Abstract
The hyperglycemia-accelerated formation of advanced glycation end-products is involved in the pathogenesis of diabetic complications. The present study evaluated the antiglycation capacities of numerous flower herbal infusions, based on their capacity to inhibit the formation of fluorescent advanced glycation end-products and N-carboxymethyllysine in in vitro albumin/glucose and albumin/methylglyoxal systems. The antiglycation capacities of Sweet Osmanthus and Rose infusions were better than that of Chrysanthemum infusion, which is a well-documented antiglycation herb. Lavender and Chamomile infusions have an equivalent antiglycation capacity to that of Chrysanthemum, with a moderate effect. Moreover, the total phenolic content of a particular flower infusion was significantly correlated with its antiglycation capacity. This result indicated that phenolic compounds are responsible for the antiglycation activity of flower infusions.
INTRODUCTION
Diabetes mellitus is one of the common chronic diseases. The global prevalence of diabetes among adults is 6.4%. By 2030, it will have increased to 7.7%, influencing around 439 million adults.[ Citation 1 ] Since diabetes predisposes people to vascular complications that affect the eyes, blood vessels, nerves, and kidneys, the future health care burden will be huge in many countries. Consequently, dietary agents to be used in diabetic primary and secondary prevention must urgently be developed. Epidemiological evidence shows that prolonged hyperglycemia is a critical risk factor for microvascular complications of diabetic patients. Moreover, increases in the amounts of advanced glycation end-products (AGEs) was detected in tissue specimens of diabetes and correlated with the severity of complications, including retinopathy, nephropathy, neuropathy, and atherosclerosis. Therefore, hyperglycemia-accelerated protein glycation is believed to be involved in the pathogenesis of such diabetic complications.[ Citation 2 − Citation 4 ]
Protein glycation is initiated by a reaction between the amino group of proteins and the aldehyde group of sugars, forming a schiff's base. The schiff's base spontaneously transforms into Amadori products within a few days and these products undergo a serial cascade of irreversible reactions, including dehydration, oxidation, cyclization, and fragmentation, finally forming various AGEs. Over a dozen AGEs have been identified in vivo. They fall into three broad classes—fluorescent cross-linking, such as pentosidine; non-fluorescent cross-linking, such as methylglyoxal lysine dimer; and non-cross-linking, such as N-carboxymethyllysine (CML).[ Citation 5 ,Citation 6 ] Although the mechanism by which an individual AGE underlies diabetic complications has not been elucidated, the negative impact of all AGEs on diabetic complications has been verified.[ Citation 3 − Citation 5 ] The build-up of AGEs on body proteins directly causes permanent structural abnormalities, inactivation of enzymes, and alteration of immunogenicity.[ Citation 7 ,Citation 8 ] Additionally, by binding to specific receptors, AGEs cause intracellular oxidative stress and activate nuclear factor-κB (NF-κB) signaling, inducing the expression of adhesive molecules and secretion of proinflammatory cytokines.[ Citation 9 − Citation 11 ] AGEs-elicited chronic inflammation further exacerbates these complications.[ Citation 7 ,Citation 12 ] Apparently, the inhibition of protein glycation is a basis for the development of agents that can be used in diabetic primary and secondary intervention. Indeed, many AGEs inhibitors, including aminoguanidine, 2-isopropylidenehydrazono-4-oxo-thiazolidin-5-ylacetanilide (OPB-9195), phenyl thiazolium bromide, and pyridoxamine, have obtained promising results from animal studies.[ Citation 13 − Citation 15 ] However, the practical application of aminoguanidine, the first exploited inhibitor, was frustrated by a clinical trial in 1999.[ Citation 16 ] Nowadays, only pyridoxamine, a natural vitamin, has entered into clinical trials.[ Citation 15 ] Consequently, it is more effective to exploit antiglycation agents from food or traditional medicine due to safety concerns.
According to the report of the World Health Organization, about three-quarters of global people seek traditional medicine for their primary health care.[ Citation 17 ] Herbs are a major part of traditional medicine that has been practiced to control chronic diseases including diabetes.[18–20] Numerous traditional herbs, including Buckwheat Hull, Luobuma (Apocynum venetum L.), Mate (Ilex paraguariensis), Balm (Melissa officinalis), Mint (Mentha longifolia), and Chrysanthemum (Chrysanthemum morifolium) exhibit potent antiglycation capacities.[ Citation 21 − Citation 25 ] Flower-based herbal infusions are widely consumed because of not only their pleasing aroma, but also their potential biomedical function. A comprehensive database of the antiglycation functions of herbal infusions would be invaluable. This study evaluated the antiglycation capacities of several commonly consumed flower-based herbal infusions and compared them with that of Chrysanthemum tea, an identified anti-glycation floral infusion, based on their inhibition of the formation of fluorescent AGEs and CML in an in vitro bovine serum albumin (BSA)/glucose or BSA/methylglyoxal system.[ Citation 25 ] Correlations among phenolic, flavonoid amount, and antiglycation activity were analyzed to determine which components contribute to the antiglycation activity of these floral herbal infusions.
MATERIALS AND METHODS
Chemicals
BSA, D-glucose, aminoguanidine, dimethyl sulfoxide (DMSO), Folin-Ciocalteu phenol reagent, fluorescein, 2,2-azobis (2-amidinopropane) dihydrochloride (AAPH), ferrozine, nitro blue tetrazolium chloride/5-bromo-4-chloro-30-indolyphosphate p-toluidine salt (NBT/BCIP) tablets, and the reference standards trolox, catechin, gallic acid, and ethylenediamine tetraacetic acid (EDTA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All chemicals used were of analytical grade.
Herbal Infusions
Nine dried edible or medicinal flowers, Calendula (Calendula officinalis), Chamomile (Matricaria recutitai), Chrysanthemum (Chrysanthemum morifolium), Cornflower (Centaurea cyanus), Jasmine (Jasminum sambac), Lavender (Lavandula angustifolia), Neroli (Citrus aurantium), Rose (Rosa rugosa), and Sweet Osmanthus (Osmanthus fragrans Lour) were purchased from a local market (Hsinchu, Taiwan). Flower-based herbal infusions were made by steeping 2.5 g of dried flowers in 50 mL of double distilled water at 95°C for 10 min. After they had been strained and filtered through a layer of gauze, the infusions were collected and the volume was made up to 50 mL by adding double distilled water.
Total Phenolic Content
Folin-Ciocalteu spectrophotometric approach was used to measure the amount of phenolic compounds as described previously.[ Citation 26 ] Diluted infusion (0.1 mL) was reacted with Folin-Ciocalteu phenol reagent (0.5 mL) and 7.5% sodium carbonate (0.4 mL) for 1 h. Then, the absorbance at 765 nm was measured. Gallic acid (0–250 mg/L) was used to produce a standard calibration curve. The total phenolic content was expressed in mg of gallic acid equivalents (GAEs)/L of infusion.
Total Flavonoid Content
A spectrophotometric method described by Zhishen et al.[ Citation 27 ] was employed: 1.25 mL diluted infusion, 0.075 mL 5% NaNO2, 0.075 mL 10% AlCl3, 0.5 mL 1 M NaOH, and 0.6 mL distilled water was added into a glass tube and absorbance at 510 nm was measured. Catechin (0–500 mg/L) was used to produce a standard calibration curve. The total flavonoid content was expressed in mg of catechin equivalents (CEs)/L of infusion.
Oxygen Radical Absorbance Capacity (ORAC)
The ORAC assay was performed following the method of Huang et al.[ Citation 28 ] The flower herbal infusion, trolox standard, and distilled water blank (25 μL) were added to wells of a 96-well plate and then 150 μL fluorescein solution was added. The wells were kept in the dark for 30 min. After 25 μL of AAPH solution (153 mM) was added, solution fluorescence readings were taken at 2 min intervals for 120 min using a microplate multimode detector (Zenyth 3100; Anthos Labtec Instruments, Inc., Wals, Austria). The difference between the area under the fluorescence decay curve of each herbal infusion, the standard and the blank yields the antioxidant capacity. A calibration curve was established using trolox and experimental results were expressed in mmoles of trolox equivalents (TEs)/L of infusion.
Metal Chelating Capacity
Undiluted, two- and four-fold diluted infusions and EDTA were pipetted into wells of a 96-well plate and reacted with 40 μM ferrous chloride and 200 μM ferrozine for 10 min. The absorbance at 540 nm was then determined. The metal chelating capacity (%) of the flower infusion was calculated by the following equation:
Measurement of Fluorescent AGEs Formation
The glycation protocol for measuring the formation of fluorescent AGEs was as follows: 0.5 mL of 10-, 20-, and 40-fold diluted infusions was reacted with 2 mL freshly mixed glycation solution to yield final concentrations of BSA, glucose, NaN3, and phosphate buffer (pH 7.4) of 20 mg/mL, 0.5 M, 0.02%, and 0.1 M, respectively. The final concentration of another glycating agent, methylglyoxal, was set to 1 mM. Following incubation at 37°C for 3 weeks, fluorescent intensity was measured using a fluorescent spectrometer (F-2500, Hitachi, Tokyo, Japan) with an excitation wavelength of 370 nm and an emission wavelength of 440 nm. The capacity (%) of each flower infusion to inhibit the formation of fluorescent AGEs was calculated by the following equation:
Measurement of CML Formation
A two-fold diluted infusion (0.5 mL) was used in the glycation reaction, as described above. Following incubation at 37°C for 3 weeks, the levels of CML were determined by immunoblotting according to Tsuji-Naito et al.[ Citation 25 ] Glycation solution was diluted 20-fold by adding sample buffer (2% SDS, 5% β-mercaptoethanol, 2 mM EDTA in 125 mM Tris buffer, pH 6.8) and boiled for 5 min. Sample was loaded and separated in a 10% SDS-polyacrylamide gel and then transferred to polyvinylidene fluoride filters. The filters were blocked, probed with anti-CML antibodies (Trans Genic Inc., Tokyo, Japan), exposed to alkaline phosphatase-conjugated secondary antibodies, and then visualized by incubation with an NBT/BCIP solution. Additionally, the levels of loaded total protein in the gel were visualized by staining with Coomassie blue. Finally, the intensities of developed CML bands on the filters were quantified using a software-supported photoimager (ImageQuant300, GE Healthcare Bioscience, Piscataway, NJ, USA) and normalized to total protein.
Statistical Analysis
All experimental data were analyzed using SPSS software (SPSS 12.0 for Windows; SPSS, Inc., Chicago, IL, USA) and presented as mean ± SD from three independent tests. ANOVA and Duncan's multiple range test were used to compare treatments. The Pearson test was used to evaluate the correlation between two variants. The level of significance was set to p < 0.05.
Table 1 Total amounts of phenolic compounds and flavonoid in flower-based herbal infusions and their ORAC
RESULTS
Phenolic and Flavonoid Content in Flower Infusions and Their Antioxidant Capacity
presents the total amount of phenolic compounds and flavonoid in different flower infusions. The total amount of phenolic compounds varies markedly among flower infusions. Rose and Sweet Osmanthus flower infusions that contained 2.75 and 2.13 g GAEs/L, respectively, are rich in phenolic compounds. Conversely, Chamomile and Chrysanthemum flower infusions contained only 0.53 and 0.50 g GAEs/L, respectively, of phenolic compounds—around 18% of that in Rose. However, except for Sweet Osmanthus, the tested flower infusions contained moderate amounts of flavonoid ranging from 0.14 to 0.54 g CEs/L, with a mean of 0.27. The Sweet Osmanthus flower infusion was rich in flavonoid with a value of 3.56 g CE/L, almost 13 times that in the other tested flower infusions. ORAC was used to evaluate the antioxidant capacities of flower infusions and the related analytical results were presented in . The antioxidant capacities followed the order: Sweet Osmanthus (634) > Rose (422) > Neroli (352) > Chamomile (298) ≧ Lavender (276) ≧ Jasmine (268) > Chrysanthemum (209) ≧ Cornflower (192) ≧ Calendula (177). The antioxidant capacity of Sweet Osmanthus infusion was 3.6 times that of the Calendula infusion.
Metal Chelating Capacities of Flower Infusions
summarizes the metal chelating capacity of each flower infusion. EDTA is a frequently used metal chelating agent, chelated 49–100% of ferrous ions at concentrations of 0.05–1 mM. All flower infusions had a dose-dependent metal chelating capacity at one- to four-fold dilutions. When undiluted, the order of the metal chelating capacities of the flower infusions was Jasmine (61.9) > Neroli (38.1) ≧ Lavender (36.1) and Chrysanthemum (31.7) ≧ Rose (28.1) > Chamomile (17.6), Calendula (17.1), Cornflower (11.8), and Sweet Osmanthus (10.0). Notably, all of the tested flower infusions, even when undiluted, exhibited a metal chelating capacity that was lower than that of 0.1 mM EDTA.
Table 2 Metal chelating capacity of un-diluted, two- and four-fold diluted flower-based herbal infusions
Anti-Glycation Capacities of Flower Infusions, Determined from Their Inhibition of the Formation of Fluorescent AGEs in a BSA/Glucose System
presents the capacities of the flower infusions to inhibit the formation of total fluorescent AGEs in a BSA/glucose system. All flower infusions suppressed total fluorescent AGEs formation in a dose dependent manner at 10- to 40-fold dilution. At a 10-fold dilution, Sweet Osmanthus, Lavender, and Rose exhibited inhibitory capacities of >80% and excellent antiglycation effects. The other flower infusions, Chamomile, Cornflower, Neroli, Calendula, Jasmine, and Chrysanthemum exhibited inhibitory capacities of >50% against the formation of fluorescent AGEs and were moderately effective glycation inhibitors.
Table 3 Inhibitory capacity of 10-, 20-, and 40-fold diluted herbal infusions on the fluorescent AGEs formation in a BSA/glucose or in a BSA/methylglyoxal system
Anti-Glycation Capacities of Flower Infusions, Determined from Their Inhibition of the Formation of Fluorescent AGEs in a BSA/Methylglyoxal System
The inhibitory capacities of flower infusions against the formation of fluorescent AGEs in a BSA/methylglyoxal system are presented in . For comparison, in this experiment, the inhibitory activity of aminoguanidine, a well-known antiglycation drug with a dicarbonyl compound scavenging property was observed. Aminoguanidine inhibited 86–107% of the formation of methylglyoxal-mediated fluorescent AGEs at concentrations between 0.5 and 2 mM. All flower infusions suppressed the methylglyoxal-mediated formation of fluorescent AGEs in a dose-dependent manner. At 10-fold dilution, the anti-glycation capacities of flower infusions followed the order Sweet Osmanthus (93.0) > Rose (79.1) > Lavender (60.7) > Calendula (52.4) > Neroli (49.4) > Chrysanthemum (47.0), Cornflower (46.4), and Jasmine (45.2) > Chamomile (41.6). Notably, the inhibitory capacity of 10-fold diluted Sweet Osmanthus infusions was higher than that of 0.5 mM aminoguanidine.
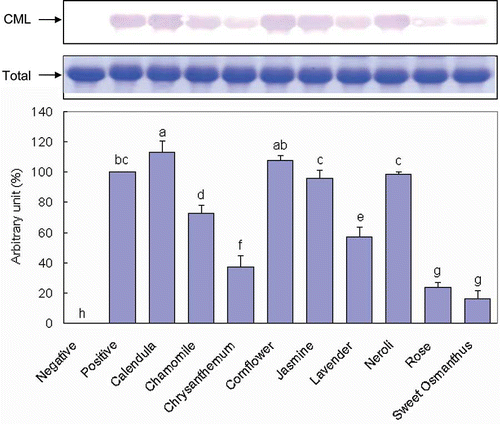
Anti-Glycation Capacities of Flower Infusions, Determined from Their Inhibition of the Formation of CML in a BSA/Glucose System
As shown in , when BSA was incubated with glucose at 37°C for 3 weeks (positive control), significant CML formation was detected; no such significant formation was detected without incubation (negative control). Of the nine tested flower infusions, Calendula, Cornflower, Neroli, and Jasmine did not inhibit the formation of CML as determined by comparison with the positive control. All of the other tested flower infusions significantly inhibited CML formation as determined by comparison with the positive control. The inhibitory capacities of these flower infusions followed the order Sweet Osmanthus (83.7%), Rose (76.1%), Chrysanthemum (62.6%), Lavender (43.0%), and Chamomile (27.1%).
Correlative Analysis
The amounts of both phenolic compounds (r = 0.779, p = 0.013) and flavonoid (r = 0.842, p = 0.004) in a particular flower infusion were significantly correlated with antioxidant capacity (ORAC). Additionally, the phenolic content of a particular flower infusion was significantly correlated with their capacity to inhibit formations of fluorescent AGEs (r = 0.748, p = 0.021) and CML (r = 0.683, p = 0.043) in a BSA/glucose system and correlated with their capacity to inhibit formation of fluorescent AGEs (r = 0.889, p = 0.001) in a BSA/methylglyoxal system. In contrast, the total amount of flavonoid was correlated only with one of the anti-glycation indicators, the capacity to inhibit the formation of fluorescent AGEs (r = 0.788, p = 0.012). The metal chelating capacity of flower infusions was not associated with either the amount of phenolic compounds or that of flavonoid. It was also independent of antiglycation capacity.
DISCUSSION
Of the nine tested flower infusions, Sweet Osmanthus and Rose most strongly inhibited the formation of both fluorescent AGEs and CML. Chamomile, Chrysanthemum, and Lavender also exhibited moderate capacities to inhibit the formations of both fluorescent AGEs and CML. However, Calendula, Cornflower, Jasmine, and Neroli inhibited the formation of only fluorescent AGEs and not CML and were therefore categorized as poor glycation inhibitors. Consistent with previous studies, this study found that the total amount of phenolic compounds in a particular herbal infusion was significantly correlated with its antiglycation activity, as determined by the formation of both fluorescent AGEs and CML.[24,29–31] Again, the result supports the hypothesis that phenolic compounds contribute to and that their total amount is a key determinant of the antiglycation capacity of plant foods. With some inconsistency, the total amounts of phenolic compounds in Chamomile and Chrysanthemum infusions were the lowest among the tested flower infusions, but these infusions moderately inhibited CML formation. Clearly, in addition to the total amount of phenolics, other factors, such as the class of phenolic compounds, influence the antiglycation capacity of flower infusions.[ Citation 32 − Citation 34 ] Matsuda et al.[32] reported that flavones have a stronger antiglycation capacity than do corresponding flavonols, flavanones, and isoflavones. Moreover, hydroxyl groups at the 3′-, 4′-, 5′-, and 7′-positions can promote and the methylation and glucosylation of the 4′-hydroxyl group can reduce, the antiglycation activity of flavonoids.[ Citation 32 ] Chrysanthemum infusion contains abundant flavones, including apigenin, acacetin, luteolin, and especially apigenin, which accounts for more than 50% of all of the flavonoids.[ Citation 25 ] Similarly, apigenin and luteolin represent around 20% of the total flavonoids in Chamomile infusion.[ Citation 35 ] The high amount of flavones presented in Chrysanthemum and Chamomile infusions may be responsible for this unique phenomenon.
Metal chelating and free radical scavenging abilities are generally considered to be two crucial determinants of glycation inhibition.[ Citation 36 − Citation 39 ] However, since the metal chelating ability of flower infusions was herein far less than 1 mM EDTA and was not associated with antiglycation capacity, metal chelating ability seems unlikely to be a crucial determinant of the antiglycation capacity of flower infusions. In contrast, the significant relationship between ORAC and antiglycation capacity implies that free radical scavenging ability is a key determinant of the antiglycation capacity of flower infusions.
Although Chrysanthemum infusion is traditionally used to treat fever and inflammatory diseases of the eye in China, its potential anti-diabetic effects, including reducing glucose absorption and anti-glycation and hypoglycemic activities, have also been recently identified in vitro and in vivo.[ Citation 25 ,Citation 40 ,Citation 41 ] Moreover, Chrysanthemum infusion has been proven to perform the anti-diabetic inhibition of the key enzyme of the polyol pathway, which is aldose reductase.[ Citation 42 ] Chamomile is a well recognized folk medicine for treating anxiety and cutaneous inflammation in the West.[ Citation 35 ] Similarly, chamomile tea has been found to reduce blood glucose levels and help to prevent hepatic glycogen degradation in diabetic rats and to suppress aldose reductase activity.[ Citation 43 ] Rose flower decoctions have been commonly used for effective washing and skin care and can also be used for treating acne and relieving irritated skin. Rose petals contain abundant anthocyanidins and flavonol glycosides.[ Citation 44 ] The flower extract from a species of rose (Rosa damascena Mill) has been found to exert an anti-diabetic effect by inhibiting α-glucosidase and reducing postprandial hyperglycemia.[ Citation 45 ] Related studies along with the results herein, suggest that regular consumption of Chrysanthemum, Chamomile, and Rose flower infusions could help to prevent diabetic complications. Although related investigation on the antidiabetic effects of Lavender and Sweet Osmanthus is scarce, the abundance of phenolics in these flower infusions leads to the reasonable expectation of their possible anti-diabetic effects. Heavy consumption of coffee and tea has been found to be able to reduce risk of diabetes and based on accumulated epidemiological and research data, phenolic compounds are undoubtedly some of the best candidates that are responsible for the anti-diabetic effects of coffee and tea.[ Citation 46 − Citation 48 ] Indeed, in addition to having anti-glycation and anti-oxidation properties, phenolic compounds have been revealed to possess other anti-diabetic effects, such as inhibiting the intestinal digestion and absorption of carbohydrate, increasing the secretion of insulin, regulating glucose metabolism, retarding the polyol pathway, and suppressing inflammation.[ Citation 49 − Citation 53 ]
Although the global consumption of individual herbal infusion is less than that of coffee or tea, herbal infusions are popular and add large amounts of antioxidant phenolics to people's diets.[ Citation 54 ] Not only has their antiglycation function been shown herein, but also the anti-inflammatory activity of flower herbal infusion has been identified.[ Citation 55 ] This latter effect could mitigate inflammation response of diabetes. Although these results need further approval by in vivo investigation, it is suggested that the regular consumption of such flower-based herbal infusions would protect against diabetic microvascular complications based on their antiglycation capacities.
ACKNOWLEDGMENTS
The authors would like to thank the National Science Council (Taipei, Taiwan) for financially supporting this research under Contract No. NSC-100-2313-B-264-001.
REFERENCES
- Shaw , J.E. , Sicree , R.A. and Zimmet , P.Z. 2010 . Global estimates of the prevalence of diabetes for 2010 and 2030 . Diabetes Research and Clinical Practice , 87 ( 1 ) : 4 – 14 .
- Vlassara , H. , Bucala , R. and Striker , L. 1994 . Pathogenic effects of advanced glycosylation: Biochemical, biologic and clinical implications for diabetes and aging . Laboratory Investigation , 70 : 138 – 151 .
- Brownlee , M. , Cerami , A. and Vlassara , H. 1998 . Advanced glycosylation end products in tissue and biochemical basis of diabetic complications . New England Journal of Medicine , 318 : 1315 – 1321 .
- Singh , R. , Barden , A. , Mori , T. and Beilin , L. 2001 . Advanced glycation end products: A review . Diabetologia , 44 : 129 – 146 .
- Ahmed , N. 2005 . Advanced glycosylation end products—Role in pathology of diabetic complications . Diabetes Research and Clinical Practice , 67 ( 1 ) : 3 – 21 .
- Peyroux , J. and Sternberg , M. 2006 . Advanced glycation endproducts (AGEs): Pharmacological inhibition in diabetes . Pathologie Biologie (Paris) , 54 : 405 – 419 .
- Brownlee , M. 2001 . Biochemistry and molecular cell biology of diabetic complications . Nature , 414 : 813 – 820 .
- Vlassara , H. and Palace , M.R. 2002 . Diabetes and advanced glycation endproducts . Journal of Internal Medicine , 251 : 87 – 101 .
- Yan , S.D. , Schmidt , A.M. , Anderson , G.M. , Zhang , J. , Brett , J. , Zou , Y.S. , Pinsky , D. and Stern , D. 1994 . Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors binding proteins . Journal of Biological Chemistry , 269 : 9889 – 9897 .
- Stern , D.M. , Yan , S.D. , Yan , S.F. and Schmidt , A.M. 2002 . Receptors for advanced glycation endproducts (RAGE) and the complications of diabetes . Ageing Research Reviews , 1 : 1 – 15 .
- Mrudula , T. , Suryanarayana , P. , Srinivas , P.N. and Reddy , G.B. 2007 . Effect of curcumin on vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina . Biochemical and Biophysical Research Communications , 361 ( 2 ) : 528 – 532 .
- Bengmark , S. 2007 . Advanced glycation and lipoxidation end products–amplifiers of inflammation: The role of food . Journal of Parenteral and Enteral Nutrition , 31 : 430 – 440 .
- Edelstein , D. and Brownlee , M. 1992 . Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine . Diabetes , 41 : 26 – 29 .
- Vasan , S. , Zhang , X. , Zhang , X. , Kapumiotu , A. , Bernhagen , J. , Teichberg , S. , Basgen , J. , Wagle , D. , Shih , D. , Terlecky , I. , Bucala , R. , Cerami , A. , Egan , J. and Ulrich , P. 1996 . An agent cleaving glucose-derived protein crosslinks in vitro and in vivo . Nature , 382 : 275 – 278 .
- Stitt , A. , Gardiner , T.A. , Alderson , N.L. , Canning , P. , Frizzell , N. , Duffy , N. , Boyle , C. , Januszewski , A.S. , Chachich , M. , Baynes , J.W. and Thorpe . 2002 . S.R. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes . Diabete , 51 : 2826 – 2832 .
- Freedman , B.I. , Wuerth , J.P. , Cartwright , K. , Bain , R.P. , Dippe , S. , Hershon , K. , Mooradian , A.D. and Spinowitz , B.S. 1999 . Design and baseline characteristics for the Aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II) . Controlled Clinical Trials , 20 : 493 – 510 .
- Egan , C.D. 2002 . Addressing use of herbal medicine in the primary care setting . Journal of the American Academy of Nurse Practitioners , 14 ( 4 ) : 166 – 171 .
- Li , W.L. , Zheng , H.C. , Bukuru , J. and De Kimpe , N. 2004 . Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus . Journal of Ethnopharmacology , 92 : 1 – 21 .
- Mukherjee , P.K. , Maiti , K. , Mukherjee , K. and Houghton , P.J. 2006 . Leads from Indian medicinal plants with hypoglycemic potentials . Journal of Ethnopharmacology , 106 : 1 – 28 .
- Yeh , G.Y. , Eisenberg , D.M. , Kaptchuk , T.J. and Phillips , R.S. 2003 . Systematic review of herbs and dietary supplements for glycemic control in diabetes . Diabetes Care , 26 : 1277 – 1294 .
- Yokozawa , T. and Nakagawa , T. 2004 . Inhibitory effects of Luobuma tea and its components against glucose-mediated protein damage . Food and Chemical Toxicology , 42 : 975 – 981 .
- Gugliucci , A. , Markowicz Bastos , D.H. , Schulze , J. and Ferreira Souza , M.F. 2009 . Caffeic and cholorogenic acids in Ilex paraguariensis extracts are the main inhibitors of AGE generation by methylglyoxal in model proteins . Fitoterapia , 80 : 339 – 344 .
- Zielinska , D. , Szawara-Nowak , D. and Zielinski , H. In press . “ Antioxidative and anti-glycation activity of buckwheat hull tea infusion ” . In International Journal of Food Properties 2011 doi: 10.1080/10942912.2010.551308
- Ho , S.C. , Wu , S.P. , Lin , S.M. and Tang , Y.L. 2010 . Comparison of anti-glycation capacities of several herbal infusions with that of green tea . Food Chemistry , 122 ( 3 ) : 768 – 774 .
- Tsuji-Naito , K. , Saeki , H. and Hamano , M. 2009 . Inhibitory effects of Chrysanthemum species extracts on formation of advanced glycation end products . Food Chemistry , 116 : 854 – 859 .
- Pekal , A. , Drozdz , P. and Pyrzynska , K. In press . “ Comparison of the antioxidant properties of commonly consumed commercial teas ” . In International Journal of Food Properties 2011 doi: 10.1080/10942912.2010.514642
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Huang , D. , Ou , B. , Hampsch-Woodill , M. , Flanagan , J.A. and Prior , R.L. 2002 . High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format . Journal of Agricultural and Food Chemistry , 50 : 4437 – 4444 .
- Hsieh , C.L. , Lin , Y.C. , Ko , W.S. , Peng , C.H. , Huang , C.N. and Peng , R.Y. 2005 . Inhibitory effect of some selected nutraceutic herbs on LDL glycation induced by glucose and glyoxal . Journal of Ethnopharmacology , 102 : 357 – 363 .
- Peng , X. , Zheng , Z. , Cheng , K.W. , Shan , F. , Ren , G.X. , Chen , F. and Wang , M. 2008 . Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts . Food Chemistry , 106 : 475 – 481 .
- Dearlove , R.P. , Greenspan , P. , Hartle , D.K. , Swanson , R.B. and Hargrove , J.L. 2008 . Inhibition of protein glycation by extracts of culinary herbs and spices . Journal of Medicinal Food , 11 ( 2 ) : 275 – 281 .
- Matsuda , H. , Wang , T. , Managi , H. and Yoshikawa , M. 2003 . Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities . Bioorganic & Medicinal Chemistry , 11 : 5317 – 5323 .
- Wu , C.H. and Yen , G.C. 2005 . Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts . Journal of Agricultural and Food Chemistry , 53 : 3167 – 3173 .
- Urios , P. , Grigorova-Borsos , A.M. and Sternberg , M. 2007 . Flavonoids inhibit the formation of the cross-linking AGE pentosidine in collagen incubated with glucose, according to their structure . European Journal of Nutrition , 46 : 139 – 146 .
- McKay , D.L. and Blumberg , J.B. 2006 . A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.) . Phytotherapy Research , 20 ( 7 ) : 519 – 530 .
- Oya , T. , Osawa , T. and Kawakishi , S. 1997 . Spice constituents scavenging free radicals and inhibiting pentosidine formation in a model system . Bioscience Biotechnology & Biochemistry , 6 : 263 – 266 .
- Sajithlal , G.B. , Chithra , P. and Chandrakasan , G. 1998 . The role of metal catalyzed oxidation in the formation of advanced glycation end products: An in vitro study on collagen . Free Radical Biology & Medicine , 25 ( 3 ) : 265 – 269 .
- Price , D.L. , Rhett , P.M. , Thorpe , S.R. and Baynes , J.W. 2001 . Chelating activity of advanced glycation end-product inhibitors . Journal of Biological Chemistry , 276 : 48967 – 48972 .
- Chace , K.V. , Carubelli , R. and Nordquist , R.E. 1991 . The role of nonenzymatic glycosylation, transition metals, and free radicals in the formation of collagen aggregates . Archives of Biochemistry and Biophysics , 288 ( 2 ) : 473 – 480 .
- Kim , J.S. , Ju , J.B. , Choi , C.W. and Kim , S.C. 2006 . Hypoglycemic and antihyperlipidemic effect of four Korean medicinal plants in alloxan induced diabetic rats . American Journal of Biochemistry and Biotechnology , 2 ( 4 ) : 154 – 160 .
- Wang , Z. , Clifford , M.N. and Sharp , P. 2008 . Analysis of chlorogenic acids in beverages prepared from Chinese health foods and investigation, in vitro, of effects on glucose absorption in cultured Caco-2 cells . Food Chemistry , 108 : 369 – 373 .
- Matsuda , H. , Morikawa , T. , Toguchida , I. , Harima , S. and Yoshikawa , M. 2002 . Medicinal flowers. VI. Absolute stereostructures of two new flavanone glycosides and a phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L.: Their inhibitory activities for rat lens aldose reductase . Chemical & Pharmaceutical Bulletin , 50 ( 7 ) : 972 – 975 .
- Kato , A. , Minoshima , Y. , Yamamoto , J. , Adachi , I. , Watson , A.A. and Nash , R.J. 2008 . Protective effects of dietary chamomile tea on diabetic complications . Journal of Agricultural and Food Chemistry , 56 : 8206 – 8211 .
- Velioglu , Y.S. and Mazza , G. 1991 . Characterization of flavonoids in petals of Rosadamascena by HPLC and spectral analysis . Food Chemistry , 39 : 463 – 467 .
- Gholamhoseiniana , A. , Fallah , H. and Sharififar , F. 2009 . Inhibitory effect of methanol extract of Rose damascena Mill . flowers on α-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine , 16 ( 10 ) : 935 – 941 .
- van Dam , R.M. and Feskens , E.J. 2002 . Coffee consumption and risk of type 2: The epidemiology of diabetes mellitus . Lancet , 360 : 1477 – 1478 .
- Iso , H. , Date , C. , Wakai , K. , Fukui , M. and Tamakoshi , A. 2006 . The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults . Annals of Internal Medicine , 144 ( 8 ) : 554 – 562 .
- Ferruzzi , M.G. 2010 . The influence of beverage composition on delivery of phenolic compounds from coffee and tea . Physiology & Behavior , 100 ( 1 ) : 33 – 41 .
- McDougall , G.J. , Shpiro , F. , Dobson , P. , Smith , P. , Blake , A. and Stewart , D. 2005 . Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase . Journal of Agricultural and Food Chemistry , 53 : 2760 – 2766 .
- Shu , X.S. , Lv , J.H. , Tao , J. , Li , G.M. , Li , H.D. and Ma , N. 2009 . Antihyperglycemic effects of total flavonoids from Polygonatum odoratum in STZ and alloxan-induced diabetic rats . Journal of Ethnopharmacology , 124 ( 3 ) : 539 – 543 .
- Valentova , K. , Truong , N.T. , Moncion , A. , de Waziers , I. and Ulrichova , J. 2007 . Induction of glucokinase mRNA by dietary phenolic compounds in rat liver cells . Journal of Agricultural and Food Chemistry , 55 : 7726 – 7731 .
- Kawanishi , K. , Ueda , H. and Moriyasu , M. 2003 . Aldose reductase inhibitors from the nature . Journal of Medicinal Chemistry , 10 ( 15 ) : 1353 – 1374 .
- Mueller , M. , Hobiger , S. and Jungbauer , A. 2010 . Anti-inflammatory activity of extracts from fruits, herbs and spices . Food Chemistry , 122 : 987 – 996 .
- Dimitrios , B. 2006 . Sources of natural phenolic antioxidants . Trends in Food Science & Technology , 17 : 505 – 512 .
- Tsai , P.J. , Tsai , T.H. , Yu , C.H. and Ho , S.C. 2007 . Comparison of NO-scavenging and NO-suppressing activities of different herbal teas with those of green tea . Food Chemistry , 103 : 181 – 187 .