. 1979 . Protein-water interactions and functional properties . Journal of American Oil Chemists' Society , 56 : 53A – 62A .
- Melander , W. and Horvat , C. 1977 . Salt effect on hydrophobic interactions in precipitation and chromatography of proteins: an interpretation of the lyotropic series . Archives of biochemistry and biophysics , 183 : 200 – 215 .
- Van Megan , W.H. 1974 . Solubility behavior of soybean globulins as a function of pH and ionic strength . Journal of Agricultural and Food Chemistry , 22 : 126 – 129 .
- Arogundade , L.A. , Akinfenwa , M.O. and Salawu , A.A. 2004 . Effect of NaCl and its partial or complete replacement with KCl on some functional properties of defatted Colocynthis citrullus L. seed flour . Food Chemistry , 84 : 187 – 193 .
- Liu , L.H. and Hung , T.V. 1998 . Functional properties of acetylated chickpea proteins . Journal of Food Science , 63 : 331 – 337 .
- El-Adaway , T.A. , Rahma , E.H. , Bedauey , A.A. and Gafar , A.F. 2001 . Nutritional potential and functional properties of sweet and bitter lupin seed protein isolates . Food Chemistry , 74 : 455 – 462 .
- Eknayake , S. , Jansz , E.R. and Nair , B.M. 1999 . Proximate composition, mineral and amino acid content of mature Canavalia gladiata seeds . Food Chemistry , 66 : 115 – 119 .