Abstract
Tyrosinase is the key enzyme in the biosynthesis of melanin. In order to investigate the effect of apple polyphenols on tyrosinase activity, the enzyme kinetic method was used. The concentration of apple polyphenols, which caused the tyrosinase activity to be lost by 50%, was 1.0 mg ml−1 for monophenolase and 0.9 mg ml−1 for disphenolase. When the concentration of apple polyphenols was 2.0 mg ml−1, the lag time before reaction of monophenolase was extended from 1.2 to 4.5 min. Inhibition kinetics analysis suggested that apple polyphenols were reversible and mixed inhibitors for tyrosinase with an inhibition equilibrium constant 2.3 mmol l−1. The results indicated that apple polyphenols were ideal tyrosinase inhibitors and could be used in food and cosmetics.
INTRODUCTION
Tyrosinase (EC 1.14.18.1), the key enzyme in melanin biosynthesis, is a copper metal oxidase that is distributed widely in nature. Tyrosinase has both monophenolase and diphenolase activities, which can hydroxylate tyrosine into levodopa (L-DOPA) and oxidate L-DOPA into dopa quinine.[Citation1] Abnormal expression of tyrosinase in the human body will result in various diseases, such as skin disease and Parkinson’s disease.[Citation2] Tyrosinase in plants has a significant effect on metabolism and biosynthesis of compounds, such as L-DOPA, phenolic compounds, and alkaloids. Tyrosinase can cause browning of fruits and vegetables as well. Therefore, considerable attention has been paid to the study of tyrosinase inhibitors.
Tyrosinase inhibitors can prevent the biosynthesis of melanin by inhibiting tyrosinase activity, protect fruits and vegetables from browning, and prevent diseases caused by melanin. Tyrosinase inhibitors also have whitening effects when they are used in cosmetics. It has been reported that phenolic compounds and substances containing a 4-hydroxy-3-methoxyphenyl structure have an inhibitory effect on tyrosinase.[Citation3] Gong et al.[Citation4] showed that ferulic acid had a significant inhibitory effect on tyrosinase.
Apple polyphenols were chosen as the effector in this study. The inhibitory effect of apple polyphenols on catalyzing reaction of tyrosinase and the associated inhibitory kinetics were investigated. Kinetic parameters were obtained in order to provide a theoretical basis for the molecular design and practical application of tyrosinase inhibitors, and provide new methods for the development of efficient tyrosinase inhibitors.
MATERIALS AND METHODS
Materials and Reagents
Apple pomace (Guoguang apple) was obtained from Yuantong Fruit Juice Factory in Yantai on October 25, 2008, and was vacuumized before use in analysis. Tyrosinase was purchased from the American Worthington Company (specific activity was 2000 U mg−1); L-DOPA was purchased from the American NOVO Company; L-tyrosine was purchased from the American Sigma Company; ethanol, DMSO, disodium hydrogen phosphate, and sodium dihydrogen phosphate were all sourced from China.
Methods
Extraction and purification of apple polyphenols
An ultrasonic extraction method and macroporous adsorptive resin (NKA-9) were used to extract and purify phenolic compounds from apple pomace. The ultrasonic-assisted extracting condition of phenolic compounds from apple pomace was as follows: extracting solvent 60% ethanol; extracting time 30 min; solid-liquid ratio 1:6 (g/ml); extracting temperature 60°C; and extracting once. The dynamic adsorption condition of NKA-9 was as follows: apple polyphenol concentration 2.5 mg ml−1; pH value 4.5; and velocity of flow 1.0 ml min−1. The desorption condition was as follows: ethanol concentration 70%, velocity of flow 1.0 ml min−1, desorption volume 10 BV. Under the above parameters, the purity of apple polyphenols was 70%.
Effect of apple polyphenols on monophenolase and diphenolase activity
Tyrosinase activity was measured by a method modified from Jones et al.[Citation5] Tyrosinase was prepared in 50 mM of phosphate buffer, pH 6.8 (assay buffer) at 2000 U ml−1 and stored at -20°C in 1-ml aliquots prior to use. For use in assays, stock enzyme solutions were thawed and diluted to 200 U ml−1 with assay buffer. A 2-mM working solution of substrate, L-DOPA, was prepared in assay buffer for each assay. Samples were dissolved in 10% DMSO (0.5 ml) and diluted to 5 ml with assay buffer. The reaction mixture consisted of 0.050 ml 2 mM L-DOPA, 0.050 ml 200 U ml−1 tyrosinase, and 0.050 ml apple polyphenol solution. Reaction volume was adjusted to 0.200 ml with assay buffer. Assays were performed in 96 well Falcon 3097 flat-bottom microtiter plates (Beckton Dickinson, NJ, USA). Appearance of dopachrome was measured with a Bio-Tek Instruments EL311 microplate reader (Winooski, VT, USA). Average velocity was determined from linear enzyme rate as measured by change in absorbance (A475) at 475 nm min−1. Relative enzyme activity of tyrosinase by test samples was determined by comparison of A475 of samples versus control using the formula:
Relative enzyme activity (%) = (A1 − A0)/(A2 − A0) × 100%,
where A0 is reaction system without apple polyphenols or tyrosinase; A1 is reaction system with apple polyphenols, tyrosinase and substrate; and A2 is reaction system without apple polyphenols.
Inhibitory mechanism of apple polyphenols on tyrosinase
The concentration of L-DOPA was 0.5 mmol l−1. Solutions containing different concentrations of tyrosinase were added into the reaction solution and the effect of different apple polyphenol concentrations on tyrosinase catalyzing L-DOPA was recorded to estimate the inhibitory effect of apple polyphenols on tyrosinase.
Kinetics analysis of apple polyphenols inhibiting tyrosinase
The method used to determine inhibition type and the constant of apple polyphenols on tyrosinase was the same as the method used by Huang et al.[Citation6] In the activity determination system, tyrosinase concentration was fixed at 5 mg l−1, and the inhibitory effect of apple polyphenols on tyrosinase was determined by changing the L-DOPA concentration. The kinetics parameters of the tyrosinase catalyzing reaction were obtained by Lineweaver-Burk double reciprocal cartography. The inhibition type was estimated by comparing the changes in the kinetics parameters, including Michaelis constant (Km) and maximum reaction rate (Vm).
RESULTS AND ANALYSIS
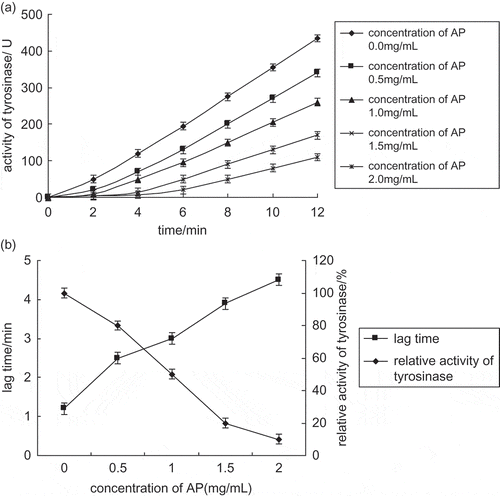
Effect of Apple Polyphenols on Monophenolase Activity
There is a lag time before the reaction of monophenolase.[Citation7] The length of the lag time depends on the concentrations of tyrosinase and monophenol in the reaction solution. The lag time will be shortened or eliminated when catechol or transition metal ions are added into the reaction solution,[Citation8] and it will be prolonged when compounds, such as phenolic ketones or cycloheptatriene, are added into the reaction solution.[Citation9] showed the inhibitory effect course curve of apple polyphenols on monophenolase (concentration of tyrosinase: 10 mg l−1; L-tyrosine concentration: 5 mmol l−1). At the beginning of this reaction, the reaction rate was very slow. After a lag period, there was a linear increase in the reaction rate and a constant rate was achieved, which indicated that the reaction had reached a steady state. The steady state was shown by the linear part of this curve, and the lag time was shown by the abscissa intercept of the linear part. When the concentration of apple polyphenols increased, the lag time was extended and the activity of the tyrosinase decreased. According to the above result, apple polyphenols had an inhibitory effect on monophenolase. showed the effect of apple polyphenols on lag time and steady enzyme activity. When the concentration of apple polyphenols increased, the lag time was extended significantly. The lag time of the enzyme reaction without apple polyphenols was 1.2 min, while the lag time of the reaction with 2.0 mg ml−1 apple polyphenols was 4.5 min. The lag time had increased to almost four times of that without apple polyphenols, and the steady enzyme activity decreased 90%. The concentration of apple polyphenols causing enzyme activity to decrease 50% (IC50) was 1.0 mg ml−1.
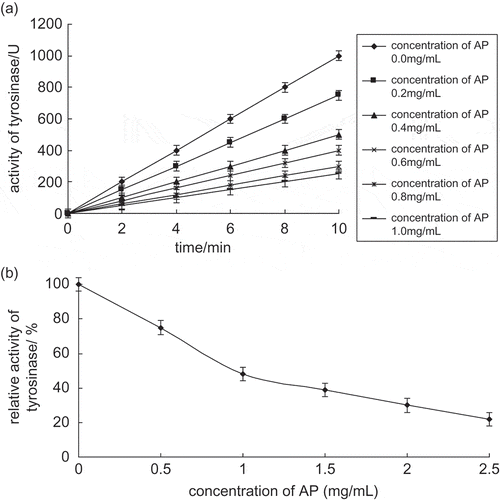
Effect of Apple Polyphenols on Diphenolase Activity
Initially, 5 mmol l−1 L-DOPA was chosen as a substrate; the concentration of tyrosinase was 10 mg l−1, and the effect of apple polyphenols on diphenolase activity was investigated and the result was shown in . The course curve of the tyrosinase reaction was a straight line passing through the origin. There was no lag time during this reaction. When the concentration of apple polyphenols increased, slope of the line decreased, which means that the reaction rate decreased. Therefore, apple polyphenols had an inhibitory effect on diphenolase. showed the inhibitory effect curve of apple polyphenol concentration on diphenolase. When the concentration of apple polyphenols increased, relative enzyme activity decreased. The concentration of apple polyphenols causing enzyme activity to decrease 50% (IC50) was 0.9 mg ml−1.
Inhibitory Mechanism of Apple Polyphenols on Tyrosinase
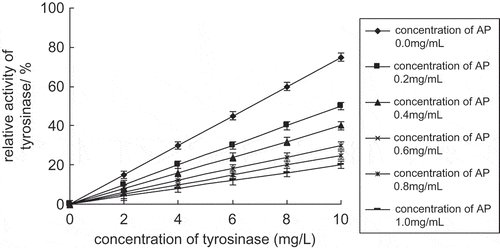
According to inhibitory effect, enzyme inhibitor can be classified as irreversible inhibitor and reversible inhibitor. If enzyme activity is inhibited by covalent combining of inhibitor and enzyme, and cannot be renewed by simple methods (such as dialysis), the inhibitor is irreversible. When a figure is made with relative enzyme activity and enzyme molar concentration, a group of straight lines with the same slope will be obtained. As inhibitor concentration is increasing, the lines move rightward. If enzyme activity is inhibited by reversible combining of enzyme and non-covalent bond of inhibitor, and can be renewed by simple methods (such as dialysis), the inhibitor is reversible. When a figure is made with relative enzyme activity and enzyme molar concentration, a group of straight lines will be obtained. As inhibitor concentration increases, the slopes of the lines decrease.
The concentration of substrate was 0.5 mmol l−1. Solutions containing different concentrations of tyrosinase were added into the reaction solution and the effect of different apple polyphenol concentrations on tyrosinase catalyzing L-DOPA was recorded. showed a group of straight lines passing through the origin and indicated the relationship between enzyme activity and enzyme concentration. When the concentration of apple polyphenols increased, the slope of the lines decreased. It indicated that the inhibitory effect of apple polyphenols on tyrosinase was reversible.
Kinetics Analysis of Apple Polyphenols Inhibiting Tyrosinase
Reversible inhibition includes competitive inhibition, non-competitive inhibition, and inverse competitive inhibition. The characteristic of competitive inhibition is: Michaelis constant (Km) increases, while maximum reaction rate (Vm) does not change. The characteristic of non-competitive inhibition is: Michaelis constant (Km) does not change, while maximum reaction rate (Vm) decreases. The characteristic of inverse competitive inhibition is: Michaelis constant (Km) decreases and maximum reaction rate (Vm) decreases too.
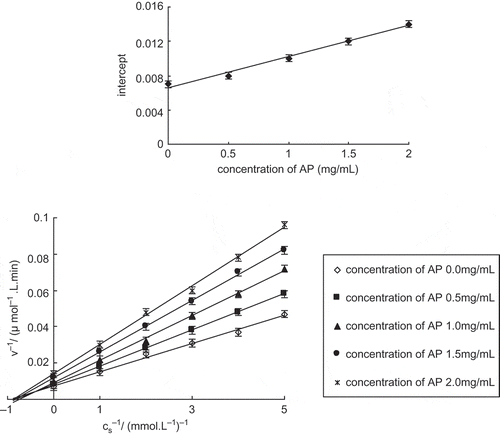
The type of inhibition caused by apple polyphenols on tyrosinase was investigated. In the activity determination system, tyrosinase concentration was fixed at 5 mg l−1, the inhibitory effect of apple polyphenols on tyrosinase was determined by changing the L-DOPA concentration. The kinetics parameters of the tyrosinase catalyzing reaction were obtained by Lineweaver-Burk double reciprocal cartography. The inhibition type was estimated by comparing the changes in the kinetics parameters, including Michaelis constant (Km) and maximum reaction rate (Vm).
showed that a group of straight lines having the same abscissa intercept were obtained by Lineweaver-Burk double reciprocal cartography. When the concentration of apple polyphenols increased, Km did not change, while Vm decreased. This suggests that apple polyphenols, when acting as tyrosinase inhibitor, are of non-competitive inhibitor. The combination of apple polyphenols and tyrosinase was separated from the combination of substrate and tyrosinase, and apple polyphenols did not change the affinity of tyrosinase for the substrate. A straight line was obtained by cartography of 1/Vm to apple polyphenol concentration, and an inhibition constant (Ki) (2.3 mmol/−1) was obtained from the slope (Ki = Kis).
DISCUSSION
Tyrosinase possesses both monophenol hydroxylase activity and diphenol oxidase activity.[Citation10] There are three types of tyrosinase during the course of catalysis: a reduction type (Emet), an oxidation type (Eoxy), and a deoxygenation type (Edeoxy). Eoxy possesses both monophenol hydroxylase activity and diphenol oxidase activity. Emet possesses only diphenol oxidase activity. Complexes without any activity will be obtained when Emet is combined with monophenol, which turns the tyrosinase catalyzing reaction into an endless circulation. That is why there is a lag time when tyrosinase catalyzes monophenol. Edeoxy can combine oxygen.[Citation11] As this experiment was carried out under the condition of oxygen saturation, the Km and Vm obtained from this experiment were all apparent constants, and the effect of oxygen concentration on these constants was not clear yet.
The type of apple polyphenols inhibiting tyrosinase is reversible inhibition, which illuminates that the reversible combination of apple polyphenols and tyrosinase causes the tyrosinase activity being inhibited, but does not cause tyrosinase inactivation. Apple polyphenols possess non-competitive inhibitory effect on diphenolase, which means apple polyphenols can combine not only free enzyme (Emet or Eoxy) but also enzyme-substrate complex (EmetS or EoxyS), and the binding constants are the same. The combination of apple polyphenols and tyrosinase happens outside of the enzyme active site, which leads to the creation of a substrate-tyrosinase-apple polyphenols ternary complex (SEI). In most cases, the SEI is an endpoint complex without any catalytic activity and cannot be decomposed. The effect of apple polyphenols cannot be eliminated even if the concentration of substrate is increased. Sometimes the SEI can be decomposed, but there is still an inhibitory effect on the catalytic reaction rate. Monophenolase possesses a lag time and the steady-state rate in this reaction system is obtained after the lag time. Apple polyphenols can prolong the lag time and cause the steady-state rate declining (), which means monophenolase activity is reduced by apple polyphenols. The possible reason is as follows: Emet and Eoxy can combine monophenol substrate, and the combination of Eoxy and monophenol results in a normal circular reaction, while the combination of Emet and monophenol results in an endless circular reaction. All of these reactions are reversible, but the balance of the combination and the whole reaction will prolong the time of catalytic reaction system being turned into a steady state, which creates lag time. The length of lag time is also closely related to the above course.
CONCLUSION
The inhibition mechanism of apple polyphenols on tyrosinase was elucidated in theory. A theoretical basis was provided for the development of food and cosmetics containing apple polyphenols. As apple polyphenols can be extracted from apple pomace produced by apple juice factories, and apple pomace is environmentally acceptable and easy to obtain, the food and cosmetics containing apple polyphenols possess broad application prospect and high economic potential.
REFERENCES
- Robb, D.A.; Lontie, R. ( Eds). Copper Protein and Copper Enzyme; CRC Press: Boca Raton, FL, 1984; 207–241.
- Xu, Y.; Stokes, A.H.; Freeman, W.M.; Kumer, S.C.; Vogt, B.A.; Vrana, K.E. Tyrosine mRNA is expressed in human substantia nigra. Molecular Brain Research 1997, 45, 159–162.
- Kubo, I.; Ikuyo, K.H. 2-Hydoxy-4-methoxybenzaldehyde: A potent tyrosinase inhibitor from African medicinal plants. Planta Medica 1999, 65, 19–22.
- Gong, S.Z.; Cheng, J.; Yang, Z.R. Inhibitory effect of ferulic acid on oxidation of L-DOPA catalyzed by mushroom tyrosinase. Chinese Journal of Chemistry 2005, 13 (6), 771–775 (in English).
- Jones, K.; Hughes, J.; Hong, M.; Jia, Q.; Orndorff, S. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigment Cell Research 2002, 15 (5), 335–340.
- Huang, H.; Liu, X.; Chen, Q. Studies on mushroom tyrosinase activity inhibited by benzaldehyde family compounds. Journal of Xiamen University: Natural Science 2003, 42 (1), 98–101.
- Cooksey, C.J.; Garratt, P.J.; Land, E.J.; Pavel, S.; Ramsden, C.A.; Riley, P.A.; Smit, N.P. Evidence of the indirect formation of the catecholici termediate substrate responsible for the autoactivatin kinetics of tyrosinase. Journal of Biological Chemistry 1997, 272, 26226–26235.
- Cabanes, J.; Garcia-Canovas, F.; Lozano, J.A.; Garcia-Carmona, F. A kinetic study of the melanization pathway between L-tyrosinase and dopachrome. Biochimica and Biophysica Acta (BBA)—General Subjects 1987, 923 (2), 187–195.
- Sancher, F.A.; Rodriguze, L.J.N.; Garcia, C.F. Tyrosinase: A comprehensive review of its mechanism. BBA Protein and Molecular Enzymology, 1995, 124 (7), 1–11.
- Chen, Q.X.; Ke, L.N.; Song, K.K.; Huang, H.; Liu, X.D. Inhibitory effects of hexylresorcinol and dodecylresorcinol on mushroom tyrosinase. The Protein Journal 2004, 23 (2), 135–141.
- Xie, L.P.; Chen, Q.X.; Huang, H.; Liu, X.D.; Chen, H.T.; Zhang, R.Q. Inhibitory effects of cupferron on the monophenolase and diphenolase activity of mushroom tyrosinase. The International Journal of Biochemistry & Cell Biology 2003, 35, 1658–1666.