Abstract
Five mung bean cultivars varying in seed coat color were analyzed for polyphenol content. The effect of soaking and germination on the polyphenol content and polyphenol oxidase activity was investigated. The level of total polyphenols was found to be in the range of 310–340 mg/100 g in whole seeds. Soaking reduced the total polyphenols, whereas germination for 48 h increased total polyphenol content by 41–76%. The hydrolysable tannins, condensed tannins, and hydrolysable tannins/condensed tannins index increased with an increase in period of germination. The total and percent condensed tannins content slightly decreased while hydrolysable tannins increased during germination. Maximum polyphenol oxidase activity of 102–108 units was observed in 24 h germinated seeds. There was no significant correlation found between the polyphenolic content and polyphenol oxidase activity in germinating mung bean seeds. These findings demonstrated that the mung beans are fair sources of polyphenols, thus having great potential as a source of natural antioxidants.
INTRODUCTION
Legumes are good source of starch, dietary fiber, protein, and minerals and also serve as a rich source of bioactive constituents.[1] Recently, legumes are gaining interest because they are excellent sources of bioactive compounds, such as polyphenols. Most legumes are known to contain appreciable levels of polyphenolic substances broadly referred to as tannins, which may be divided into two main groups: hydrolysable (HTs) and condensed (CTs) tannins.[Citation2] HTs can be easily extracted with methanol or acetone, whereas CTs with acidified methanol.[Citation3] Many studies have demonstrated detrimental effects of polyphenols in humans and in animals including their ability to form complexes with digestible protein in general and digestive enzymes in particular,[Citation4] growth retardation by decreasing protein and carbohydrate digestibility,[Citation5] and reducing ionizable iron absorption by acting as a natural iron chelating agent.[Citation6] Traditional processing methods can improve nutritional quality by increasing food palatability and digestibility or increasing nutritional availability by destroying antinutritional factors or minimizing their effects. These involve mechanical, physical, chemical, and biological modes of operation.[Citation7] Polyphenols constitute a diverse group of secondary metabolites that have been traditionally thought of as antinutritional compounds. However, in recent years, an increasing number of beneficial effects have been recognized for polyphenols from very different plant sources. For instance, the antioxidant, antiproliferative, and apoptosis-inducing properties of polyphenols have been related to the prevention of diseases, such as cancer[Citation8] and cardiovascular[Citation9] or degenerative diseases.[Citation10] Polyphenol oxidase (PPO) (EC 1.10.3.1) is a widely distributed enzyme in the plant kingdom, including different leguminous plants and are thought to be involved in oxidation of phenolic compounds.[Citation11]
Mung bean (Phaseolus aureus L.) is the most important legume due to its high carbohydrate, protein, and mineral content. Its protein quality is similar to or better than other legumes, such as chickpea, black gram, peas, pigeon pea, etc.[Citation12,Citation13] India is the major pulse producing country and among various pulses, mung bean stands as third, [Citation14] and is the principal crop from which edible bean sprouts, noodles, and weaning foods are prepared. The polyphenol content of mung bean cultivars differ in seed coat color, as reported earlier.[Citation15] The systematic investigation on polyphenolic content of mung bean sprouts is lacking. The present work was aimed at studying the effect of soaking and germination on total polyphenol content in terms of HTs and CTs and to correlate with PPO activity.
MATERIALS AND METHODS
The green mung bean cultivars, Vaibhav and Hum-1, were procured from the Agriculture Research Station, Aland Road, Gulbarga, India. The three yellow cultivars, ALM-1, ALM-2, and ALM-3, were procured from farmers of the Hyderabad-Karnataka region in India. The samples were cleaned and stored in the laboratory at 4°C. All chemicals used were of analytical grade.
Extraction and Determination of Polyphenols
Clean, whole dry seeds were finely powdered to pass through a 0.6-mm mesh screen and defatted with petroleum ether. One gram of defatted meal was extracted twice with 10 ml of methanol each time at 25 ± 2°C for 20 min. The residue was re-extracted twice with 10 ml of methanol containing 1% HCl.[Citation3] Methanol and acidic methanol extracts were analyzed for polyphenol content according to the method of Burns.[Citation16] The concentration of polyphenols was expressed in milligram of gallic acid equivalents (GAE) per 100 g of sample.
Processing of Mung Bean
Soaking
The seeds were first surface sterilized for 3 to 4 min with 0.1% mercuric chloride. The sterilized seeds were washed under running tap water and rinsed with distilled water to remove traces of mercuric chloride. The surface sterilized seeds were soaked in distilled water (1:10, w/v) for 12 h at 25 ± 2°C. The soaked seeds were dried in a hot air oven at 60°C to a constant weight and finely powdered. The fine powder of processed mung bean was analyzed for polyphenols as mentioned earlier.
Germination
The soaked seeds were placed on a moist filter paper bed in petri plates and incubated at 37 ± 0.5°C in the dark. The filter paper was moistened with distilled water at regular intervals. The sprouts were sampled at 12, 24, and 48 h and dried in a hot air oven at 60°C to a constant weight and finely powdered. The fine powder of processed mung bean was analyzed for polyphenols.
Assay of polyphenol oxidase
Five gram seed samples were homogenized in pre-chilled pestle and mortar using 10 ml of ice-cold distilled water. The homogenate was filtered through muslin cloth and filtrate was centrifuged for 20 min at 20,000 g at 4°C. The supernatant was used to determine the enzyme activity. The freshly prepared pyrogallol (4 mg/ml) in 0.1 M citrate phosphate buffer of pH 6.8 was oxygenated for 5 min by bubbling oxygen through it and was used as substrate. The enzyme activity was determined by the method of Kruger.[Citation17] The assay mixture consisted of 100 μL of the enzyme extract and 2.9 ml of pyrogallol. The change in absorbance at 410 nm was recorded. One unit of enzyme activity was defined as the amount of the enzyme that causes an increase in absorbance of 0.001/min at 25°C.
Statistical Analysis
The values were expressed in mean ± standard deviation (SD). Statistical analyses were performed by analysis of variance (ANOVA) using the SPSS data analysis tool, Version 14.
RESULTS AND DISCUSSION
Polyphenol Content of Mung Bean
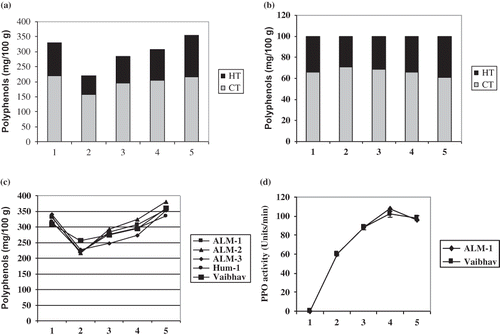
All analyzed mung bean cultivars contained significant levels of polyphenols (). The level of total polyphenols was found to be in the range of 310–340 mg/100 g in whole seeds, with an average 325 mg/100 g, while the content of HTs and CTs ranged from 56–68 and 156–188 mg/100 g, respectively. The HTs/CTs index was in between 0.34 and 0.66 and found to be high in yellow cultivars (ALM-1 and ALM-2). The tested mung bean cultivars are rich sources of polyphenols. The polyphenol content of yellow mung bean cultivars was more than the green cultivars thus yellow cultivars have great potential as a source of natural antioxidants.
Table 1 Effect of soaking on the content of polyphenols of mung bean cultivars
Effect of Soaking
Soaking of mung bean seeds in plane water for 12 h reduced the polyphenols significantly (r = 0.683, p ≤ 0.04). The loss of total polyphenols ranged from 18 to 35% (). The concentration of HTs and CTs decreased in all the cultivars. The HTs/CTs index was less than 0.4 and ranged between 0.34 and 0.38, and more reduction was observed in two yellow cultivars, ALM-1 and ALM-2. Upon soaking in plain water, the polyphenol content was reduced considerably. Reduction in polyphenol content upon soaking was reported for several legumes. A 50% reduction in polyphenols in chickpea,[Citation18] in mung bean reproduction of polyphenols about 25–50%,[Citation19] and 23% in mung bean[Citation20] after soaking was reported and the reduction was thought to be leaching out of water soluble polyphenols in soaked water. The yellow cultivars contained more HTs than green cultivars and the HTs/CTs index decreased drastically upon soaking. Being more water soluble, HTs are leached out into soaking water.
Effect of Germination
Germination is a process widely used for legumes to increase their palatability and nutritional value. Soaked mung bean seeds were germinated and analyzed for polyphenols. Gradual increase in the polyphenol content of all the mung bean cultivars was observed upon germination (). The HTs, CTs, and HTs/CTs index were increased with an increase in period of germination. The total CTs and percent CTs contents slightly decreased while that of HTs increased during germination of ALM-1 (Figs. 1a and 1b) and Vaibhav (figures are not shown). The maximum increase of 41–76% in total polyphenol content was observed after 48 h of germination. Several authors have reported the reduction in polyphenol content of legumes seeds upon germination. Reduction of polyphenol content by 32% in mung bean seeds[Citation20] and 37% in Jack bean seeds[Citation21] upon germination for 24 h was reported. Rahma et al.[Citation22] observed a reduction of about 23% in polyphenol content of faba beans after one day of germination. A decrease in the total phenolic content of mung bean by 16.72% upon 12 h of germination and by 41.73% upon 24 h of germination was observed by Hardeep et al.,[Citation23] while in black gram a decrease in the total phenolic content by 35.79% up to the first 12 h of germination and thereafter an increase of 12.84% upon further 12 h germination has been reported by the same authors.[Citation23] Our results are not in accordance with what authors reported earlier. In contrast, in the present investigation a gradual increase in the polyphenol content has been noticed in mung bean during germination. Our results are in accordance with the observations made by Shastry and John,[Citation24] who have reported a progressive increase in polyphenol content in germinating Dolichos lablab beyond 48 h of germination. Charlene et al.[Citation19] had also reported an increase in polyphenol content of mung bean sprouts upon continued germination up to 120 h. In the present investigation, a progressive and significant increase (r = 0.969, p ≤ 0.01) in polyphenol content was observed during germination up to 48 h (Fig. 1c). A significant increase in polyphenol content of germinated seeds may be due to a fresh synthesis of polyphenols or the polymerization of existing phenolic compounds or degradation of high molecular weight insoluble polymers into smaller molecular weight soluble polymers that give color reaction to the reagent.[Citation25] The content of HTs, CTs, and also HTs/CTs ratio increased during germination, which suggests fresh synthesis of both HTs as well as CTs during germination of mung beans.
Table 2 Effect of germination on the polyphenol content of mung bean cultivars
Figure 1 (a) Subdivided bar diagram of polyphenol content of ALM-1 cultivar during soaking and germination. (b) Percentage bar diagram of polyphenol content of ALM-1 cultivar during soaking and germination. (c) Effect of soaking and germination of mung bean on polyphenol content. (d) PPO activity during soaking and germination of ALM-1 and Vaibhav. 1. Dry seeds; 2. soaked seeds and sprouts after germinating for: 3. 12 h; 4. 24 h; and 5. 48 h.
PPO Activity
The PPO activity in germinated seeds of mung beans was analyzed after 12, 24, and 48 h. The PPO activity was not observed in dry mung beans (). A marked increase in PPO activity was observed upon soaking followed by germination. The PPO activity of 60 units was observed in soaked seeds, and maximum activity of 102–108 units was observed in 24-h germinated seeds. Germination beyond 24 h, to 48 h has decreased PPO activity (Fig. 1d). PPO is ubiquitous in legume seeds and is responsible for the oxidation of phenolic compounds. PPO activity during germination was reported for several legumes, such as for broad bean leaves,[Citation26] mung bean leaf,[Citation27] and field bean.[Citation28] In tested mung beans, the PPO activity increased with germination up to 24 h and total polyphenolic content, HTs and CTs and HT/CT index was also found to be increased. There was no correlation between the polyphenolic content and PPO activity in germinated mung bean seeds.
Table 3 Polyphenol oxidase activity of mung bean seeds
CONCLUSION
The total polyphenol content in the yellow and green cultivars of mung bean varied significantly. The yellow mung bean cultivars contained higher amounts of polyphenols than that of green cultivars. Soaking caused significant reduction in HTs, CTs, and also HTs/CTs index, while soaking followed by germination gradually increased the same. An increase in polyphenol contents of the seeds during the progressive germination would certainly be of nutritional importance. The polyphenol oxidase activity was not found in dry seeds, but was maximum at 24 h germination. There was no significant correlation found between the polyphenolic content and PPO activity in mung bean sprouts. Germinated mung beans are rich sources of polyphenols, thus they have great potential as a source of natural antioxidants for human nutrition.
ACKNOWLEDGMENTS
The authors are grateful to Agriculture Research Station, Aland Road, Gulbarga and farmer Gundajja for providing mung bean samples. Thanks are also due to Gulbarga University, Gulbarga for providing the laboratory facility.
REFERENCES
- Geil , P.B. and Anderson , J.W. 1994 . Nutrition and health implications of dry beans: A review . Journal of the American College of Nutrition , 13 : 549 – 558 .
- Goldstein , J.L. and Swain , T. 1963 . Changes in tannins in ripening fruits . Phytochemistry , 2 : 371 – 383 .
- Asquith , T.V. , Izuno , C.C. and Butler , L.G. 1983 . Characterization of condensed tannin (Proanthocyanidin) from a group II sorgham . Journal of Agricultural and Food Chemistry , 31 : 1299 – 1303 .
- Liener , I.E. 1987 . “ Antinutritional factors in legume seeds. State of the art ” . In Recent Advances of Research in Antinutritional Factors in Legume Seeds , 6 – 13 . Prodc: Wageningen : The Netherlands .
- Deshpande , S.S . 1992 . Food legumes in human nutrition: A personal perspective . CRC Critical Reviews in Food Science and Nutrition , 32 : 333 – 363 .
- Rao , B. S. N. and Prabhavati , T. 1982 . Tannin content of foods commonly consumed in India and its influence on ionisable iron . Journal of the Science of Food and Agriculture , 33 : 89 – 96 .
- Knorr , D. and Sinskey , A. 1985 . Biotechnology in food production and processing . Science , 229 : 1224 – 1229 .
- Ramos , S. 2008 . Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways . Molecular Nutrition and Food Research , 52 : 507 – 526 .
- Benevente-Garcia , O. and Castillo , J. 2008 . Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity . Journal of Agricultural and Food Chemistry , 56 : 6185 – 6205 .
- Singh , M. , Arsenesult , M. , Sanderson , T. , Murthy , V. and Ramassamy , C. 2008 . Challenges for research on polyphenols from foods in Alzheimer's disease: Bioavailability, metabolism, and cellular and molecular mechanisms . Journal of Agricultural and Food Chemistry , 56 : 4855 – 4873 .
- Whitaker , J. R. and Lee , C. Y. 1995 . “ Recent advances in chemistry of enzymatic browning: An overview. In: Enzymatic Browning and its Prevention ” . In ACS Symposium Series , Edited by: Chang , Y.L. and Whitaker , J.R. Vol. 600 , Washington DC : American Chemical Society .
- Jood , S. , Bishnoi , S. and Sharma , S. 1998 . Chemical analysis and physicochemical properties of chickpea and lentil cultivars . Food/Nahrung , 42 ( 2 ) : 71 – 74 .
- Kataria , A. , Chahan , B. M. and Punia , D. 1989 . Antinutrients and protein digestibility (in vitro) of mung bean as affected by domestic processing and cooking . Food Chemistry , 32 : 9 – 17 .
- Chandrasekhar , C. P. and Ghosh , J. 2002 . Food economy in disarray . Indian Food Industry , 21 : 10 – 14 .
- Tajoddin , M. , Manohar , S. and Lalitha , J. 2010 . Polyphenols of mung bean (Phaseolus aureus L.) cultivars differing in seed coat color: Effect of dehulling . Journal of New Seeds , 11 ( 4 ) : 369 – 379 .
- Burns , R. E. 1963 . Methods of tannin analysis for forage crop evaluation , Vol. 32 , Athens : Technical Bulletin. Georgia Agriculture Station .
- Kruger , J. E. 1976 . Changes in the polyphenol oxidases of wheat during kernel growth and maturation . Cereal Chemistry , 53 ( 2 ) : 201 – 213 .
- Rao , P. I. and Deosthale , Y. G. 1982 . Tannin content of pulses: Varietal difference and effect of cooking and germination . Journal of the Science of Food and Agriculture , 33 : 1013 – 1016 .
- Barroga, C , F. , Laurena , A. C. and Mendoza , E. T. 1985 . Polyphenols in mung bean (Vigna radiata (L.) Wilczek): Determination and removal . Journal of Agricultural and Food Chemistry , 33 : 1006 – 1009 .
- Grewal, A.; Jood, S. Effect of processing treatments on nutritional and antinutritional contents of green gram . 2006 . Journal of Food Biochemistry , 30 : 535 – 546 .
- Babar , V. S. , Kadam , S. S. and Chavan , J. K. 1988 . Effect of heat treatments and germination on trypsin inhibitor activity and polyphenols in jack bean (Canavalia ensiformis L . DC). Plant Foods for Human Nutrition , 38 : 319 – 324 .
- Rahma , E. M. , El-Bawey , A. A. , El-Adawy , T. A. and Guma , M. A. 1987 . Changes in chemical and antinutritional factors and functional properties of faba bean during germination . Lebensmittel-Wissenschaft und Technologie , 20 : 271 – 276 .
- Hardeep , S. G. , Mamta , A. , Paras , S. and Jaspreet , S. 2011 . Phenolic content and Antioxidant activity of germinated and cooked pulses . International Journal of Food Properties , 14 : 1366 – 1374 .
- Shastry , Y. M. and John , E. 1991 . Biochemical changes and in-vitro protein digestability of the endosperm of germinating Dolichos lablab . Journal of the Science of Food and Agriculture , 55 ( 4 ) : 529 – 538 .
- Satwadhar , P. N. , Kadam , S. S. and Salunkhe , D. K. 1981 . Effects of germination and cooking on polyphenols and in vitro digestibility of horse gram and moth bean . Qualitas Plant Foods for Human Nutrition , 31 : 71 – 76 .
- Ganesa , C. , Fox , M. T. and Flurkey , W. H. 1992 . Microheterogeneity in purified broad bean polyphenol oxidase . Plant Physiology , 98 : 472 – 479 .
- Shin , R. , Froderman , T. and Flurkey , W. H. 1997 . Isolation and characterization of a Mung bean leaf polyphenol oxidase . Phytochemistry , 45 : 15 – 21 .
- Paul , B and Gowda , L. R. 2000 . Purification and characterization of a polyphenol oxidase from the seeds of field bean (Dolichos lablab) . Journal of Agricultural and Food Chemistry , 48 : 3839 – 3846 .
