Abstract
A new methodology was developed using bromelain as a proteolytic enzyme for the production of a partially hydrolyzed bovine milk formula, without changing the milk's organoleptic and nutritional properties. This study also revealed that the in vitro angiotensin-converting enzyme inhibition increased in the modified milk as compared with the control sample, showing the highest angiotensin-converting enzyme-inhibitory activity for fractions with molecular weights between 3 and 10 kg mol−1. The Lineweaver-Burk plot revealed that the produced milk formula acted as a non-competitive inhibitor, as determined by high performance liquid chromatography.
INTRODUCTION
Milk is a biological fluid with a high nutritional value and an unquestionable importance in the human diet has increasingly been acknowledged over the last two decades as a result of new scientific findings in the field of nutrition.[Citation1] However, it is well known that some individuals, particularly young children, have difficulty digesting bovine milk proteins. For this reason, new functional milk-derived products have been introduced in the market to satisfy the needs of individuals with milk protein intolerance.[Citation2] Milk proteins are considered the most important source of bioactive peptides. These peptides are inactive within the sequence of the parent protein but can be released by enzymatic hydrolysis using digestive enzymes (pepsin, trypsin, and chymotrypsin are the most prominent), fermentation of milk with proteolytic starter cultures, and proteolysis by enzymes derived from microorganisms or plants.[Citation1] These procedures have been used industrially to create different functional milks. The bioactive peptides have been shown under in vitro and in vivo conditions to exert a number of activities affecting the gastrointestinal, endocrine, cardiovascular, immune, and nervous systems.[Citation3] Among these, angiotensin-converting enzyme (ACE) inhibitory peptides have been extensively studied due to their capacity to control hypertension.[Citation4] In recent years, there has been considerable interest in using nutraceuticals or functional foods, including bioactive peptides in its composition, as potential alternative therapies to treat hypertension,[Citation3,Citation5–Citation Citation7] taking into consideration that hypertensive patients are susceptible to heart attack and ischemic cardiac disease, and these cardiovascular diseases are the second most common cause of death in many developed countries. It is a current opinion that the most economic and beneficial way to take advantage of ACE inhibitory products will be to market the peptides as contained in the crude protein hydrolysate and consumed on a long-term basis for desired therapeutic effects (e.g., Wu and Ding[Citation8]).
Bromelain is a collective name for proteolytic enzymes or proteases found in tissues, including stem, fruit, and leaves, of the pineapple plant of the Bromeliaceae family,[Citation9] a traditional plantation crop in the S. Miguel Island (Azores). Information about the use of bromelain to modify the bovine milk protein is very scarce. However, some authors have used bromelain to produce hydrolyzed proteins from other raw materials, such as Acaudina molpadioidea.[Citation10] On the other hand, since in the Azores Islands there are ca 110,000 milk cows for a population of 240,000 people, milk-based products are very common in the Azorean diet.[Citation11]
The objectives of this study were to develop a new type of functional bovine milk with partially hydrolyzed proteins, using bromelain as a proteolytic enzyme, keeping all of its organoleptic properties and to evaluate the hydrolysis extension using a reverse-phase high performance liquid chromatography (RP-HPLC) with ultra-violet (UV) detection. In addition, the study's goals were also to investigate the suitable substrate to enzyme ratio (S:E) in order to modify the milk proteins, to analyze the in vitro inhibitory effects of this new milk formula against ACE in comparison to commercial ultra pasteurized (UHT) Azorean milk sample, and furthermore, to survey its acceptability and palatability by submitting to a sensory panel. Physicochemical and microbiological properties of the produced milk formula were also analyzed and compared with the non-hydrolyzed UHT milk.
MATERIALS AND METHODS
Milk Samples and Chemicals
High quality bovine UHT skim and half-skimmed milk samples from Azorean Holstein cows were kindly donated by UNILEITE, U.C.L.T. (Arrifes, S. Miguel, Azores). HPLC-grade acetonitrile, HPLC-grade methanol, trifluoroacetic acid (TFA), trizma base, hippuryl-histidyl-leucine (HHL), hippuric acid (HA), hydrochloric acid (HCl), ACE from porcine kidney (A2580), bromelain (EC 3.4.22.32 from pineapple stem), rennet (type II from Mucor miehei), pepsin A (EC 3.4.23.1 from porcine stomach mucosa), trypsin (EC 3.4.21.4 type I from bovine pancreas), and other chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Deionized water was obtained with an in-house Milli-Q water purification system (Millipore, Bedford, MA, USA).
Sample Preparation
The milk samples were incubated with different proteolytic enzymes (bromelain, rennet, pepsin, and trypsin) at substrate:enzyme (S:E) = 150:1 mL mg−1 (v/w) at 30°C and at pH 6.5 based on the method published by Baptista et al.,[Citation12] during 1 h in a sterilized metallic container in order to maximize the heat transfers effect using a water bath (Buchi waterbath B-480). The enzymes were then inactivated by increasing the temperature of the water bath to 75°C for 30 min that completely stop the enzymatic reaction. This temperature also allows the milk pasteurization and avoids any changes in the milk's organoleptic properties. Furthermore, the best active enzyme was investigated at different ratios S:E (200:0, 200:1, 150:1, 100:1, 75:1, and 65:1 mL mg−1, v/w) in order to evaluate the hydrolysis extension.
RP-HPLC/UV Analysis of Hydrolyzed Milk Samples
The separation and quantification of the major bovine milk proteins was performed using a high performance liquid chromatography (HPLC) system (Perkin-Elmer model 500, San José, CA, USA), using the reverse-phase (RP) Vydac Protein-C4 (250 × 4.6 mm i.d.; 5 μm) column (Grace, Baltimore, MD, USA). The mobile phase consisted of phase A: 0.1% TFA in water and phase B: 0.1% TFA in acetonitrile. Gradient elution was carried out at a flow rate of 1 mL min−1 starting with A:B = 7:3 (v/v) followed by a linear gradient during 60 min reaching A:B = 1:1 (v/v) and A:B = 3:7 (v/v) after 70 min. Prior to injection (20 μL), the samples of commercial milk and milk hydrolysate were centrifuged during 10 min at 2500 rpm (433 g) and supernatant was diluted 1:1 (v/v) with distilled water. The effluent was monitored by an ultraviolet (UV) detector at 220 nm.
Ultrafiltration Process of the Hydrolyzed Milk Formula
The partially hydrolyzed half-skimmed milk sample at S:E = 100:1 mL mg−1 was ultrafiltrated at room temperature and under nitrogen pressure using two different molecular weight cut-offs (MWCO) membranes (Millipore Co., Billerica, MA, USA). The hydrolysate was first fractionated through a 10,000 MWCO membrane and the permeate fraction was again fractionated through a 3000 MWCO membrane. The three fractions obtained (>10, 10–3, and <3 kg mol−1) were then lyophilized and further analyzed in order to determine their inhibition of ACE activity.
Determination of ACE Inhibitory Activity of Hydrolyzed Milk Samples/Fractions by HPLC Methodology
The in vitro determination of ACE inhibitory activity was performed by RP-HPLC, based on the method described by Wu and Ding[Citation8] with some modifications. ACE inhibitory activity was measured towards hydrolysis of HHL (hippuryl-L-histidyl-L-leucine) synthetic peptide and confirmed by monitoring the formation of HA (hippuric acid) at 228 nm. The reaction system contained the following solutions in a total volume of 100 μL: 10 μL of ACE from porcine kidney prepared at 0.3 units mL−1 in 100 mmol L−1 Tris-HCl buffer (pH 8.3) containing 300 mmol L−1 NaCl and 10 μmol L−1 ZnCl2; 50 μL of buffer or lyophilized sample (25 mg mL−1) and 40 μL of 12.5 mmol L−1 HHL. After incubation at 37°C for 30 min, 10 μL of the reaction solution was injected to a Hewlett Packard HPLC system series 1050 equipped with an Adsorbosphere C18 (250 × 4.6 mm i.d.; 5 μm) column (Grace, Baltimore, MD, USA) to separate the HA from HHL. The column was eluted using an isocratic mobile phase (flow rate of 0.5 mL min−1) consisting of 14% methanol, 14% acetonitrile, and 72% water with 0.1% HCl. The effluent was monitored by an UV detector at 228 nm. The chromatographic data of the quantitative determinations were performed by a Schimadzu CR501 Chromatopac integrator and the results were expressed as percentage of inhibition of ACE activity using the following formula:
Determination of ACE Inhibition Pattern of the Hydrolyzed Milk Formula
Various substrate HHL (hippuryl-L-histidyl-L-leucine) concentrations, 1.57, 3.125, 6.25, and 12.5 mmol L−1, were incubated with ACE solution in the presence and/or absence of 25 mg mL−1 of partially hydrolyzed lyophilized milk formula. Reaction solution (10 μL) was injected after incubation at 37°C for 30 min and eluted on the RP-C18 column using the analytical conditions as described in the subsection above. Standard HA solution (1.12 mmol L−1) was injected first as a reference. The acidic mobile phase stopped the ACE reaction immediately following injection and the HA was separated and quantified. The Km and V max values for the different reactions were determined by GraphPad Prism (version 5.00) according to the Lineweaver-Burk kinetics.
Sensory Analysis of the Hydrolyzed Milk Formula
Sensory analysis was performed by a panel of 28 trained volunteers (10 males and 18 females in the 20–50-year-old range) representing potential consumers with specific sensory skills. This control group was not aware of the type of milk to be tested in order for them to pinpoint any significant differences between the two types of milk. The test was performed in the sensory laboratory of the Department of Technological Sciences and Development (University of Azores), in individual cabins under normal light conditions. In the cabins, there were two plastic sample cups identified with a coded combination of numbers and a water cup to be taken between them. Each participant had an anonymous questionnaire to identify possible differences in the organoleptic characteristics between a commercial UHT half-skimmed milk sample (from UNILEITE) and the new partially hydrolyzed UHT half-skimmed milk formula.
Physicochemical and Microbiological Properties of the Hydrolyzed Milk Formula
The physicochemical and microbiological parameters were evaluated according to the official methods of analysis that regulate the assessment of milk quality in Portugal. The physicochemical parameters include fat,[Citation13] pH, and acidity.[Citation14] Protein, lactose, and nonfat dry matter were estimated using a MilkoScan FT 120 (FOSS, Hilleroed, Denmark) equipment. The microbiological parameters analyzed were total yeast and molds,[Citation15] sporulated,[Citation16] and the stability/sterility test.[Citation17]
Statistical Analysis
Data of the ACE inhibitory activity are expressed as the means ± standard deviation (SD) of three independent measurements. Statistical analysis of the sensory results was acquired by Microsoft Office Excel, version 2007, software package for Windows.
RESULTS AND DISCUSSION
Production of a Partially Hydrolyzed Bovine Milk Formula with Bromelain
Food allergy is becoming a medical, economical, and social concern. Milk protein allergy is one of the main problems in the first year of children's life and, particularly, bovine milk protein allergy is the most severe one.[Citation18] Some alternatives have been introduced in the market, such as soy-based formulas, partially and extensively hydrolyzed whey, or casein as well amino acid-derived formulas.[Citation19]
Milk contains more than 50 proteins and the major ones are implicated in a number of immunologically mediated reactions.[Citation20] Caseins and β-lactoglobulin (β-Lg) have been described as the main antigenic and allergenic components for human beings.[Citation18,Citation21] Several chemicals and enzymatic methodologies have been used in order to modify bovine milk proteins. In this study, the first step was to choose the best proteolyptic enzyme to produce a bovine milk formula with partially hydrolyzed proteins, maintaining all the organoleptic and nutritional properties (same fat, nonfat dry matter, protein, lactose content) of the original milk. Four proteolytic enzymes (bromelain, rennet, pepsin, and trypsin) were tested using the same ratio S:E (150:1 mL mg−1) and the procedure is described in the Sample Preparation part of the Methods section. The results obtained, measured by the ratio (R) between the amino acids and small peptides (AASP) released versus caseins using a HPLC system with a Vydac C4-Protein reverse-phase column, show that trypsin had the highest proteolytic activity (R = 0.41), followed by bromelain (R = 0.23), rennet (R = 0.11), and pepsin (R = 0.10). Trypsin was rejected because of a yellowish color produced and the unpleasant taste. The yellowish color could be attributed to an acceleration of Maillard reaction due to the temperature applied during the process.[Citation22] The unpleasant taste may be attributed to the release of hydrophobic amino acids and peptides that increase the bitter sensation. This observation is in accordance with previous studies by Baptista et al.[Citation12] that showed that trypsin releases more hydrophobic peptides from β-Lg with high molecular weight, which are normally more associated with milk flavor, as compared with the other tested enzymes. Bromelain was used in this study for its proteolytic activity, because of its less expensive cost compared to trypsin and pepsin, and by its unique high temperature stability characteristics that, unlike rennet, loses activity at temperatures above 50°C.
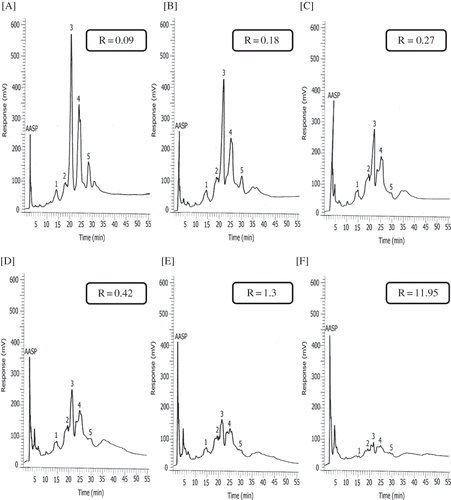
The second step in this study was to evaluate the extension of bromelain proteolytic (3–7 units/mg of protein) activity (one unit released 1.0 μmole of p-nitrophenol from Nα-Z-L-lysine p-nitrophenol ester per min at pH 4.5 at 25°C) in order to find the best S:E ratio to produce a stable modified milk and avoid clotting. shows the RP-HPLC profiles of partially hydrolyzed bovine skim milk samples at different ratios S:E (200:0, 200:1, 150:1, 100:1, 75:1 and 65:1 mL mg−1). An increase in concentration of bromelain ( to 1f) resulted in a natural increase of caseins hydrolysis, accordingly the observed raise of AASP and the consequent decrease in the content of caseins (peaks 1–5). In the chromatograms there is the indication of the R value for each S:E concentration. The high R (11.95) at S:E = 65:1 mL mg−1 () represents a clotted sample of milk in which micelles lose their stability. After several testings, the coagulation limit was established at S:E = 75:1 mL mg−1 (R = 1.3). However, the safety limit was considered at S:E = 100:1 mL mg−1 (R = 0.42) that presents 40% of caseins content reduction.
Figure 1 RP-HPLC profiles of partially hydrolyzed bovine skim milk samples at different substrate to enzyme ratios (mL mg−1): (a) S:E = 200:0; (b) S:E = 200:1; (c) S:E = 150:1; (d) S:E = 100:1; (e) S:E = 75:1; (f) S:E = 65:1. Hydrolysis conditions: S:E at 30°C during 1 h and inactivated during ½ h at 75°C. HPLC analytical conditions: Vydac C4-Protein (250 × 4.6 mm i.d., 5 μm) column. Mobile phase: 0.1% TFA in water (phase A) and 0.1% TFA in acetonitrile (phase B), starting with A:B 70:30 (v/v) followed by a linear gradient during 60 min reaching A:B 50:50 and A:B 30:70 after 70 min. Flow rate: 1 mL min−1. Injection volume: 20 μL. Detection: UV at 220 nm. Legend (peaks identification): AASP: amino acids and small peptides; 1: κ-casein; 2: αS2-casein; 3: αS1-casein; 4, 5: β-casein.
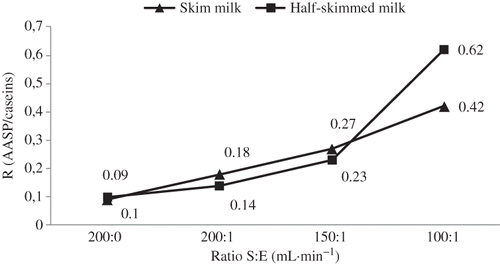
illustrates the comparison between partially hydrolyzed skim and half-skimmed milk samples. The results show that the proteolytic activity of bromelain was similar for skim and half-skimmed milk samples, with the exception of S:E = 100:1 mL mg−1, presenting the last sample with the highest hydrolysis value. This is expected because the amount of protein present in both milk samples was the same (3.2 g per 100 mL of milk), only the percentage of fat (0.1 and 1.6%, respectively) was different. According to the literature on several nutraceutical products, bromelain has been used for weight loss purposes. There are some who claim that bromelain can “digest fat” or absorb fat at an amazing rate by an unknown mechanism. Furthermore, there is no scientific evidence that explains how bromelain interacts with fat molecules in milk samples.[Citation23]
In Vitro ACE Inhibitory Activity Determination
ACE plays a key role in the renin-angiotensin system (RAS), which regulates both arterial blood pressure and fluid and salt balance in mammals.[Citation24] The inhibitory kinetic properties of food-protein-derived ACE inhibitors have been the subject of research efforts in order to elucidate the mechanism of the action of peptides. The developed continuous direct injection HPLC method for the determination of ACE inhibitory activity makes it possible to evaluate kinetic properties concisely and conveniently.[Citation8] Initial reaction velocity was obtained after plotting the amount of HA versus reaction time. The formation of HA increased with increasing reaction time.
In vitro ACE inhibitory activity of partially hydrolyzed milk formula
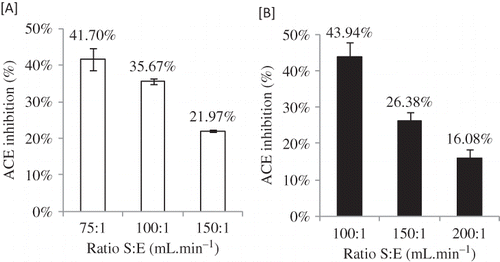
The ACE inhibitory activity of the enzymatically prepared hydrolysate makes it commercially attractive either as a functional food or as health-enhancing ingredient of a diversity of matrices.[Citation8] The ACE inhibitory activity of this new milk formula was slightly different in skim and half-skimmed milk samples, as illustrated in , which shows the percentage of ACE inhibition of milk samples at different S:E ratios. Comparing the samples at S:E = 100:1 mL mg−1 there is more activity in half-skimmed than in skim milk due to the release of more peptides from the higher hydrolysis obtained in the first one. In the half-skimmed milk, we could not analyze the sample at S:E = 75:1 mL mg−1 due to the fact that in most of the experiments the milk samples clotted. From the results obtained (), one main conclusion can be drawn: that is a high degree of hydrolysis provides hydrolysates with high ACE inhibitory activity. These results gain support from some of the existing literature (Janitha et al.[Citation25] and Jiang et al.[Citation26]).
ACE inhibitory kinetics of partially hydrolyzed milk formula
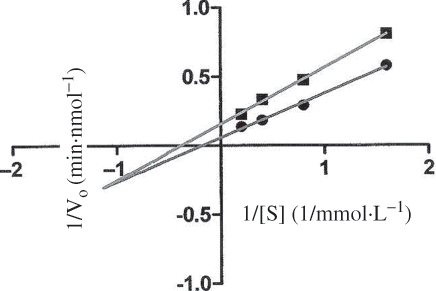
As shown in , the mode of inhibition of the ACE-catalyzed hydrolysis of HHL, evaluated by Lineweaver-Burk kinetics, was nearly non-competitive. Although most ACE inhibitors derived from food protein hydrolysates belong to the competitive mode,[Citation8] non-competitive ACE inhibitory peptides also have been reported.[Citation27,Citation28] A non-competitive inhibitor is a substance that interacts with the enzyme, but usually not at the active site. The effect of a non-competitive inhibitor is to change the shape of the enzyme and, thus, the active site so that the substrate can no longer interact with the enzyme to give a reaction.[Citation29]
ACE inhibitory activity of partially hydrolyzed milk formula fractions
The most active fraction of the partially hydrolyzed half-skimmed milk sample at S:E = 100:1 mL mg−1 was the one with molecular weight between 3 and 10 kg mol−1 (75.9% ± 2.65), followed by fraction >10 kg mol−1 (29.6% ± 1.21) and fraction <3 kg mol−1 (14.0% ± 0.17). According to some authors, the fraction <3 kg mol−1 has the highest ACE inhibitory activity (Jiang et al.[Citation26] and Hong et al.[Citation30]). However, our results show that this fraction is the less active one. The low activity of this fraction reveals that AASP are present in very small amounts in comparison with other low molecular weight compounds, such as salts, vitamins, carbohydrates, and lipids.[Citation31] Indeed, the ACE inhibitory activity of this fraction is not relevant to the study. The fraction with molecular weight >10 kg mol−1is mainly composed of partial and/or intact proteins. It is well known that ACE inhibitory peptides are generally short chain peptides.[Citation30,Citation32] This fact could explain the lower ACE inhibition of this fraction. The binding to ACE is strongly influenced not only by the extent of the peptide chain but also by the C-terminal sequence, whereby hydrophobic amino acids, such as tryptophan, tyrosine, proline, or phenylalanine, are more active if present at each of the three C-terminal positions.[Citation33] In addition, the presence of the positive charge of lysine and arginine as the C-terminal residue may contribute to the inhibitory potency. Proline as the C-terminal residue has been shown to enhance binding to the ACE, although the presence of another hydrophobic amino acid like leucine does not seem to affect the final activity.[Citation33]
Sensory Analysis of the Milk Formula
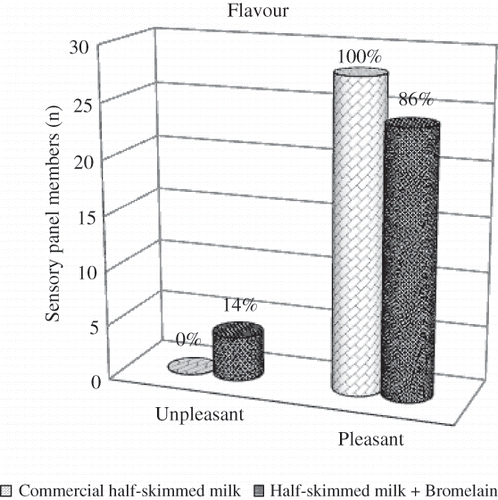
Several publications report that peptides released by proteolysis have a bitter taste and that depends on the size of the peptide and its hydrophobicity. In addition, bitterness depends on conformational factors, because only part of the peptide interacts with the gustatory receptor.[Citation34,Citation35] Some authors have stated that the 193–201 fraction of β-caseins is responsible for the bitterness of hydrolyzates. Others have asserted that bitterness depends on the proteolytic enzyme used.[Citation36,Citation37] To investigate this fact, we submitted the milk samples to a sensory analysis trained panel. shows that 86% of the participants found a pleasant taste in the modified milk and only 14% found a different taste on the milk in comparison with commercial UHT half-skimmed milk sample (the most consumed milk in the Azorean market). From the results presented, we conclude that modified milk with bromelain does not release or release less peptides with bitter taste and less hydrophobic contrasting with some proteases, such as trypsin that originates an unpleasant taste on the milk. Furthermore, the modified milk was kept during one week at a refrigerated temperature (4–5°C) with no changes in the organoleptic properties and no tendency to coagulate.
Physicochemical and Microbiological Properties of the Milk Formula
In order to evaluate the physicochemical and microbiological properties of the milk formula, skim and half-skimmed milk partially hydrolyzed with bromelain were submitted to industrial standard quality procedures. The results obtained by physicochemical analysis are shown in . All the parameters analyzed (fat, protein and lactose content, nonfat dry matter, pH, acidity) were similar for both partial hydrolyzed and non-hydrolyzed samples. With regards to the microbiological parameters, the values obtained were considered normal according to the Portuguese quality norms for milk products.
Table 1 Comparison of physicochemical properties of the hydrolyzed and non-hydrolyzed milk samples
CONCLUSION
The results obtained from this study clearly revealed the possibility of industrially producing a bovine milk formula with partial hydrolysis of caseins using bromelain at a substrate to enzyme ratio of 100:1 mL mg−1. This new product will provide an additional gain to casein intolerants, not only because it represents an innovative concept, but also due to the fact that this functional milk preserves the sensory and nutritional characteristics of the original milk. This study also demonstrated that the ACE-inhibitory activity of milk casein hydrolyzates varied with the degree of hydrolysis and the amount of lipid present in the milk. Additional physicochemical studies are necessary to adjust this new milk formula to industrial production, taking into account the complex requisites of the industrial process. Furthermore, it is crucial in the future to evaluate this modified milk in vivo as hypoallergenic and hypotensive milk formula, and to characterize the compositional characteristics of the produced protein hydrolysate.
ACKNOWLEDGMENTS
The authors are indebted to União de Cooperativas Agrícolas de Lacticínios e Produtores de Leite da Ilha de S. Miguel (UNILEITE), Arrifes, Ponta Delgada, Azores, for the milk samples. Financial support for graduate studies (Vera Medeiros) was provided by Azores University, UNILEITE, and the Government of Azores.
REFERENCES
- Kitts , D. and Weiler , K. 2003 . Bioactive proteins and peptides from food sources . Applications of bioprocesses used in isolation and recovery. Current Pharmaceutical Design , 9 : 1309 – 1323 .
- Caffarelli , C. , Plebani , A. , Poiesi , C. , Petroccione , T. , Spattini , A. and Cavagni , G. 2002 . Determination of allergenicity to three cow's milk hydrolysates and an amino acid-derived formula in children with cow's milk allergy . Clinical & Experimental Allergy , 32 : 74 – 79 .
- Korhonen , H. 2009 . Milk-derived bioactive peptides: from science to applications . Journal of Functional Foods , 1 : 177 – 187 .
- Lopez-Fandino , R. , Otte , J. and van Camp , J. 2006 . Physiological, chemical and technological aspects of milk protein derived peptides with antihypertensive and ACE inhibitory activity . International Dairy Journal , 16 : 1277 – 1293 .
- Aludatt , M. , Ereifej , K. , Abou-Zaitoun , A. , Al-Rababah , M. , Almajwal , A. , Rababeh , T. and Anti-oxidant , Yang, W. 2011 . anti-diabetic and anti-hypertensive effects of extracted phenolics and hydrolyzed peptides from barley protein fractions . International Journal Food Properties , doi: 10.1080/10942912.2010.503357
- Chen , Z. , Peng , C. , Jiao , R. , Wong , Y. , Yang , N. and Huang , Y. 2009 . Anti-hypertensive nutraceuticals and functional foods . Journal of Agricultural and Food Chemistry , 57 : 4485 – 4499 .
- Turpeinen , A.M. , Kumpu , M. , Ronnback , M. , Seppo , L. , Kautiainen , H. , Jauhiainen , T. , Vapaatalo , H. and Korpela , R. 2009 . Antihypertensive and cholesterol-lowering effects of a spread containing bioactive peptides IPP and VPP and plant sterols . Journal of Functional Foods , 1 : 260 – 265 .
- Wu , J. and Ding , X . 2002 . Characterization of inhibition and stability of soy-protein-derived angiotensin I converting enzyme inhibitory peptides . Food Research International , 35 : 367 – 375 .
- Doko , M. , Bassani , V. , Casadebaig , J. , Cavailles , L. and Jacob , M. 1991 . Preparation of proteolytic enzyme extracts from Ananas comosus L . Merr. fruit juice using semipermeable membrane, ammonium sulfate extraction, centrifugation and freeze-drying processes. International Journal of Pharmaceutics , 76 : 199 – 206 .
- Zhao , Y. , Li , B. , Dong , S. , Liu , Z. , Zhao , X. , Wang , J. and Zeng , M. 2009 . A novel ACE inhibitory peptide isolated from Acaudina molpadioidea hydrolysate . Peptides , 30 : 1028 – 1033 .
- Leite , J. , Lima , E. and Baptista , J. 2007 . Azorean bovine milk conjugated linoleic acid . Effect of green pasture feeding diet, storage and processing temperature. Lait , 87 : 167 – 179 .
- Baptista , J. , Tavares , J. , Carvalho , R. and Isolation , Jerónimo, M. 1999 . partial characterization and applications of azorean pineapple stem proteinases (bromelains) . Açoreana , 9 : 63 – 84 .
- Norma Portuguesa NP 469 . 2002 . Leites. Determinação da matéria gorda (técnica de Gerber). Processo corrente , Instituto Português da Qualidade .
- Norma Portuguesa NP 470 . 1983 . Leites. Determinação da acidez , Instituto Português da Qualidade .
- Norma Portuguesa NP 1934 . 1986 . Microbiologia alimentar. Leite e produtos lácteos. Contagem de bolores e leveduras , Instituto Português da Qualidade .
- Norma Portuguesa NP 577 . 1987 . Microbiologia alimentar. Leites, leitelhos e soros, líquidos e em pó. Pesquisa de bactérias esporuladas anaeróbias. Técnica de Weinzirl , Instituto Português da Qualidade .
- Norma Portuguesa NP 579 . 1991 . Microbiologia alimentar. Leite esterilizado e leite ultrapasteurizado (UHT). Apreciação da estabilidade e da esterilidade , Instituto Português da Qualidade .
- Docena , G. , Fernandez , R. , Chirdo , F. and Fossati , C. 1996 . Identification of casein as the major allergenic and antigenic protein of cow's milk . Allergy , 51 : 412 – 416 .
- Brill , H. 2008 . Approach to milk protein allergy in infants . Canadian Family Physician , 54 : 1258 – 1264 .
- Sampson , H.A. 1999 . Food allergy. Part 1: Immunopathogenesis and clinical disorders . Journal of Allergy and Clinical Immunology , 103 : 717 – 728 .
- Restani , P. , Gaiaschi , A. , Plebani , A. , Beretta , B. , Cavagni , G. , Fiocchi , A. , Poiesi , C. , Velonà , T. , Ugazio , A. and Galli , C. 1999 . Cross-reactivity between milk proteins from different animal species . Clinical & Experimental Allergy , 29 : 997 – 1004 .
- Jaeger , H. , Janositz , A. and The , Knorr, D. 2010 . Maillard reaction and its control during food processing . The potential of emerging technologies. Pathologie Biologie , 58 : 207 – 213 .
- Medeiros , V. , Rainha , N. , Lima , E. and Baptista , J. 2011 . “ Is the proteolytic activity of bromelain increased by milk fat content? ” . In Proceedings of the International Food Congress—Novel Approaches in Food Industry Vol. 2 , 862 – 866 . Izmir , Turquia
- FitzGerald , R. , Murray , B. and Walsh , D. 2004 . Hypotensive peptides from milk proteins . The Journal of Nutrition , 134 : 980S – 988S .
- Janitha , P. , Wanasundara , P. , Ross , A. , Amarowicz , R. , Ambrose , S. , Pegg , R. and Shand , P. 2002 . Peptides with angiotensin I-converting enzyme (ACE) inhibitory activity from defibrinated, hydrolyzed bovine plasma . Journal of Agricultural and Food Chemistry , 50 : 6981 – 6988 .
- Jiang , Z. , Tian , B. , Brodcrob , A. and Production , Huo, G. 2010 . analysis and in vivo evaluation of novel angiotensin-I-converting enzyme inhibitory peptides from bovine casein . Food Chemistry , 123 : 779 – 786 .
- Matsufuji , H. , Matsui , T. , Seki , E. , Osajima , K. , Nakashima , M. and Angiotensin , Osajima, Y. 1994 . I-converting enzyme inhibitory peptides in an alkaline protease hydrolyzed derived from sardine muscle . Bioscience, Biotechnology, and Biochemistry , 58 : 2244 – 2245 .
- Nakagomi , K. , Yamada , R. , Ebisu , H. , Sadakane , Y. , Akizawa , T. and Tanimura , T. 2000 . Isolation of acein-2, a novel angiotensin-I converting enzyme inhibitory peptide derived from a tryptic hydrolysate of human plasma . FEBS Letters , 467 : 235 – 238 .
- Leskovac , V. 2003 . Comprehensive Enzyme Kinetics , New York : Kluwer Academic/Plenum Publishers .
- Hong , F. , Ming , L. , Yi , S. , Zhanxia , L. , Yongquan , W. and Chi . 2008 . L. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides , 29 : 1062 – 1071 .
- Banks , W. and Dalgleish , D. 1990 . Dairy Microbiology—The Microbiology of Milk , 2nd , Edited by: Robinson , R.K. New York : Elsevier Science Publishers .
- Mao , X. , Ni , J. , Sun , W. , Hao , P. and Fan , L. 2007 . Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides . Food Chemistry , 103 : 1282 – 1287 .
- Cheung , H. , Wang , F. , Ondetti , M. , Sabo , E. and Cushman , D. 1980 . Binding of peptide substrates and inhibitors of angiotensin-converting enzyme . Importance of the COOH-terminal dipeptide sequence. Journal of Biological Chemistry , 255 : 401 – 407 .
- Bumberger , E. and Belitz , H.D. 1993 . Bitter taste of enzymatic hydrolysates of casein . I. Isolation, structural and sensorial analysis of peptides from tryptic hydrolysates of beta-casein. Zeitschrift für Lebensmittel-Untersuchung und –Forschung , 197 : 14 – 19 .
- Lalasidis , G. 1978 . Four new methods for debittering protein hydrolysates and a fraction of hydrolysates with high content of essential amino acids . Annales de la nutrition et de l'alimentation , 32 : 709 – 723 .
- Maehashi , K. , Matsuzaki , M. , Yamamoto , Y. and Udaka , S. 1999 . Isolation of peptides from an enzymatic hydrolysate of food proteins and characterization of their taste properties . Bioscience, Biotechnology, and Biochemistry , 63 : 555 – 559 .
- Singh , T. , Young , N. , Drake , M. and Cadwallader , K. 2005 . Production and sensory characterization of a bitter peptide from beta-casein . Journal of Agricultural and Food Chemistry , 53 : 1185 – 1189 .