Abstract
The rheological behavior of model and commercial borojó (Borojoa patinoi Cuatrec.) jams was analysed as a function of temperature (5–60°C). Model borojó jam formulations were prepared by modifying pectin concentration. Both small-amplitude oscillatory shear and steady flow measurements were performed. Rheological results were also compared with those obtained with a traditional commercial peach jam. The Herschel-Bulkley model was used to describe the steady flow behaviour. In general, all rheological parameters increased with pectin concentration and decreased with temperature. Arrhenius-type relationships were used to quantify the influence of temperature on several viscoelastic and viscous flow parameters. Model borojó jam formulations exhibited higher temperature dependence of linear viscoelastic functions than peach jam samples but lower than borojó commercial jam. The influence of temperature on the yield stress was more important in model borojó formulations than in both commercial jams.
INTRODUCTION
Borojoa patinoi Cuatrec. is an understory tree, i.e., cultivated under a shelter wood system, member of the Rubiaceae family, and restricted to tropical climates, especially in humid areas, mainly growing in Panamá, Colombia, and Ecuador. The cultivation system involves low production costs and conserves much of the biodiversity of the natural forest. Borojó is an exotic and apple-sized fruit with some medicinal and aphrodisiacal properties, which command a high price in the local markets and is traditionally used in drinks and other handcrafted preparations.[Citation1 − Citation3] The Borojó fruit weighs around 750 g, between 250 and 1000 g, and consists of pulp, seed, and peal. Often the pulp and seed represent around 83 and 10%, respectively. The pulp is acid, dense, and contains a significant level of carbohydrates, calcium, and soluble solids. Although borojó pulp is currently used to produce processed products like juices, jams, compotes, candies, ice creams, or energy drinks,[Citation3] the commercialization of such products is very limited to the native countries and their physicochemical, rheological, or textural properties are scarcely investigated. In this respect, recent works[Citation4,Citation5] have explored the possibility of using the spray-drying and freeze-drying techniques to open up new alternatives for their commercialization in international markets, avoiding some difficulties in handling, transport, and packing.
The rheological, textural, and sensory properties of jam formulations manufactured from other much more universally popular and accepted tropical fruits like pineapple and mango have been reported.[Citation6 − Citation9] The manufacture of borojó jam with suitable physicochemical, sensory, and rheological properties and dissemination of these properties is a way to increase the appraisal of this fruit in other markets. Jam is an intermediate moisture food product obtained by cooking the fruit ingredient mixed with sugar and other ingredients (preservative, colouring, or flavouring agents) and concentrating to get the right consistency, thick enough to hold the tissues.[Citation10] Jam must contain no less than 65% soluble solids.[Citation11] Pectin, sugar, acids, and water are present in approximately the following proportions: 0.5–1% added pectin, 50–75% sugar, 1% acid, and 33–38% water (on fruit pulp basis).[Citation12] One of the factors affecting jam's quality is the pulp content. For instance, in Ecuador, the legislation requires that jam formulations should be made with at least 45% wt. of original fruit.[Citation11]
Rheology of complex food products plays an important role not only in quality control, determining in many cases the consumer acceptance, but also in the design of unit operations like pumping, mixing, stirring, evaporation, heat exchange, etc.[Citation13,Citation14] In this sense, a full rheological characterization of such formulations must cover both the viscous flow behaviour and linear viscoelasticity.[Citation15 − Citation17] Jam can be considered a gel-like solid-in-liquid system with a structure that immobilizes the liquid and confers viscoelastic properties.[Citation17] However, fracture occurrence under large deformations is not clearly detected like in real gels (gelatin, jelly, surimi, etc.). The flow behaviour of most conventional fruit jams has been widely investigated in the past, especially regarding the modeling of the shear stress-shear rate relationship.[Citation8,Citation18,Citation19] However, less attention has been paid on the time-dependent flow behaviour[Citation7] and linear viscoelastic properties.[Citation9] From this existing literature, it can be deduced that the most important factors affecting the rheology of jam formulations are some compositional parameters and interactions among components like pectin and sugar contents and temperature. In this work, the linear viscoelastic response and flow behaviour of commercial and model borojó jam formulations were explored by means of small-amplitude oscillatory shear (SAOS) and steady-state flow measurements, respectively, by analyzing the influences of pectin concentration and temperature. Results were also compared to those obtained with a traditional commercial peach jam.
MATERIALS AND METHODS
Materials and Preparation of Model Jam Formulations
Commercial borojó jam was supplied by Gamboinos factory (Francisco de Orellana, Ecuador). Commercial peach jam (Helios, Spain) was purchased in a local supermarket. Model borojó jam formulations were prepared using borojó pulp previously characterized,[Citation20] commercial grade sugar purchased in a local supermarket, and high-methoxyl pectin, quick set, 153 USA SAG, Unipectine RS 150 (Cargill Texturizing Solutions, France). Borojó fruits were obtained from Sandrita farm (Los Ríos, Ecuador). Physicochemical characteristics of borojó pulp employed are included in .
Table 1 Physicochemical data of original borojó pulp and jam formulations studied
Model borojó jams were prepared using a conventional procedure. The amount of sugar to be added (45:55 pulp/sugar ratio) and the amount of water to be evaporated was calculated according to the Ecuadorian norm[Citation11] to reach at least 65 °Brix and 50% pulp in the final product. Borojo pulp used in this work contains 2.6% of pectin (wet basis), and concentrations of high-methoxyl pectin added were 0.25, 0.50, 0.75, and 1% wt. Model jam formulations were prepared on the basis of 250 g of borojó pulp. Initially, 90% of the pulp was mixed at 80°C with only 10% of the total sugar calculated to facilitate evaporation. Pectin was then added before reaching 25 °Brix, in order to favour complete dissolution, previously blended with sucrose in a 1:5 weight ratio, while stirring vigorously. Evaporation was continued at a temperature not exceeding 90°C until 35 °Brix, during 6–10 min at atmospheric pressure. At this time, the rest of the sucrose was added rapidly reaching 65–67 °Brix and then the remaining 10% of borojó pulp was added. Borojó pulp acidity () ensures a pH value lower than 3.3 during jam preparation and, therefore, further pH adjustment was not required. Afterwards, borojó jams were packed in 250-mL cylindrical jars at 88°C under vacuum, which were then cooled down to room temperature (25°C) and stored at 4°C.
Physicochemical Characterization
Physicochemical analyses of jam samples studied were performed in duplicate, using standard methods.[Citation21] The physicochemical variables studied were soluble solids, moisture, ashes, pH, and acidity. Soluble solids content was determined by means of refractometric measurements, at 20°C, in a refractometer (model Abbe, Atago Co. Ltd., Japan), according to the AOAC method 932.12. Moisture was determined in a Thermo Scientific Heraeus vacuum oven, model VT6130 M (Germany), at 70°C and 100 mm Hg, for 24 h (AOAC method 920.151). pH was determined at 25°C in an Orion pH meter (Research Inc., Boston, MA, USA), according to the AOAC method 981.12. The acidity of jam samples was determined by titration and expressed in grams of malic acid per 100 g of jam (AOAC method 942.15). Ash content was determined in a muffle furnace, model Neyo M-525 (Division Barkmeyer, Yucaipa, CA, USA), at 525°C, according to the AOAC method 940.26.
Rheological Characterization
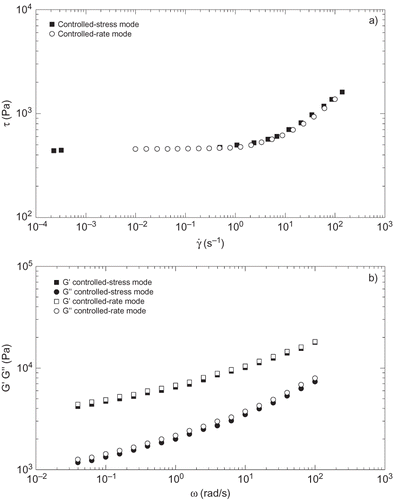
Small-amplitude oscillatory shear (SAOS) tests were conducted in a controlled-stress rheometer (model Rheoscope, ThermoHaake, Germany), within the linear viscoelastic region using a plate-plate geometry (35 mm diameter, 1 mm gap) in a frequency range of 5·10−3–16 Hz. Linear viscoelasticity range was previously determined by means of stress sweep tests at 1 Hz. Viscous flow tests were performed in a Physica MCR-501 rheometer (Anton Paar, Austria), which allows a better controlled-rate operation mode (0.1 μN m torque sensitivity), by applying a stepped-shear rate ramp in a range of shear rates of 10−2–102 s−1, using parallel plates (diameter: 25 mm, 1 and 2 mm gaps, depending on sample consistency) with grooved surfaces to overcome wall slip phenomena usually observed in colloidal food systems.[Citation22,Citation23] shows the comparison of viscous flow curves obtained using both the controlled-rate and the controlled-stress modes for a selected sample. As can be observed, the controlled-rate mode allows the consecution of a more uniform distribution of shear stress (or apparent viscosity) data in the whole shear rate range studied, which cannot be obtained in the controlled-stress mode as a consequence of the marked yielding characteristics of the samples studied. Of course, the mechanical spectra obtained in SAOS measurements, inside the linear viscoelastic range, are not influenced by the type of rheometer or operation mode employed (). All rheological measurements were carried out at temperatures comprised between 0 and 60°C. At least two replicates of each test were performed on fresh samples.
RESULTS AND DISCUSSION
Physicochemical Parameters
collects the values of parameters deduced from the physicochemical analysis of the different jam formulations studied. Physicochemical properties of borojó jams were very similar to those of both peach and borojó commercial jams. All model jams were processed to reach a soluble solid content close to 65 °Brix, which is basically the same obtained for the commercial peach jam but slightly lower than that measured in the commercial borojó jam. On the contrary, peach jam showed the highest moisture content.
SAOS Tests
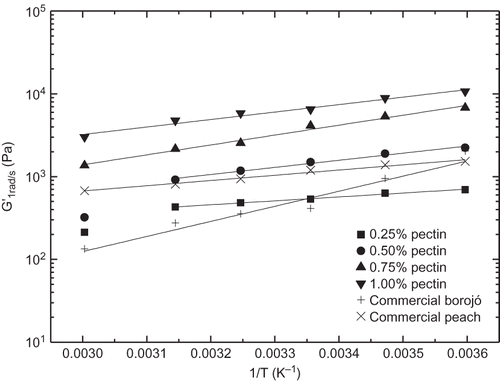
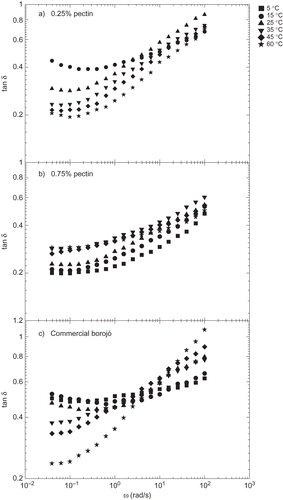
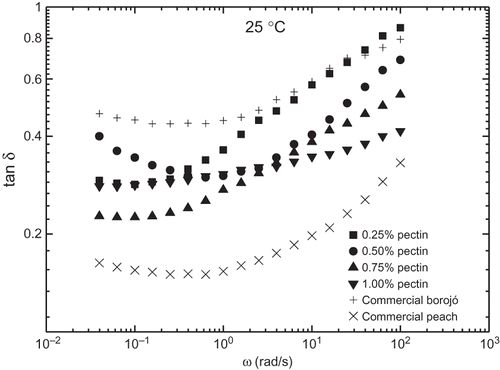
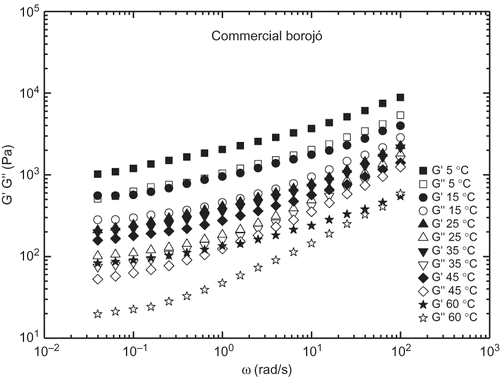
shows the evolution of the storage (G′) and loss (G″) moduli with frequency within the linear viscoelastic region for model borojó jam samples formulated with different added pectin concentration at three selected temperatures. These formulations are compared with commercial borojó and peach jams. In all cases, the typical gel-like behaviour with G′ values higher than G″ in all the frequency range studied is apparent.[Citation15] A power-law evolution (with approximate constant slope in log-log plots) with frequency was found for G′, whereas a tendency to reach constant values at low frequencies, i.e., a plateau region, was detected for G″. The same behaviour was reported for mango jams containing sucrose or sorbitol.[Citation9] Therefore, neither these viscoelastic functions nor G* are susceptible to be described through power-law models as previously proposed[Citation9,Citation24,Citation25] in the whole frequency range. Besides, SAOS functions increased with added pectin concentration. Differences of more than one decade were found by modifying the added pectin content from 0.25 to 1.0%, especially at low temperatures. Moreover, the frequency dependence of both moduli for model formulations is approximately the same to that found in the commercial borojó jam, with very similar values of SAOS functions in the case of jam formulation containing 0.5% pectin at 5°C. However, jam containing 0.25% pectin is more similar to the commercial sample above 25°C. This means that the temperature effect is greater for the commercial borojó jam. A rather different behaviour was shown by the commercial peach jam, which exhibited a much lower frequency dependence of both SAOS functions. Thus, for instance, G′ values for this jam are similar to those obtained with model borojó jam formulation containing 0.5% pectin in the low frequency range but are similar to those shown by the borojó jam with 0.25% pectin in the high frequency range, independently of the temperature (). Moreover, the relative elasticity is slightly affected by the pectin content. As can be observed in , the loss tangent (tan δ = G″/G′) generally decreased with increasing the pectin content, especially at high frequencies. However, all model formulations exhibited tan δ values comprised between those found in both commercial formulations analysed.
Figure 2 Frequency dependence of SAOS functions for the different jam formulations studied at selected temperatures.
Figure 3 Evolution of the loss tangent with frequency for the different jam formulations studied at 25°C.
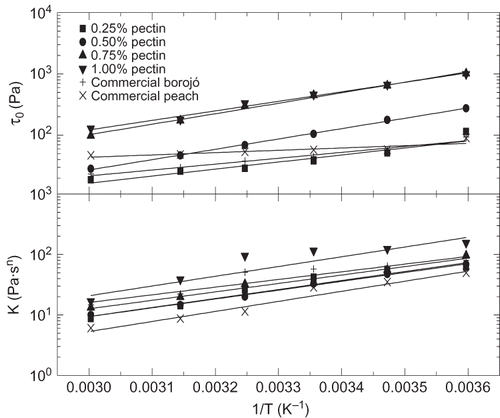
The effect of temperature on some rheological parameters of different jams has been previously reported,[Citation7,Citation8,Citation19] although mainly focusing on the viscous flow behaviour. From , a decrease in the SAOS functions by increasing temperature can be detected. As previously mentioned, the temperature effect seems to be different for model and commercial borojó jams. Temperature influence is much better illustrated in and for a model (0.75% added pectin) and commercial borojó jams, respectively. As can be seen, the linear viscoelastic functions of commercial borojó jam are more affected with temperature than observed for the model formulation. On the contrary, a very low thermal dependence was found for the commercial peach jam. The G′ value at 1 rad/s was selected to quantify the influence of temperature by using an Arrhenius-type equation:
Table 2 Activation energy values obtained from the fittings to EquationEqs. (1), Equation(3), and Equation(4) for the different jam formulations studied
Figure 4 Frequency dependence of SAOS functions as a function of temperature for a model borojó jam containing 0.75% added pectin.
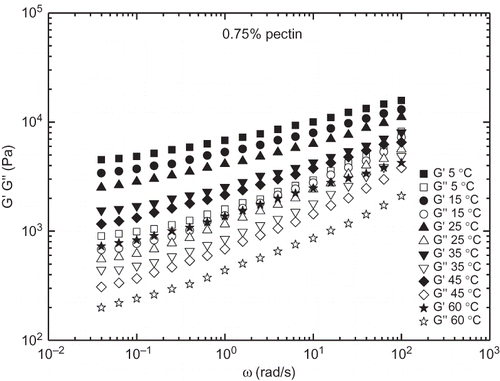
Figure 5 Frequency dependence of SAOS functions as a function of temperature for the commercial borojó jam formulation.
Viscous Flow Behaviour
As previously reported,[Citation8,Citation18,Citation19] jams typically display a shear-thinning behaviour associated to a tendency to reach a yield stress value. This flow behaviour has been frequently expressed in terms of the Herschel-Bulkley model:[Citation29]
Table 3 Herschel-Bulkley parameters for the different jam formulations studied as a function of temperature
Figure 8 Viscous flow curves and Herschel-Bulkley's fittings (solid lines) for the different jam formulations studied at 25°C.
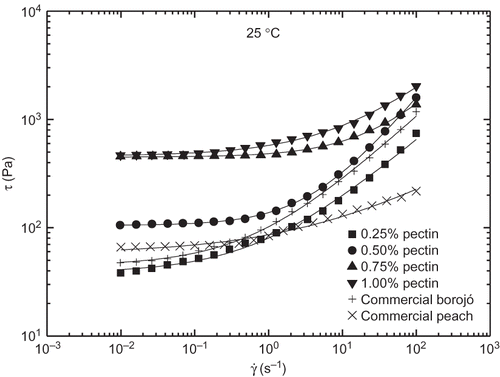
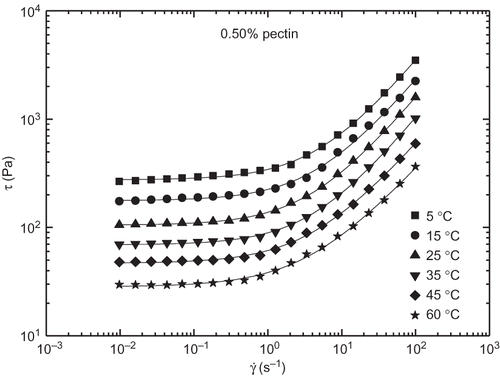
Besides this, as illustrated in , for a selected model jam formulation apparent viscosity (or stress) values decreased with increasing temperature but, however, the shear rate dependence was not significantly modified. Hence, as can be observed in , τ o and values decreased with increasing temperature but, however, n did not shown any systematic variation with temperature. Similarly, to that proposed above for G′, two Arrhenius-type relationships (R 2 > 0.895) were used to quantify the influence of temperature on both τ o and k parameters ():
CONCLUSIONS
The rheological behaviour of borojó (Borojoa patinoi Cuatrec.) jams was studied as a function of temperature (5–60°C) and added pectin content (0.25–1.0% wt.). SAOS response reveals a typical gel-like behaviour, whereas the viscous flow can be fairly well described by the Herschel-Bulkley model. In general, linear viscoelastic functions and viscosity values increased with pectin concentration and decreased with increasing temperature. Model jam formulations containing low pectin concentrations (0.25–0.5% wt.) are more similar to both borojó and peach commercial samples used as references. Thus, the yield stress values for both commercial jam formulations are generally comprised between the values obtained for model borojó jams containing 0.25 and 0.5% added pectin. However, commercial peach jam exhibited a much lower frequency dependence of both SAOS functions than borojó jams. Arrhenius-type relationships were used to quantify the influence of temperature on the storage modulus (G′), yield stress (τ o ), and consistency index (k). Model borojó jam formulations show higher temperature dependence of linear viscoelastic functions than peach jam sample but lower than borojó commercial jam. On the contrary, the influence of temperature on the yield stress is lower for both commercial jams, whereas the effect on the consistency index is very similar for all jam samples studied.
REFERENCES
- Arango , A.G.J. and Quijano , T.J. 1986 . Estudio de los frutos de Boroja patinoi (Cuatrec.). Revista Latinoamericana de Química , 17 ( 3–4 ) : 167 – 169 .
- Ricker , M. , Jessen , J.H. and Daly , D.C. 1997 . The case for Borojoa patinoi (Rubiaceae) in the Chocó region, Colombia . Economic Botany , 51 : 39 – 48 .
- Biocomercio , CORPEI. 2004 . Ecociencia , Quito , Ecuador : Estudio de mercado para el Borojó; Final report .
- Mosquera , L.H. , Moraga , G. and Martínez-Navarrete , N. 2010 . Effect of maltodextrin on the stability of freeze-dried borojó (Borojoa patinoi Cuatrec.) . powder. Journal of Food Engineering , 97 : 72 – 78 .
- Mosquera , L.H. , Moraga , G. , Fernández , de Córdoba and Martínez-Navarrete , P. 2011 . N. Water content–water activity–glass transition temperature relationships of spray-dried borojóas related to changes in color and mechanical properties . Food Biophysics , 6 : 397 – 406 .
- Pelegrine , D. H. , Silva , F.C. and Gasparetto , C.A. 2002 . Rheological behaviour of pineapple and mango pulps . LWT–Food Science and Technology , 35 : 645 – 648 .
- Basu , S. , Shivhare , U.S. and Raghavan , G.S.V. 2007 . Time dependent rheological characteristics of pineapple jam . International Journal of Food Engineering , 3 : 1
- Basu , S. and Shivhare , U.S. 2010 . Rheological, textural, microstructural and sensory properties of mango jam . Journal of Food Engineering , 100 : 357 – 365 .
- Basu , S. , Shivhare , U.S. , Singh , T.V. and Beniwal , V.S. 2011 . Rheological, textural and spectral characteristics of sorbitol substituted mango jam . Journal of Food Engineering , 105 : 503 – 512 .
- Baker , R.A. , Berry , N. , Hui , Y.H. and Barrett , D.M. 2005 . “ Food preserves and jams ” . In Processing Fruits , 2nd , Edited by: Barrett , D.M. , Somogyi , L. and Ramaswamy , H.S. Boca Raton , FL : CRC Press . InEdEds.
- Norma Técnica , INEN. 1988 . “ Ecuatoriana ” . In 419. Conservas vegetales , Quito , Ecuador : Mermeladas de frutas. Requisitos .
- Lal , G. , Siddappaa , G.S. and Tandon , G.L. 1998 . Preservation of fruits and vegetables , Vol. 197 , New Delhi : ICAR .
- Steffe , J.F. 1996 . Rheological Methods in Foods Process Engineering , 2ndEd , East Lansing , MI : Freeman Press .
- Barbosa-Canovas , G.V. , Kokini , J.L. , Ma , L. and Ibarz , A. 1996 . The rheology of semiliquid foods . Advances in Food and Nutrition Research , 39 : 1 – 69 .
- Gallegos , C. and Franco , J.M. 1999 . Rheology of food, cosmetics and pharmaceuticals . Current Opinion in Colloid & Interface Science , 4 : 288 – 293 .
- Gallegos , C. , Franco , J.M. and Partal , P. 2004 . “ Rheology of food dispersions ” . In Rheology Reviews; , Edited by: Binding , D.M. and Walter , K. 19 – 65 . UK : The British Society of Rheology: Aberystwyth . InEds.
- Tabilo-Munizaga , G. and Barbosa-Canovas , G.V. 2005 . Rheology for the food industry . Journal of FoodEngineering , 67 : 147 – 156 .
- Carbonell , E. , Costell , E. and Durán , L. 1991 . Caracterización del flujo de mermeladas comerciales españolas . Revista Agroquímica y Tecnología Alimentaria , 31 : 227 – 235 .
- Alvarez , E. , Cancela , M.A. and Maceiras , R. 2006 . Effect of temperature on rheological properties of different jams . International Journal of Food Properties , 9 : 135 – 146 .
- Díaz-Ocampo , R. , García-Zapateiro , L. and Franco , J.M. “ Caracterización de la pulpa fresca de borojó ” . In (Borojoa patinoi Cuatrec.) Ciencia y Tecnología 2012, 5, 17–24.
- AOAC . 2005 . Official Methods of Analysis , 18th , Gaithersburg , MD : Association of Official Analytical Chemists .
- Franco , J.M. , Gallegos , C. and Barnes , H.A. 1998 . On slip effects in steady-state flow measurements of oil-in-water food emulsions . Journal of Food Engineering , 36 : 89 – 102 .
- Sánchez , M.C. , Valencia , C. , Franco , J.M. and Gallegos , C. 2001 . Wall slip phenomena in oil-in-water emulsions: Effect of some structural parameters . Journal of Colloid and Interface Science , 241 : 226 – 232 .
- Subramanian , R. , Muthukumarappan , K. and Gunasekaran , S. 2006 . Linear viscoelastic properties of regular- and reduced-fat pasteurized processed cheese during heating and cooling . International Journal of Food Properties , 9 : 377 – 393 .
- Gabriele , D. , De Cindio , B. and D'Antona , P. 2001 . A weak gel model for foods . Rheologica Acta , 40 : 120 – 127 .
- Tsoga , A. , Richardson , R.K. and Morris , E.R. 2004 . Role of cosolutes in gelation of high-methoxy pectin . Part 1. Comparison of sugars and polyols. Food Hydrocolloids , 18 : 907 – 919 .
- Agoub , A.A. , Giannouli , P. and Morris , E.R. 2009 . Gelation of high methoxy pectin by acidification with D-glucono-d-lactone (GDL) at room temperature . Carbohydrate Polymers , 75 : 269 – 281 .
- Haghighi , M. , Rezaei , K. , Labbafi , M. and Khodaiyan , F. On the formulation design and rheological evaluations of pectin-based functional gels . Journal of Food Science , 76 E15 – E22. . 2011
- Herschel , W.H. and Bulkley , R. 1926 . Konsistenzmessungen von gumibenzollosungen . Kolloid-Z , 39 : 291 – 300 .