Abstract
Three Himalayan medicinal plants (Habenaria intermedia, H. edgeworthii, and Roscoea procera), widely used in vitality strengthening Ayurvedic formulations in India, were assessed for nutritional phytochemical constituents, and antioxidant activity. These target species emerged as a good source of minerals and possessed important micro elements. Individually, H. intermedia contained a high content of total phenols, thiamins, tannins, and calcium; R. procera was rich in potassium and iron content; and H. edgeworthii emerged as a good source of sodium. While various antioxidant assays provided evidences on the antioxidant potential of target species, greater antioxidant potential of H. intermedia as compared to the other two species was revealing. This study, therefore, highlighted the possibilities of harnessing nutritional and antioxidant potential of these species.
INTRODUCTION
Among traditional Ayurvedic medicines, ‘Astavarga’ (a group comprising of eight medicinal plants, namely, Ridhi [Habenaria intermedia], Vriddhi [H. edgeworthii], Jeevak [Malaxis acuminata], Rishvak [M. muscifera], all four in family Orchidaceae; Kakoli [Roscoea procera—family Zingiberaceae]; and Kshirakakoli [Lilium polyphyllum], Meda [Polygonatum verticillatum], and Mahameda [P. cirrhifolium], all three in family Liliaceae) is well recognised for strengthening vital force of the body, cell regeneration capacity, and immunity system.[ Citation 1 − Citation 3 ] Individually, these species have been reported highly efficacious in a range of serious health problems, including constipation, heart diseases, urinary disorders, diabetes, fever, healing fractures, and abnormal thirst.[ Citation 4 − Citation 6 ] Owing to their medicinal values, ‘Astavarga’ plants are used in different forms, such as Taila (oils), Gritam (medicated clarified butters), and Churna (powders), in the traditional Indian System of Medicine (ISM).[ Citation 2 ] Among others, the group forms a major constituent of Chyavanprash (a herbal combination used in ISM), widely known to protect degenerative diseases and to maintain youthfulness, vigour, vitality, etc.[ Citation 2 ,Citation 6 ]
With the growing awareness among consumers, the natural antioxidants are attracting attention of users and researchers. In this context, among others, the medicinal plants are considered as an easily available natural source of antioxidants.[ Citation 7 ] However, detailed screening for chemical composition and antioxidant activity in most of the known medicinal plants is lacking. The ‘Astavarga’ species are no exception.
Considering this gap in systematic investigation on nutritional and antioxidant potential, three ‘Astavarga’ species, namely, H. intermedia, H. edgeworthii, and R. procera were investigated. The target species occurs between an altitude of 1700–2800 m asl in the Himalayan region. Besides being used in different medicinal formulations, these species are also consumed as wild edible.[ Citation 1 ,Citation 5 ,Citation 8 ] The details of their ethnotherapeutical and medicinal importance are given in . Considering the proven traditional uses as medicine and food supplements, the present study, for the first time, attempts to systematically investigate nutritional and antioxidant potential of the target species from the Indian Himalayan Region (IHR).
Table 1 Ethnobotanical uses and medicinal importance of selected medicinal plants used in Astavarga
MATERIAL AND METHODS
Plant Material
Tubers/rhizomes of the target species were collected during August 2007 from Dhanoulti (N 30° 27′ 02″; E 78° 15′ 07″; 2100 m asl), district Tehri in Uttarakhand (Indian west Himalaya). Voucher specimens, identified after consulting the herbarium at Botanical Survey of India, Dehradun were deposited in the herbarium of G. B. Pant Institute of Himalayan Environment and Development (GBPIHED), Kosi-Katarmal, Almora, India. The collected plant material was dried in a hot air oven (55°C) and powdered using a grinder mill (Macro Scientific, India). Powdered samples were stored in airtight polythene bags containing silica gel in the dark at room temperature (20−25°C) till further analysis.
Nutritional and Phytochemical Analysis
The powdered samples (10 g) were extracted in 100 ml of 20% v/v acetic acid (in ethanol) for 4 h to determine alkaloids. The extract was filtered to remove cellulose debris and then concentrated to about one-fourth of the original volume. One percent NH4OH was added drop by drop until a precipitate occurred. After filtration, the crude alkaloid in precipitated form was obtained.[ Citation 9 ] Total phenolic content of methanolic extracts (80%) were quantified by using Folins-Ciocalteu reagents method[ Citation 10 ] with minor modification.[ Citation 11 ] Gallic acid was used as standard for quantification and results were expressed as gallic acid equivalent per gram dry weight. Total flavoniod content was determined following aluminium chloride method.[ Citation 12 ] Quercetin was used as standard for quantification and results were expressed as quercetin equivalent per gram dry weight. Tannin content was estimated by using the Folins-Dannis reagent method.[ Citation 13 ] In methanolic extract, an equal volume of Folins-Dannis reagent was added and neutralized with saturated sodium carbonate solution. The standard curve of tannic acid was used for quantification and results were expressed as tannic acid equivalent per gram dry weight. Crude fat was determined by extracting 2.0 g plant material with diethyl ether in a soxhlet extractor for 6 h, and after drying the solvent, extracted crude fat was estimated.[ Citation 14 ] Crude fibre was determined on defatted samples, which involved a sequential extraction with 1.25% NaOH, drying the residue (4 h; 102°C) followed by incineration (30 min; 600°C). The difference between dry weight and ash content of the residue was taken as an estimation of the crude fibre content.[ Citation 15 ] For quantification of thiamine content, samples (5 g) were homogenized with 50 ml of ethanolic sodium hydroxide and filtered. In 10 ml of filtered solution, 10 ml of potassium dichromate was added for colour development, and the resulting absorbance was measured at 360 nm.[ Citation 16 ] For riboflavin content, each sample (5 g) was extracted with 100 ml of 50% ethanol solution and shaken for 1 h; the solution was filtered. Then, 10 ml of this extract was added to 10 ml of 5% potassium permanganate (KMNO4). Thereafter, 10 ml of 30% hydrogen peroxide (H2O2) was added and allowed to stand over a hot water bath for 30 min. The total volume (50 ml) was made by adding 40% sodium sulphate. Absorbance was measured at 510 nm in a spectrophotometer (Hitachi U-2001, Japan).[ Citation 16 ]
Analysis of Mineral Elements
Potassium (K), sodium (Na), calcium (Ca), and lithium (Li) were determined by a flame photometer (Systronic-128) following Allen.[ Citation 17 ] The microelements (i.e., copper [Cu], zinc [Zn], iron [Fe], magnesium [Mg], and cobalt [Co]) were determined using Atomic Absorption Spectrophotometer (Varian AA 28-0Z).
HPLC Analysis of Phenolic Compounds
Analysis was conducted using a HPLC system (Merck Hitachi) equipped with an L-7100 series pump and L-7400 series UV-VIS detector; facilitated with Winchrome 99 software (Infotech Instrument, Mumbai, India). The chromatographic column 250 × 4.6 mm i.d., Lichrosphere® 100; RP-8e (5 μM) column (Merck Pvt. Ltd., Germany) was used. The mobile phase was water:methanol:acetic acid (80:20:1) in isocratic mode, flow rate was 0.8 ml per minute and injection volume was 20 microliter. The spectra of gallic acid, catechin, 3-hydroxybenzoic acid, ρ-coumaric acid, and ellagic acid were recorded at 254 nm and ρ-caffeic acid and chlorogenic acid at 370 nm. The identification of phenolic compounds was done with respect to retention time of corresponding external standard. UV-VIS spectra of pure standard at different concentrations were used for plotting standard calibration curve for quantification of phenolic compounds. The repeatability of quantitative analysis was <3.0% for each phenolic compound. The results were expressed as milligram per 100 g of dry weight.
Antioxidant Activity
Radical scavenging activity by ABTS assay
Total antioxidant activity was measured following improved 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method as described in Cai et al.[ Citation 18 ] ABTS salt (7.0 μM) and potassium persulphate (2.45 μM) was added for the production of ABTS cation (ABTS· +) and kept in the dark for 16 h at 23°C. ABTS· + solution was diluted with 80% (v/v) ethanol till an absorbance of 0.700 ± 0.005 at 734 nm was obtained. Diluted ABTS· + solution (3.90 ml) was added in 0.10 ml of methanolic extract, and the resulting mixture was homogenised thoroughly. The reaction mixture was allowed to stand for 6 min in the dark at 23°C and absorbance was recorded at 734 nm using UV-VIS spectrophotometer with respect to a blank that was prepared with 0.10 ml 80% (v/v) methanol. A standard curve of various concentrations of ascorbic acid was prepared in 80% v/v methanol for the equivalent quantification of antioxidant potential with respect to ascorbic acid. Results were expressed in millimole (mM) ascorbic acid equivalent (AAE) per 100 g dry weight.
Radical scavenging activity by DPPH assay
Traditional DPPH (1,1-diphenyl-2-picrylhydrazyl) assay was carried out following Brand-William et al.[ Citation 19 ] with minor modification.[ Citation 11 ] Briefly, a 25 ml of 400 mM DPPH was added in 25 ml of 0.2 M MES buffer (pH 6.0 adjusted with NaOH) and 25 ml 20% (v/v) ethanol. A DPPH cation solution (2.7 ml) was mixed with 0.9 ml of sample extract and kept in the dark at room temperature for 20 min. Reduction in the absorbance at 520 nm was recorded by UV-VIS spectrophotometer. Results were expressed in millimole (mM) ascorbic acid equivalent (AAE) per 100 g dry weight.
Reducing ability by FRAP assay
Ferric reducing antioxidant power (FRAP) assay was performed following Faria et al.[ Citation 20 ] with minor modifications.[ Citation 11 ] FRAP reagent was prepared by adding 10 vol. of 300 mM acetate buffer (i.e., 3.1 g of sodium acetate and 16 ml of glacial acetic acid per liter), 1 vol. of 10 mM 2,4,6-tri-2-pyridyl-1,5-triazin (TPTZ) in 40 mM HCl and 1 vol. of 20 mM ferric chloride. The mixture was preheating at 37°C and 3.0 ml of the mixture added to 0.10 ml methanolic extract and kept at 37°C for 8 min. Absorbance was taken at 593 nm by using UV-VIS spectrophotometer. A blank was prepared by ascorbic acid and results were expressed in millimole (mM) of ascorbic acid equivalent (AAE) per 100 g dry weight.
RESULTS AND DISCUSSION
Nutritional and Phytochemical Constituents
Significant variations (p < 0.01) in phytochemicals was revealing among the target species (). While alkaloids were recorded more in Habenaria species (H. intermedia 0.52 mg/g and H. egdeworthii 0.47 mg/g), tannin content was found to be significantly more (p < 0.01) in the case of R. procera (4.75 mg tannic acid equivalent/g dry weight). Habenaria species also exhibited more total phenolic content (H. intermedia: 6.89 mg gallic acid equivalent/g and H. egdeworthii: 5.31 mg gallic acid equivalent/g dry weight) whereas total flavonoids were more in the case of R. procera (7.64 mg quercetin equivalent/g dry weight). Crude fibre and fat was observed at maximum in the case of H. egdeworthii (4.61 mg/g and 6.27 mg/g dry weight, respectively). H. intermedia possessed significantly more thiamine content (8.46 mg/g dry weight) as compared to H. egdeworthii (5.75 mg/g dry weight) and R. procera (4.75 mg/g dry weight). Riboflavin was recorded at the highest in R. procera (3.80 mg/g dry weight).
Table 2 Phytochemical composition of target species in Indian Himalayan region
While comparing with other reports, the total phenolic content of R. procera was comparable with the reported values in other Zingiberaceae members, such as Alpinia galanga, Curcuma zedoaria, C. aeruginosa, C. mangga, Etlingera maingayi, Hedychium spicatum, Kaempferia galanga, and Zingiber spectabile.[ Citation 11 ,Citation 21 ,Citation 22 ] R. procera, however, possessed higher amount of total phenolic content as compared to reported values for Alpinia chinensis, Curcuma longa, C. xanthorhiza, Etlingera elatior, and Zingiber officinale.[ Citation 21 ,Citation 23 ,Citation 24 ] Regarding family Orchidaceae very few reports are available on phytochemicals. Specifically considering the genus Habenaria studies have mainly focused on H. petalodes, H. intermedia, and H. edgeworthii.[ Citation 25 ,Citation 26 ] Total phenolic content in the case of Habenaria edgeworthii has been reported as 4.90 mg/g dw in tubers collected from wild sources,[ Citation 27 ] which is comparatively lower than in the present study. The present values of total phenolic content in H. intermedia and H. edgeworthii were comparable with values reported for some well known medicinal plant species in the region, such as Acorus calamus, Aconitum heterophyllum, Artimisia abrotanum, Ocimum sanctum, Hedychium spicatum, etc.[ Citation 11 ,Citation 21 ]
Among phytochemicals, the flavonoids represent a most common and widely distributed group of plant phenolics[ Citation 28 ] and are generally known as free radical scavengers preventing oxidative cell damage and having strong anticancer activity. Likewise, alkaloids are generally known as most efficient therapeutic phytochemicals used for analgesic, antispasmodic, and antibacterial properties.[ Citation 29 ] Presence of phenolic content and alkaloids can be attributed to beneficial effects of target species in curing various diseases and enhancing the nutritional value. Such reports are available world over for different species of plants, thus determining their potential in food and pharmaceutical industries.[ Citation 30 ,Citation 31 ]
Mineral Elements
In spite of significant (p < 0.01) variation amongst the species, target species have emerged as good source of potassium, sodium and calcium (). H. intermedia contains a higher amount of calcium (792.40 mg/100 g dry weight) and R. procera was found rich in potassium (410.45 mg/100 g dry weight). However, H. egdeworthii contains the highest amount of sodium (62.90 mg/100 g dry weight). Among the micronutrients, Roscoea procera revealed maximum content of iron (95.91 mg/100 g dry weight), magnesium (12.43 mg/100 g dry weight), zinc (9.28 mg/100 g dry weight), and lithium (5.18 mg/100 g dry weight). However, H. intermedia contains a higher amount of cobalt (7.58 mg/100 g dry weight). While comparing present results with available reports on mineral contents following was revealing: (1) the target species contained more mineral elements as compared to many other medicinal plants in the region;[ Citation 14 ] (ii) mineral contents of target species were comparable with some of the well known wild edible species of IHR;[ Citation 32 − Citation 34 ] and (iii) mineral contents of the target species were also comparable with highly valued (medicinal as well as nutritional) Berberis species fruits in the region.[ Citation 35 ] Therefore, realizing that the mineral contents play important role in human nutrition[ Citation 36 ,Citation 37 ] the target species have emerged as a good source of minerals. Further, these species also possess micro elements, such as magnesium and zinc, which are required in many enzyme-catalyzed reactions and plasma and extracellular fluid to maintain osmotic equilibrium.[ Citation 14 ,Citation 38 ]
Table 3 Mineral elements of target species in Indian Himalayan region
Phenolic Compounds
Species specific responses in phenolic compounds were revealing (). For example, gallic acid was observed highest in R. procera (92.84 mg/100 g dry weight), whereas hydroxybenzoic acid was detected only in H. intermedia (18.51 mg/100 g dry weight) and H. edgeworthii (7.56 mg/100 g dry weight). Likewise, catechin and ρ-coumaric acid were detected only in R. procera (12.05 and 0.14 mg/100 g dry weight, respectively). Phenolic compounds are the most diverse group of phytochemicals responsible for various health benefits.[ Citation 28 ] They act as antioxidants by donating a hydrogen atom, as an acceptor of free radical, by interrupting chain oxidation reaction or chelating metals.[ Citation 39 ] Among the different phenolic compounds, gallic acid (3,4,5-tri-hydroxy benzoic acid) with three hydroxyl group bonded to aromatic ring in the ortho position possess more scavenging properties toward DPPH. Chlorogenic acid is an antioxidant, and is effective in preventing oxidative injury to human gastric epithelial cell in vitro, however, it is poorly absorbed in the human body.[ Citation 40 ] Catechins possess a similar ortho- dihydroxy moiety bonded to the aromatic ring in ortho position and also have more affinity toward free radicals. The lowest antiradical and antioxidant activity of some compounds having one hydroxyl group, such as 3-hydroxy benzoic acid, 2-hydroxy benzoic acid (salicylic acid), and ρ-coumeric acid (4-hydroxy cinnamic acid), is due to the fact that antioxidant and anti radical activity of phenolic acid has positively correlated with a number of hydroxyl groups to the aromatic ring.[ Citation 41 ]
Table 4 Phenolic compounds (mg/100 g dry weight) in target species in IHR
Antioxidant Activity
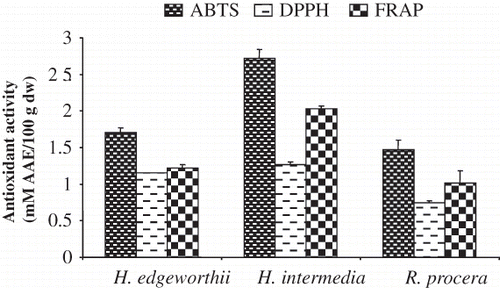
Antioxidant activities, assessed by three different in vitro assays, showed significant (p < 0.01) variations among species (). ABTS radical scavenging assay showed significantly (p < 0.01) greater antioxidant potential in H. intermedia (2.723 mM AAE/100 g dry weight) followed by H. edgeworthii (1.709 mM AAE /100g dry weight) in methanol extract. The other two antioxidant assays followed similar trends and revealed significantly higher antioxidant activity in the case of H. intermedia as compared to H. egdeworthii and R. procera. These results of are also in agreement with Surveshwaran et al.,[ Citation 21 ] who reported a range of antioxidant activity (0.75 to 2.50 mM TAEC/100 g) in methanolic extract, using the similar in vitro antioxidant assays, in a number of medicinal plants of India (i.e., Aconitum heterophyllum, A. forex, Acorus calamus, Alpinia chinensis, A. galanga, Kaempferia galanga, Nardostachys jatamansi, and Valeriana officinalis, etc.). Likewise rhizome samples of Hedychium spicatum collected from different localities of IHR revealed similar values of antioxidant activity using the same antioxidant assays (ABTS assay: 2.19 mM; DPPH assay: 0.84 mM; FRAP assay: 1.19 mM AAE/100 g dry weight).[ Citation 11 ]
CONCLUSION
In spite of the fact that all the target species have emerged as a source of minerals, phytochemicals, and possess higher antioxidant properties, H. intermedia appears a potent source of total phenols, thiamine, tannins, and calcium, R. procera contained a higher amount of potassium and iron, and H. egdeworthii emerged as a good source of sodium. Presence of various phenolic compounds, such as gallic acid, hydroxyl benzoic acid, catechin, and ρ-coumaric acid, in these species would serve as potent free radical scavengers, thereby establishing these species as a promising source of antioxidants. The information generated in this study would enrich the existing information base on antioxidant activities of Himalayan medicinal plants.
ACKNOWLEDGMENTS
The authors wish to thank Dr. L.M.S. Palni, the Director of the Institute for providing facilities and encouragement. Thanks are also due to colleagues of Biodiversity Conservation and Management Thematic group for their support and help. Comments of an anonymous reviewer on an earlier manuscript helped improve content. The partial financial support from National Medicinal Plants Boards, New Delhi (File No. Z.18017/187/Pr.GO/UA-01/2006-07) and Uttarakhand Council of Science and Technology, Dehradun (File No. UCS andT/RandD/LS-21/06/775) is greatly acknowledged.
REFERENCES
- Shah , R. 2006 . Nature's Medicinal Plants of Uttaranchal: Herbs, Grasses and Ferns , 1 – 531. . Nainital , , India : Vol. II; Gyanodaya Prakashan .
- Dhyani , A. , Nautiyal , B.P. and Nautiyal , M.C. 2010 . Importance of Astavarga plants in traditional systems of medicine in Garhwal, Indian Himalaya . International Journal of Biodiversity Science and Ecosystem Management , : 1 – 7 . iFirst doi: 10.1080/21513732.2010.521490
- Singh , A.P. 2007 . Astavarga-Ayurvedic drug of controversial origin . Journal of Medicinal and Aromatic Plant Science , 29 : 35 – 39 .
- Dey , A.C. 1988 . “ Bishen Singh Mahindra Pal Singh ” . In Indian Medicinal Plants in Ayurvedic Preparations Dehradun , , India
- Prajapati , N.S. , Purohit , S.S. , Sharma , A.K. and Kumar , T. 2003 . A Handbook of Medicinal Plants: A Complete Source Book , Jodhpur , , India : Agrobios .
- Govindarajan , R. , Singh , D.P. and Rawat , A.K.S. 2007 . High performance liquid chromatographic method for the quantification of phenolics in ‘Chyavanprash’, a potent Ayurvedic drug . Journal of Pharmaceutical and Biomedical Analysis , 43 : 527 – 532 .
- Ramlaxmi , K. , Kubno , I.R. and Rao , J.M. 2008 . Antioxidant potential of low grade coffee beans . Food Research International , 41 : 96 – 103 .
- Das , V.B. 1994 . Materia Medica of Tibetan Medicine , Vol. 130 , Delhi , India : Sai Satguru Publication .
- Obadoni , B.O. and Ochuko , P.O. 2001 . Phytochemical studies and comparative efficacy of crude extracts of some homeostatic plants of Edo and Delta states of Nigeria . Global Journal of Pure and Applied Sciences , 8 : 203 – 208 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Rawat , S. , Bhatt , I.D. and Rawal , R.S. 2011 . Variation in total phenolic content and antioxidant potential of Hedychium spicatum Buch . Ham. ex D. Don in west Himalaya, India. Journal of Food Composition and Analysis , 24 : 574 – 579 .
- Chang , C.C. , Yang , M.H. , Wen , H.M. and Chern , J.C. 2002 . Estimation of total flavonoid content in Propolis by two comparative colorimetric methods . Journal of Food and Drug Analysis , 10 : 178 – 182 .
- Nwinuka , N.M. , Ibeh , G.O. and Ekeke , G.I. 2005 . Proximate composition and levels of some toxicants in four commonly consumed species . Journal of Applied Science, Environment and Management , 9 : 150 – 155 .
- Indrayan , A.K. , Sharma , S. , Durgapal , D. , Kumar , N. and Kumar , M. 2005 . Determination of nutritive value and analysis of mineral elements for some medicinally valued plants from Uttaranchal . Current Science , 89 : 1252 – 1255 .
- Rangana , S.C. 1979 . “ Tata McGraw Hill Publishing Co Ltd ” . In Manual of Analysis of Fruit and Vegetable Products New Delhi , India
- Okwu , D.E. and Morah , F.N. 2004 . Mineral and phytochemical values of Dennrttia tripelata . fruits , 59 : 437 – 442 .
- Allen , E.S. 1989 . Chemical Analysis of Ecological Material , Oxford : Blackwell Scientific Publication .
- Cai , Y. , Luo , Q. , Sun , M. and Corke , H. 2004 . Antioxidant activity and phenolic compounds in 112 traditional Chinese medicinal plants associated with anticancer . Life Science , 75 : 2157 – 2184 .
- Brand-William , W. , Cuvelie , M.E. and Berset , C. 1995 . Use of a free radical method to evaluate antioxidant activity . Lebensmittel Wissenschaft Technologie , 28 : 5 – 30 .
- Faria , A. , Oliveira , J. , Neves , P. , Gamerio , P. , Santos-Buelga , C. , De-Freitas , V. and Mateus , N. 2005 . Antioxidant properties of prepared blueberry (Vaccinium myrtillus) extracts . Journal of Agricultural and Food Chemistry , 53 : 6896 – 6902 .
- Surveswaran , S. , Cai , Y. , Corke , H. and Sun , M. 2007 . Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants . Food Chemistry , 102 : 938 – 953 .
- Bhatt , I.D. , Prasad , K. , Rawat , S. and Rawal , R.S. 2008 . Evaluation of antioxidant phytochemical diversity in Hedychium spicatum: A high value medicinal plant of Himalaya . Pharmacognosy Magazine , 4 : S202 – S205 .
- Vankar , P.S. , Tiwari , V. , Singh , L.W. and Swapana , N. 2006 . Antioxidant properties of some exclusive species of Zingiberaceae family of Manipur . Electronic Journal of Environment, Agriculture and Food Chemistry , 5 : 1318 – 1322 .
- Chen , E.W.C. , Lim , Y.Y. , Wong , L.F. , Lianto , F.S. , Wong , S.K. , Lim , K.K. , Joe , C.E. and Lim , T.Y. 2008 . Antioxidant and Tyrosinase inhibition properties of leaves and rhizomes of ginger species . Food Chemistry , 109 : 477 – 483 .
- Cota , B.B. , Magalhaes , A. , Pimenta , A.M.C. , Siqueira , E.P. , Alves , T.M.A. and Zani , C.L. 2008 . Chemical constituents of Habenaria petalodes Lindl (Orchidaceae) . Journal of Brazilian Chemical Society , 19 : 1098 – 1104 .
- Rath , C. , Suman , K. , Dhar , B. , Mohanty , R.C. and Singh , A. 2009 . Pharmacognostical and phytochemical evaluation of rare and endangered Habenaria spp . (Riddhi and Vriddhi). Pharmacognosy Journal , 1 : 94 – 102 .
- Giri , L. , Jugran , A. , Rawat , S. , Dhyani , P. , Andola , H. , Bhatt , I.D. , Rawal , R.S. and Dhar , U. 2011 . Acta Physiologia Plantarum . In vitro propagation, phytochemical and genetic assessment of Habenaria egdeworthii: An important astavarga plant , doi: DOI 10.1007/s11738-011-0884-8
- Narayana , R.K. , Reddy , M.S. , Chaluvadi , M.R. and Krishna , D.R. 2001 . Bioflavonoid classification, pharmacological, biochemical effects and therapeutic potential . Indian Journal Pharmacology , 33 : 2 – 16 .
- Stray , F. 1998 . “ Tiger Book International ” . In The Natural Guide to Medicinal Herbs and Plants , 12 – 16 . London .
- Boligon , A.A. , Janovik , V. , Boligon , A.A. , Pivetta , C.A. , Pereira , R.P. , Rocha , J.B.T.D. and Athayde , M.L. 2011 . HPLC-analysis of polyphenolic compounds and antioxidant activity in Nasturtium officinale . International Journal of Food Properties , doi: 10.1080/10942912.2010.528111
- Wang , R.J. and Hu , M.L. 2010 . Antioxidant capacities of fruit extracts of five Mulberry genotypes with different assays and principal component analysis . International Journal of Food Properties , 14 ( 1 ) : 1 – 8 .
- Maikhuri , R.K. 1991 . Nutritional value of some lesser- known wild food plants and their role in tribal nutrition: A case study in north east India . Tropical Science , 31 : 397 – 405 .
- Samant , S.S. and Diversity , Dhar, U. 1997 . endemism and economic potential of wild edible plants of Indian Himalaya . International Journal of Sustainable Development and World Ecology , 4 : 179 – 191 .
- Sundriyal , M. and Sundriyal , R.C. 2001 . Wild edible plants of the Sikkim Himalaya, nutritive values of selected species . Economics Botany , 55 : 377 – 390 .
- Andola , H.C. , Rawal , R.S. and Bhatt , I.D. In press . “ Food Research International 2011 ” . In Comparative studies on the nutritive and anti-nutritive properties of fruits in selected Berberis , India : species of West Himalaya . doi: 10.1016/j.foodres.2010.07.017
- Edeoga , H.O. , Okwu , D.E. and Mbaebie , B.O. 2003 . Minerals and nutritive value of some Nigerian medicinal plants . Journal of Medicinal and Aromatic Plant Science , 25 : 1010 – 1015 .
- Shad , M.A. , Pervez , H. , Zafar , Z.I. , Zia-ul-Haq , M. and Nawaz , H. 2009 . Evaluation of biochemical composition and physicochemical parameters of oil from seeds of desi chickpea varieties cultivated in arid zone of Pakistan . Pakistan Journal of Botany , 41 : 655 – 662 .
- Al-Ghamdi , S.M. , Cameron , E.C. and Sutton , R.A. 1994 . Magnesium deficiency: Pathophysiologic and clinical overview . American Journal of Kidney Diseases , 24 : 737 – 754 .
- You , K.M. , Jong , H.G. and Kim , H.P. 1999 . Inhibition of cyclooxygenase/lipoxygenase from human platelets by polyhydroxylated/methoxylated flavonoids isolated from medicinal plants . Archives of Pharmaceutical Research , 22 : 18 – 24 .
- Veberic , R. , Colaric , M. and Stamper , F. 2008 . Phenolic acids and flavonoids of fig fruit (Ficus carica L.) . in northern Mediterranean region. Food Chemistry , 106 : 153 – 157 .
- Cisowski , W. and Sroka , Z. 2003 . Hydrogen peroxides scavenging, antioxidant and anti-radical activity of some phenolic acids . Food and Chemical Toxicology , 41 : 753 – 758 .