Abstract
Onion is used for treating diabetes in Chinese civil practices; however, the anti-diabetic mechanism was not clear. To investigate the potential mechanism of onion in diabetes treatment, total phenolic content, antioxidant activity, and α-glucosidase and α-amylase inhibitory activity were assessed. Both 70% ethanol extracts and boiling water extracts from white onion, red onion, and commercial dry onion powder, bitter melon, pumpkin, yam, and medlar exhibited inhibitory activities against α-glucosidase. Total phenolic content values of samples were positively related to α-glucosidase inhibitory activities (p < 0.05). Antioxidant analyses suggested that onions possessed strong free radical scavenging capacities. These results indicated that onions showed potential to be effective anti-diabetic agents.
INTRODUCTION
Treatments on diabetes have been described extensively in the ancient Chinese medicinal documents. Besides plenty of traditional Chinese medicines, many folk prescriptions (including certain common foods) are believed to be useful in diabetes management. For example, onion marinated in red wine is said to possess benefits in preventing and treating diabetes in folk practices. Bitter melon is another typical sample; it has a long history as food and medicine, and the fruit and seed of bitter melon contains active components, which are considered to be able to lower human blood-glucose. However, the acting mechanisms of these civil practices on blood sugar-lowering are not fully understood.
Modern medicine indicates that one of the most effective therapeutic approaches for controlling blood-glucose level is to inhibit absorption of glucose by suppressing of carbohydrate-hydrolyzing enzymes, such as α-amylase and α-glucosidase.[ Citation 1 ,Citation 2 ] Acarbose, miglitol, and voglibose are examples of enzyme inhibitors used in clinical treatments.[ Citation 3 ] However, side effects of these compounds, such as liver disorder, flatulence, abdominal fullness, and diarrhea, have been reported.[ Citation 4 − Citation 6 ] Therefore, there is an increasing need for development of naturally occurring, non-toxic, and readily accessible enzyme inhibitors. Investigations on natural α-glucosidase and α-amylase inhibitors from natural products have become a popular research area in the past two decades. Suppressing α-glucosidase and/or α-amylase activity delays the hydrolysis of starch or disaccharides to monosaccharides, resulting in a reduction of the blood sugar level. Therefore, development of effective food-derived enzyme inhibitors would be beneficial to type-2 diabetes. Studies indicated that some of dietary plants possessed inhibitory effect against α-glycosidase and/or α-amylase, such as sorghum, foxtail millet and proso millet,[ Citation 6 ] guava leaves,[ Citation 7 ] and eggplant.[ Citation 8 ]
Several commonly consumed foods, such as onion, bitter melon, pumpkin, yam, and medlar, have long been used for preventing or treating diabetes in Chinese folk practices. Onions also have been valued for their medicinal qualities by many other cultures. This has led some researchers to test whether the proposed medicinal attributes of onions are valid. Potential benefits of onion consumption to human health are still under investigation. In terms of onion's anti-diabetic effects, Moore[ Citation 9 ] proposed a hypothesis that antioxidants in onion are probably responsible for its treating diabetes function, and protect body tissue damage caused by hyperglycemia. In order to verify Moore's hypothesis, further check effectiveness of onions, and finally elucidate potential mechanism of onion in treating diabetes, in vitro inhibitory activities of onions as well as several referenced foods against type 2 diabetes related enzymes, α-glucosidase and α-amylase, were evaluated. The total phenolic contents and antioxidant activities of selected samples were also analyzed to seek a potential relationship between enzyme inhibitory activities and phenolic antioxidative components.
MATERIAL AND METHODS
Materials and Chemicals
Fresh white onion, fresh red onion, commercial dry onion powder, bitter melon, pumpkin, yam, and medlar were purchased from a local supermarket in Zhuhai, China. The edible part of each food was sampled. Porcine pancreatic α-amylase (EC 3.2.1.1), α-glucosidase (EC 3.2.1.20), p-nitrophenyl-α-glucopyranoside, and Trolox were supplied by Sigma Chemicals Co. (USA). 1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich Co. (Germany). Folin-Ciocalteu reagent was supplied by Sinopharm Chemical Reagent Co. Ltd. (Beijing, China). The other chemicals were purchased from Guangzhou Chemical Reagent Company (Guangzhou, China). Unless otherwise stated, all the chemicals used were of analytical grade.
Sample Preparation for Phenolic and Antioxidant Assay
Dry (0.5 g) or fresh (5 g) samples were extracted in a capped centrifuge tube with 5 mL of acetone/ water/ acetic acid (70:29.5:0.5, v/v/v). The mixture was shaken at 200 rpm at ambient temperature on an orbital shaker for 3 h followed by 12 h extraction overnight. The extracts were centrifuged at 5300 rpm (2983× g RCF) for 10 min, and the supernatants were transferred into new tubes. Residues were extracted with 5 mL of the respective solvents for the second time. Two times extracts were combined and stored at 4°C in the dark for assays of total phenolic content and antioxidant activity.[ Citation 10 ]
Total Phenolic Assay
The total phenolic content (TPC) was determined by a Folin-Ciocalteu assay[ Citation 11 ] using gallic acid (GA) as the standard. The mixture of the sample solution (50 μL), distilled water (3 mL), 250 μL of Folin-Ciocalteu's reagent solution, and 7% NaCO3 (750 μL) was vortexed and incubated for 8 min at room temperature. Then, a dose of 950 μL of distilled water was added. The mixture was allowed to stand for 2 h at room temperature. The absorbance was measured at 765 nm against distilled water as a blank. The total phenolic content was expressed as gallic acid equivalents (mg GAE/g sample) through the calibration curve of gallic acid. Linear range of the calibration curve was 10 to 200 μg/mL (r = 0.99).
DPPH Free Radical Scavenging Assay
DPPH free radical scavenging capacity of sample extract was evaluated according to the method of Djordjevic et al.[ Citation 12 ] with slight modifications. Briefly, the percent of DPPH· discoloration of the sample was calculated according to the equation: percent discoloration = [1 – (Asample /Acontrol )] × 100. The free radical scavenging activity of sample extract was expressed as an equivalent of that of L-ascorbic acid. Every sample was extracted in triplicate, and the results were expressed as micromoles L-ascorbic acid equivalents per gram of sample using the calibration curve of L-ascorbic acid. Linear range of the calibration curve was 0.01 to 1 mM (r = 0.99).
Sample Preparation for α-Glucosidase and α-Amylase Inhibitory Assay
Another portion of samples (10 g in fresh weight) was first extracted with 50 mL of 70% ethanol for 24 h at room temperature and filtered. After removing 70% ethanol extractable components, the sample residues were then extracted with 50 mL of boiling distilled water at 97°C for 20 min to obtain hot-water soluble components, such as polysaccharides. The filtrate of 70% ethanol extract was evaporated with a rotary evaporator to about 5 mL for removing organic solvent. The concentrated 70% ethanol extracts as well as the boiling water extracts were freeze-dried with a FreeZone Bench Top Freeze-Dryer System (Labconco Corporation, USA) at about −40°C. The dried powder of both 70% ethanol and boiling water extracts were re-dissolved in distilled water, respectively, and subjected to α-glucosidase and α-amylase inhibitory activity assays.
α-Amylase Inhibitory Assay
Alpha-amylase inhibitory activity of sample extract was evaluated according to the method of Kwon et al.,[ Citation 8 ] with slight modifications. A mixture of 200 μL of 20 mg/mL extract and 200 μL of 0.02 M sodium phosphate buffer (pH 6.9) containing porcine pancreatic α-amylase (EC 3.2.1.1) solution (0.5 mg/mL) were incubated at 25°C for 10 min. After pre-incubation, 200 μL of 1% starch solution in 0.02 M sodium phosphate buffer (pH 6.9) was added to each tube at 5-s intervals. The reaction mixtures were then incubated at 25°C for 10 min. The reaction was terminated by adding 400 μL of dinitrosalicylic acid (DNS) color reagent. The reaction mixtures in test tubes were then incubated in a boiling water bath for 5 min and then cooled to room temperature. The reaction mixture was then diluted after adding 4 mL distilled water and absorbance was measured at 540 nm. Then, 200 μL of distilled water (without amylase inhibitor) replaced 200 μL of the sample extracts; the other reagents were kept the same as the sample test, and such a reaction system was set up as control. To remove matrix sugar interference, the absorbance of the mixture consisted of 200 μL of sample (may contain sugars), 200 μL of PBS buffer (no amylase), 200 μL of starch, 400 μL DNS, and 4 mL of distilled water was recorded at 540 nm as blank. The α-amylase inhibitory activity was expressed as % inhibition and was calculated as the equation below:
α-Glucosidase Inhibitory Assay
Alpha-glucosidase inhibitory activity of sample extract was evaluated based on the method of Kwon et al.[ Citation 8 ] Briefly, a volume of 50 μL of 20 mg/mL sample extract and 100 μL of 0.1 M phosphate buffer (pH 6.9) containing α-glucosidase (EC 3.2.1.20) solution (1.0 U/mL) were incubated in a 96-well plate at 25°C for 10 min. After pre-incubation, 50 μL of 5 mM p-nitrophenyl-α-D-glucopyranoside solution in 0.1 M phosphate buffer (pH 6.9) was added to each well at 5-s intervals. The reaction mixtures were incubated at 25°C for 5 min. Absorbance was recorded at 405 nm by micro-array reader (Thermo Electron Co., USA) before and after incubation, and the absorbance of a control, which contained 50 μL of buffer solution instead of the extract, was recorded under the same experimental conditions. The % inhibition of α-glucosidase activity was calculated by the following equation:
Statistical Analysis
Means and standard deviations from replicates, as well as Pearson's correlation analyses, were done using Microsoft Excel 2003. Significant differences of the results among the variances were statistically analyzed by SPSS 14.0 software. Statistical significance was accepted at a level of p < 0.05.
RESULTS
Total Phenolic and Antioxidant Activity
The total phenolic contents (TPCs) of samples were presented in . The medlar exhibited the highest amount (693.3 mg GAE/100 g on dry weight basis) of TPC, followed by the bitter melon (644.0 mg GAE/100 g on dry weight basis). TPCs of white onion, red onion, and commercial onion powder were close, and ranged from 508.9 to 531.4 mg GAE/100 g on dry weight basis. As compared to the USDA national nutrient database, the values of TPCs in white onion (30.86 mg GAE/100 g on wet weight basis) and red onion (39.48 mg GAE/100 g on wet weight basis) in the present experiment were close to the values recorded in the database, in which white onion was 27 mg GAE/100 g and red onion was 48 mg GAE/100 g on wet weight basis, respectively.[ Citation 13 ] As compared to TPC values of the onions, pumpkin and yam exhibited relatively less phenolics, which contained 183.1 and 134.8 mg GAE/100 g on dry weight basis, respectively. The antioxidative activities of the samples were tested using the DPPH free radical scavenging assay. The commercial onion powder possessed the highest DPPH radical scavenging capacity followed by bitter melon, white onion, medlar, red onion, yam, and pumpkin on dry weigh basis ().
Table 1 Total phenolic contents and antioxidant activities of onions and reference foodsFootnote a
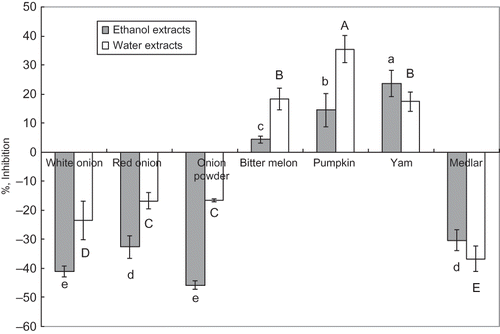
Porcine α-Amylase Inhibitory Activity
The extracts from seven samples were tested through the α-amylase inhibitory assay to predict a potential anti-diabetic effect. The results were shown in . Only bitter melon, pumpkin, and yam possessed an inhibitory effect against porcine α-amylase. The porcine α-amylase inhibitory activity of 70% ethanol extract from bitter melon was 4.4%, and the inhibitory activity of its boiling water extract was 18.3%. The inhibitory activity of 70% ethanol extract from pumpkin was 14.4%, and the inhibitory activity of its boiling water extract reached 35.5%. The inhibitory activity of 70% ethanol extract from yam reached to 23.6%; meanwhile, the inhibitory activity of its boiling water extract was 17.4%.
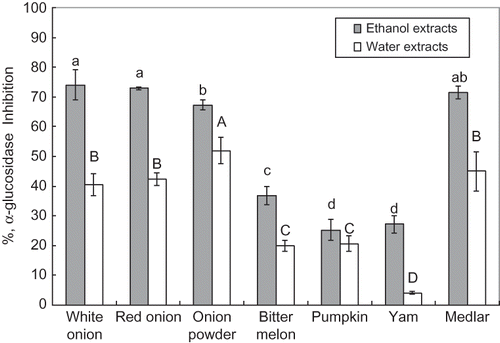
α-Glucosidase Inhibitory Activity
The extracts were also tested through the α-glucosidase inhibitory assay and the results were shown in . All 70% ethanol and boiling water extracts showed α-glucosidase inhibitory activities. Among 70% ethanol extracts, white onion exhibited the highest α-glucosidase inhibitory rate (74.0%), followed by red onion (72.9%) and medlar (71.5%). Their inhibitory effects were much more pronounced than those of commercial onion powder (67.3%), bitter melon (36.7%), yam (27.2%), and pumpkin (25.2%). Similarly, among boiling water extracts, the α-glucosidase inhibitory rates of commercial onion powder (52.0%), medlar (44.9%), red onion (42.3%), and white onion (40.5%) were apparently higher than those of pumpkin (20.7%), bitter melon (19.9%), and yam (4.1%). Furthermore, the α-glucosidase inhibitory activities of 70% ethanol extracts were higher than those of boiling water extracts. It was possible that the various classes of compounds in the extracts play different roles in α-glucosidase inhibition. This also indicated that the active components mostly existed in 70% ethanol extract rather than in boiling water extracts. However, hot water soluble components partially contributed the α-glucosidase inhibitory activities.
Correlation Between Phenolic Content, Antioxidant Activity, and α-Glucosidase Inhibitory Activity
Linear correlation analyses indicated that DPPH radical scavenging activity of samples was positively correlated with total phenolic content of the samples; the Pearson's correlation coefficient was 0.86 at p < 0.05. These results suggest that higher phenolic content does confer higher antioxidant activity linked to free radical scavenging potential. The inhibitory effect of bitter melon against digestive enzymes seemed not to depend on phenolic content or its antioxidative capacity. The potential reasons will be analyzed in the Discussion section. After excluding data of bitter melon, the correlation analyses between α-glucosidase inhibitory effect of 70% ethanol extracts or boiling water extracts and DPPH scavenging capacity or total phenolic content were conducted. The analyses of linear correlation between enzymes’ inhibitory activities and total phenolic content (not including bitter melon) showed that the inhibitory effects of samples against the activity of α-glucosidase could be due to the levels of phenolic compounds existing in the extracts. The Pearson's correlation coefficients () were 0.86, 0.94, 0.87, and 0.92 at p < 0.05, respectively. Inhibitory activities of 70% ethanol extracts or boiling water extracts from the samples against porcine α-amylase were negatively proportional to both DPPH scavenging capacity and total phenolic content, and Pearson's correlation coefficients () were −0.94, −0.83, −0.84, and −0.94 at p < 0.05, respectively.
Table 2 The correlation between various variables
DISCUSSION
Some previous studies have concluded that high dietary intake of fruits and vegetables could benefit a low risk of degenerative diseases due to the various antioxidative compounds presented in them. The most abundant types of antioxidative compounds in plants are phenolic compounds. This suggests that the phenolic compounds may play important roles in inhibitory effect on digestive enzymes. Actually, phenolic compounds have been reported to possess inhibitory activities on α-glucosidase or α-amylase in several cases. One study reported that flavonoids, such as quercetin and anthocyanins from the Allium cepa (white and red onion), had inhibitory activity on α-amylase.[ Citation 14 ] Another investigation showed that the leaves of Gymnema montanum were rich in phenolic composition and these phenolics positively correlated with inhibitory effect of α-glucosidase and α-amylase activity.[ Citation 15 ] One more case was tea catechines, especially (-)-epigallocatechine gallate, which possessed anti-diabetes effects.[ Citation 16 ] Hence, it is rational to conjecture the presence of phenolics would contribute toward α-glucosidase and/or α-amylase inhibition. In the current study, onions and several referenced foods were tested for their α-glucosidase and α-amylase inhibitory activities, their total soluble phenolic contents and antioxidant activities were further tested for comparison purposes. Medlar and onions exhibited considerable higher total phenolic contents, free radical scavenging activities, and α-glucosidase inhibitory activities. The results from a current in vitro study provide the biochemical rationale that phenolics in samples are likely to link their antioxidative capacities. This suggests that the consumption of phenolic-rich foods like onion and medlar protects against human diseases associated with oxidative stress.
The correlation analyses showed that α-glucosidase inhibitory effects of both 70% ethanol extracts and boiling water extracts positively correlated with DPPH scavenging capacities, and positively correlated with total phenolic content when bitter melon was excluded from analyses. This could be counted as the biochemical rationale that phenolic-linked ingredients of samples have the potential for α-glucosidase inhibition, this indicates the potential to reduce glucose absorption. Further, both 70% ethanol extracts and boiling water extracts exhibited inhibitory activities against α-glucosidase, which indicate that the active substances may not be a single class of substances. Namely, both phenolic compounds from 70% ethanol extract and water soluble polysaccharides from boiling water extracts may play the roles in α-glucosidase inhibitory activity. Therefore, the extracting or eating methods can directly influence glucose absorption. In other words, the way of food intake is very important in gaining health promoting effects from foods. Current results showed that 70% ethanol extract from onion significantly suppressed α-glucosidase activity, and the inhibitory activity was much higher than boiling water extracts from onions. This may partially explain that onion marinated in red wine has a good curative effect for diabetes.
On the other hand, only 6 of the 14 sample extracts showed positive inhibitory effects against porcine α-amylase. The other eight of the sample extracts showed negative values, which indicated that no inhibition occurred in the current experimental system. Some of the negative values were quite large, e.g., the 70% ethanol extract and hot water extract of commercial onion powder gave a negative value of −45.8 and −16.6%, respectively, which indicated that the porcine α-amylase was activated rather than inhibited. If this were to occur in vivo, it would aggravate rather than alleviate the diabetic condition since the rate of maltose production would be increased and thereby the serum levels have a risk to rise more rapidly if α-glucosidase activity is also activated. However, an increase in the reaction product levels may be due to a conformational change derived from binding of compounds to the enzyme.[ Citation 17 ,Citation 18 ] In addition, starch is digested to glucose in two basic steps and α-amylase only contributes to partial hydrolysis of starch rather than complete hydrolysis. In the human body, α-amylase first reduces starch to three different oligosaccharides—maltose, maltotriose, and dextrin. While α-glucosidase ultimately hydrolysis oligosaccharides into glucose monomers, the glucoses are then transported into the enterocytes in the small intestine. Onion displayed the inhibitory activity against α-glucosidase, and thus even it activates α-amylase, the final overall effects could be inhibitory activity in terms of conversion starch to glucose. This verified previous studies in which onion exhibited in vitro and in vivo pharmacological activities, which might benefit to diabetes. However, as more studies were done, researchers found that the properties of different Allium species varied, and not all onions showed favorable inhibitory effect on porcine α-amylase. In the Bahman and Nasibeh's study,[ Citation 19 ] among six Allium species tested, four species were found to possess favorable inhibitory effects on hydrolysis of starch in vitro, while the other two did not display valuable inhibitory activity.
The truth, food possesses an inhibitory effect against α-glucosidase activity while does promotion effect against α-amylase, is not restricted to the current research. Some researchers also observed that some foods did not show α-glucosidase and α-amylase inhibitory activities simultaneously. For instance, Ranilla found that purple corn (Zea mays L.) exhibited high free radical scavenging-linked antioxidant activity and possessed the high total phenolic content and α-glucosidase inhibitory activity, but did not exhibit α-amylase inhibitory activity.[ Citation 20 ] Another study suggested that cinnamic acid derivatives inhibited rat intestinal α-glucosidase activity but activated pancreatic α-amylase activity.[ Citation 21 ]
Phenolic content in samples was positively related to α-glucosidase inhibitory activity, but negatively related to porcine pancreatic α-amylase inhibitory activity (p < 0.05). Although further experiments are required to identify the active compounds in sample extracts, it can be proposed that phenolic compounds in onion, such as quercetin, gallic acid, protocatechuic acid, and caffeic acid, are possible active compounds linked to α-glucosidase inhibitory activity of onion. However, the polysaccharides are also likely active compounds, because after removing of 70% ethanol extractable components, the boiling water extract from the residues still possess certain α-glucosidase inhibitory activity in some cases.
Another phenomenon implied that phenolic compounds might not be the only class of active compounds to contribute to the anti-diabetes property of food. Bitter melon was not involved in correlation analyses due to its unique chemical compositions and effects. In the α-glucosidase inhibitory assay, it showed much lower inhibitory activity than that expected. Comparing to onions and medlar, bitter melon exhibited the similar antioxidative capacity and TPC (both on dry weight basis). In light of the above hypothesis—phenolic compounds may be the active compounds linked to α-glucosidase inhibitory activity, these samples should have similar results, while the fact was not. Furthermore, in the porcine α-amylase inhibitory assay, the response of bitter melon was opposite to onions and medlar. There are a couple of possible reasons to explain these phenomena. To start with, not all the phenolic compounds contribute to the inhibitory effects on enzymes and only some specific types have such ability. Second, plants act on various mechanisms either by acting directly on pancreas and stimulate insulin levels in blood or by inhibiting the activities of key enzymes in different pathways like glycolysis, gluconeogensis and thus having favorable effect on controlling diabetes.[ Citation 22 ] Bitter melon may have different mechanism from other samples in this study, and thus it has shown different degrees of anti-diabetic activity. Based on the book, “Diabetes to Wholeness: A Natural and Spiritual Approach to Disease Prevention & Healing!”, at least three different active compounds in bitter melon have been reported to have blood glucose lowering or other actions of potential benefit in diabetes. These include alkaloids, insulin-like peptides (polypeptide-p), and a mixture of steroidal saponins known as charantin. It is still unclear which of these is the most effective, or whether all three compounds work together.[ Citation 23 ]
CONCLUSIONS
The traditional popular folk remedies have mentioned several antidiabetic foods. Many of these foods are used for the treatment of diabetes with no mechanistic basis known of their functionality. The present investigation shows that the antidiabetic properties of onions and medlar, least in part, can be related with their α-glucosidase inhibitory effects. These results also indicate that there is positive linear correlation between total phenolic content and α-glucosidase inhibitory effects. However, phenolic compounds may not be the only class of active compounds to contribute to antidiabetes effects of onions.
ACKNOWLEDGMENTS
This work was supported by a grant (No. UIC-R201003) from Beijing Normal University–Hong Kong Baptist University United International College.
REFERENCES
- Bhandari , M.R. , Niubon , J. , Gao , H. and Kawabata , J. 2008 . α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliate Haw) . Food Chemistry , 106 : 247 – 252 .
- Heo , S.J. , Hwang , J.Y. , Choi , J.I. , Han , J.S. , Kim , H.J. and Jeon , Y.J. 2009 . Diphlorethohydroxycarmalol isolated from Ishige okamurae, brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice . European Journal of Pharmacology , 615 : 252 – 256 .
- Bailey , C.J. 2003 . “ New approaches to the pharmacotherapy of diabetes ” . In Textbook of Diabetes , Edited by: Pickup , J.C. and William , G. Vol. 2 , 73.1 – 73.21 . UK : Blackwell Science Ltd . Third
- Fujisawa , T. , Ikegami , H. , Inoue , K. , Kawabata , Y. and Ogihara , T. 2005 . Effect of two alpha-glucosidase inhibitors, voglibose and acarbose, on postprandisl hyperglycemia correlates with subjective abdominal symptoms . Metabolism , 54 : 387 – 390 .
- Shobana , S. , Sreerama , Y.N. and Malleshi , N.G. 2009 . Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase . Food Chemistry , 115 : 1268 – 1273 .
- Kim , J.S. , Hyun , T.K. and Kim , M.J. 2011 . The inhibitory effects of ethanol extracts from sorghum, foxtail millet and proso millet on α-glucosidase and α-amylase activities . Food Chemistry , 124 : 1647 – 1651 .
- Wang , H. , Du , Y.J. and Song , H.C. 2010 . a-Glucosidase and a-amylase inhibitory activities of guava leaves . Food Chemistry , 123 : 6 – 13 .
- Kwon , Y.I. , Apostolidis , E. and Shetty , K. 2008 . In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension . Bioresoure Technology , 99 : 2981 – 2988 .
- Moore, J. Chinese medical approaches to the treatment of diabetes mellitus. 2008 http://www.docin.com/p-10098009.html (http://www.docin.com/p-10098009.html)
- Xu , B.J. and Chang , S.K.C. 2007 . Comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents . Journal of Food Science , 72 ( 2 ) : S159 – S166 .
- Singleton , V.L. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent . Methods in Enzymology , 299 : 152 – 178 .
- Djordjevic , T.M. , Siler-Marinkovic , S.S. and Dimitrijevic-Brankovic , S.I. 2010 . Antioxidant activity and total phenolic content in some cereals and legumes . International Journal of Food Properties , 14 ( 1 ) : 175 – 184 .
- USDA National Nutrient Database: Oxygen Radical Absorbance Capacity (ORAC) of selected Foods—2007 www.ais.usda.gov/nutrientdata (http://www.ais.usda.gov/nutrientdata)
- Mohamed , G.A. and Alliuocide , G. 2008 . A new flavonoid with potent α-amylase inhibitory activity from Allium cepa L. Archive for Organic Chemistry 202 – 209 . XI
- Ramkumar , K.M. , Thayumanavan , B. , Palvannan , T. and Rajaguru , P. 2009 . Inhibitory effect of Gymnema montanum leaves on α-glucosidase activity and α-amylase activity and their relationship with polyphenolic content . Medicinal Chemistry Research , 19 ( 8 ) : 948 – 961 .
- Kao , Y.H. , Chang , H.H. , Lee , M.J. and Cheng , C.L. 2006 . Tea, obesity, and diabetes . Molecular Nutrition & Food Research , 50 ( 2 ) : 188 – 210 .
- Kim , J.S. , Kwon , C.S. and Son , K.H. 2000 . Inhibition of α-glucosidase and amylase by luteolin, a flavonoid . Bioscience, Biotechnology, and Biochemistry , 64 : 2458 – 2461 .
- Ali , H. , Houghtona , P.J. and Soumyanath , A. 2006 . α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus . Journal of Ethnopharmacology , 107 : 449 – 455 .
- Bahman , N. and Nasibeh , Y. 2009 . Inhibitory effects of six Allium species on α-amylase enzyme activity . Journal of Pharmacy Research , 8 ( 1 ) : 53 – 57 .
- Ranilla , L.G. , Apostolidis , E. , Genovese , M.I. , Lajolo , F.M. and Shetty , K. 2009 . Evaluation of indigenous grains from the Peruvian Andean Region for antidiabetes and antihypertension potential using in vitro methods . Journal of Medicinal Food , 12 : 704 – 713 .
- Adisakwattana , S. , Chantarasinlapin , P. , Thammarat , H. and Yibchok-Anun , S. 2009 . A series of cinnamic acid derivatives and their inhibitory activity on intestinal α-glucosidase . Journal of Enzyme Inhibition and Medicinal Chemistry , 24 : 1194 – 1200 .
- Kumar , B.D. , Mitra , A. and Manjunatha , M. 2009 . In vitro and in vivo studies of antidiabetic Indian medicinal plants: A review . Journal of Herbal Medicinal Toxicology , 3 ( 2 ) : 9 – 14 .
- Ley , B.M. 2002 . Diabetes to Wholeness: A Natural and Spiritual Approach to Disease Prevention & Healing!; , Detroit Lakes , MN : BL Publications .