Abstract
Indian grape wines are analyzed for total phenolic content and antioxidant activity along with other parameters, such as pH, alcohol content, and reducing sugars. Concentration of polyphenols, like tannic acid, catechol, vanillin, caeffic acid, ferullic acid, and resveratrol, was quantified using reverse phase-high performance liquid chromatography and ultra high performance liquid chromatography. The red wines showed the highest concentration of phenolic content (6.5 ± 0.1 mg/ml) and antioxidant activity (84.60 ± 1%) as compared to white and port wines, while red wine R2 showed the highest radical scavenging activity among red wines and R4 showed the lowest total phenolic content. The white wine W3 showed less total phenolic content and antioxidant activity. Further, a positive correlation between phenolic content and antioxidant activity was observed.
INTRODUCTION
Free radicals are extremely harmful to living organisms; they attack different constituents of the cell, accelerating its aging and destruction. Several human disease conditions, such as cancer, cardiovascular diseases, aging, and neurodegenerative disorder, are the result of high oxidative stress.[Citation1] Hence, certain amounts of natural or synthetic antioxidants are necessary to maintain an adequate level of antioxidants to balance the sources of reactive oxygen species (ROS). It has been reported that the moderate consumption of wine, particularly red wine, reduces the incidences of cardiovascular disease, atherosclerosis, platelet aggregation, diabetes type II, stroke, cataracts, and cancer.[Citation2 − Citation5] These beneficial effects of wine are due to the presence of rich polyphenolic components present in it, which acts as antioxidants or reactive oxygen species scavengers.[Citation1]
Phenolic compounds present in wine range from relatively simple compounds to complex tannin substances, which contribute to its color, taste, structure, and make the product suitable for aging.[Citation6] There are almost 20 polyphenols that are identified in wine samples and are divided into two groups: flavonoids and non-flavonoids. The most common flavonoids are tannic acid, vanillin, ferullic acid, catechin, caeffic acid, epiallocatechins, quercitin, myricetin, kaempferol, anthocyanins, morins, and p-coumaric acid, and non-flavonoids are stillbenes (resveratrol), benzoic acid, etc.[Citation7]
The richness of phenolics in wine originates from grapes; it depends upon skin, degree of maturity, environmental factors, and wine making technique, which affect wine qualitatively and quantitatively. Red and white wine differs in phenolic composition due to the difference in red and green grape varieties and in wine making procedures.[Citation7,Citation8] India is one of the fastest growing markets for wine on the global map and per capita level consumption of wine in India is increasing at about 25 to 30% every year.[Citation9] Various factors, such as modern society culture, lifestyle, tourism, and favorable government policies, allow for the steady growth of wine consumption in India. According to the statistical evaluation in 2011, consumption of red wines in India is nearly 52%, white wines 40%, and port wines 3%.[Citation9] These figures will change drastically in the near future due to an increase in the wine drinking culture across the country. The consumption of wine is increasing day by day, but the literature search has revealed that no work has been carried out on biochemical characterization and antioxidant activity of the wines produced in India.
In the present study, selected Indian wines were evaluated to determine the biochemical characterization, polyphenolic compounds, and radical scavenging activity. Biochemical parameters, such as pH, alcohol content, and reducing sugars, were determined. Total phenolic content was determined by using Folin-Ciocalteu reagent and HPLC analysis, whereas radical scavenging activity was determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay; furthermore, a correlation was set up between these parameters for all wine samples.
MATERIALS AND METHODS
Wine Sample Collection
Three different types of Indian wine samples, such as red, white, and port, were collected from following wine yards tabulated below, and the parameters for collection of the sample were maintained during the entire analysis ().
Table 1 Indian wines collected from different vineyards and their harvesting period
Preparation of Wine Samples for Phenolic Estimation
The wine samples were de-alcoholized by concentrating them using rotary evaporation at 25°C. An equal volume of distilled water was added to wine and concentrated to its original volume in order to remove alcohol without destroying the phenolic compounds.[Citation8]
pH measurement
pH estimation was carried out according to Joslyn.[Citation10]
Estimation of alcohol content
Alcohol content was estimated using potassium dichromate reagent with a slight modification in the method as per Seo et al.[Citation11] First, 10 ml of potassium dichromate reagent (0.2 M potassium dichromate and 4 M sulphuric acid) was added to 700 μl of wine (1:700 diluted) and was incubated for 30 min at room temperature. After incubation, 100 ml of distilled water and 4 ml of potassium iodide (25%) was added in each flask. The solution was titrated against sodium thiosulfate (0.1 N) with starch (1%) as an indicator. The excess dichromate was determined by titration against sodium thiosulfate. Subtracting the amount of excess dichromate from the initial amount gives the amount of alcohol present. Accuracy can be improved by calibrating the dichromate solution against a blank. All titrations were carried out in triplicate and values were reported as mean of the experiment.
Reducing Sugar Assay
Reducing sugar was determined by modified 3,5-dinitrosalicylic acid (DNSA) method as described by Sengupta et al.[Citation12] A volume of 0.5 μl of the wine sample was mixed with 1.5 ml of distilled water and the solution was mixed with 2 ml of the DNSA solution; after addition of the DNSA, the solution was incubated in a water bath for 5 min, cooled, and then 10 ml of the distilled water was added. Absorbance was measured spectrophometrically at 520 nm. The standard curve was prepared using maltose. Total reducing sugar is expressed as a maltose equivalent (mg/ml), which is a common reference compound.
Determination of Total Phenolic Content
Folin-Ciocalteu reagent was used for the determination of total phenolic content (TPC) as per Chaturvedi et al.[Citation13] Wine samples of different varieties were mixed with Folin-Ciocalteu reagent and aqueous sodium carbonate. The mixtures were kept for 30 min at room temperature and then observations were taken by spectrophotometer at 650 nm. The standard curve was prepared considering tannic acid as the standard. Total phenolic values are expressed as mg/ml using tannic acid as standard, which is a common reference compound.
Radical Scavenging Activity
The free radical scavenging activity was estimated using DPPH as described by Chaturvedi et al.[Citation13] and Wang et al.[Citation14] A solution of 0.3 mM DPPH in methanol was prepared and 0.5 ml of this solution was mixed with 100 μl of the wine sample dissolved in distilled water. The reaction mixture was left in the dark at room temperature for 30 min. The absorbance of the mixture was measured spectrophotometrically at 518 nm. The ability to scavenge DPPH radical was calculated as percent inhibition of control.
Lipid Peroxidation Assay
Wistar rats (weighing about 240 ± 20 g and 3 months old) were used due to their close resemblance to the human system for the preparation of mitochondria. Rat liver was excised and homogenized in 0.25 M sucrose containing 1 mM EDTA. The homogenate was centrifuged at 3000× g for 10 min, to remove cell debris and the nuclear fractions. The supernatant was centrifuged at 10,000× g for 10 min to sediment the mitochondria.[Citation13] Bradford reagent was prepared according to Chaturvedi et al.[Citation13] by dissolving 100 mg of Coomassie Brilliant Blue G-250 in 50 ml 95% ethanol, and add 100 ml 85% (w/v) phosphoric acid. Then, dilute the prepared reagent to 1 liter when the dye has completely dissolved, and filter it through Whatman No.1 paper just before use. Filtration may have to be repeated to get rid of the blue components. Protein was estimated at 595 nm and mitochondrial pellets were suspended in potassium phosphate buffer at the concentration of 10 mg protein/ml. Oxidative damage was induced by an ascorbate-FeCitation2 + system as described by Devasagayam.[Citation15] The pink color of thiobarbituric acid reactive substances formed was estimated spectrophotometrically at 532 nm as malondialdehyde equivalents after accounting for appropriate blanks. Malondialdehyde standard was prepared by the acid hydrolysis of tetraetoxypropane. All the results are the mean of the triplicate reading.
HPLC Characterization of Wines for Polyphenol Content and Resveratrol Detection
Analysis of individual phenolic compounds present in the different wine samples was performed by Waters HPLC (Model 2487), using a hypersil C18 reversed phase column 15 cm with 5 μ particle size. A constant rate of 0.75 ml/min was used with two mobile phases: (a) 25% methanol in 1% acetic acid and solvent (b) 75% methanol in 1% acetic acid. The elution gradient was linear starting with (a) and ending with (b) over 60 min, using a UV detector set at wavelength 280 nm.
Resveratrol detection was carried out by UltiMate 3000 RSLC System for HPLC analysis, manufactured by DIONEX, which consists of Column Acclaim PA2, C18, 3μ, and dimensions 4.6 * 150 mm. Phenolic compound and resveratrol for each sample were identified by comparing their relative retention time with the standards of mixture. Standard phenolic compounds were obtained from Sigma (USA). The concentration of an individual compound was calculated on the basis of peak area measurements and then converted to ppm. All the chemicals and solvents used were HPLC spectral grade.
Statistical Analysis
All the observations were taken in triplicate and the data were analyzed using analysis of variance technique (ANOVA) (p < 0.001) and the means were separated by Duncan's multiple range test using SPSS (version 14.0).
Table 2 Estimation of pH, alcohol content by potassium dichromate, reducing sugars by DNSA method, polyphenols, and resveratrol concentration by RP-HPLC and UHPLC analysis
RESULTS AND DISCUSSION
pH of Wine Samples
pH of the wine samples was observed to be acidic in nature. Red wine (R4) is most acidic in nature (3.39) among all the samples of the red wines, whereas white wine (W4) with a pH of 3.05 is most acidic compared to all other white wines, and port wine (P1) showed a pH of 3.56, which is highest in port wines respectively (). pH is an important factor that strongly influences the properties of wine, such as color, odor, taste, biological and chemical stability. Lower pH values are known to improve the stability and inhibit microbial and bacterial growth, so wine makers usually prefer a pH range of 3.0 to 3.5 as a crucial guideline in wine making. Wines from South Africa reported to have a pH of 2.5 to 5.0.[Citation16]
Alcohol Content
It was found that port wines have a high percentage of alcohol (16.64%) followed by red wines (13.20%) and white wines (11.48%), respectively (). Alcohol in wine is produced naturally from the fermentation process when yeast is added. Wines from South Africa and France reported to have an alcohol content of 14.5 and 12.5%, respectively.[Citation17] The presence of alcohol in wine aids polyphenol absorption and contributes to their bioavailability because before absorption, polyphenols are hydrolyzed by intestinal enzymes or by colonic micro flora, then undergo intestinal and liver metabolism; hence, the presence of alcohol protects phenols from their loss.[Citation18]
Reducing Sugar
In the wine-making process, during fermentation yeast converts sugar through enzymatic action into CO2 and alcohol. Once the fermentation is complete, sugar enhances sweetness in wine.[Citation19] Reducing sugar of port wine (P1) was observed to be 16.58 ± 0.001 mg/ml followed by red wine (R1) (3.47 ± 0.002 mg/ml) and white wine (W1) (3.58 ± 0.0 mg/ml) (). Total reducing sugar is expressed as a maltose equivalent (mg/ml), which is a common reference compound. Similar studies showed that the concentration of reducing sugar in Italian wines was 0.8 mg/ml.[Citation7]
Total Phenolic Content
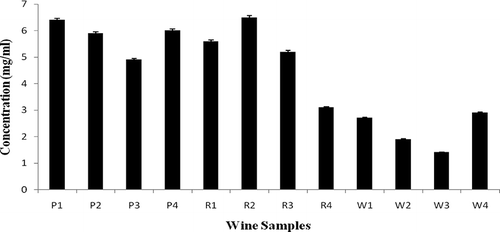
The red wines showed the highest phenolic content to that of port wine and white wine. R2 showed phenolic content of 6.5 ± 0.1 mg/ml whereas R4 showed minimum concentration of phenolic compounds (3.1 ± 0.02 mg/ml). Similarly, P1 has 6.4 ± 0.4 mg/ml of total phenolic content to that of other port wines; a lesser concentration of phenolic content was observed in W3 (1.4 ± 0.04 mg/ml) among all of the other white wines (). The concentration of phenolics decreases in the order: red > port > white wine. These results are in agreement with those available in the literature.[Citation6,Citation20] Red wines are rich in simple and complex phenolic compounds mainly represented by phenolic acids, flavonols, monomeric catechins, and tannins compared to white wines and port wines. The grape phenol composition and content are affected by several factors, such as variety, ripening time, climate, soil, and place of growing. The composition of phenolics in wine also depends on the type of fruits used for vinification, their extraction procedures employed for wine making, and the chemical reactions that occur during aging of the wine.[Citation6] In addition, wine making technologies with other aging modifications also affect both phenolic composition and amount of antioxidant activity. Similarly, the phenolic content in the Italian red wine was observed to be 5.8 ± 0.43 mg/ml.[Citation7]
Radical Scavenging Activity
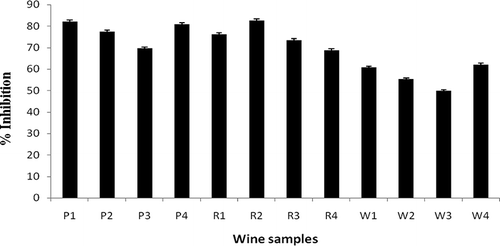
Wine contains a variety of low molecular mass of polyphenol molecules and many of these have a primary antioxidant role. In order to survive, these antioxidative compounds counteract with reactive oxygen species (ROS).[Citation13] The addition of the wine to the DPPH solution induces a rapid decrease in absorbance at 517 nm. All wine samples had significant DPPH scavenging ability. The % inhibition of DPPH activity for R2, P1, and W4 was 84.60 ± 1%, 82.06 ± 1%, and 62.08 ± 2%, respectively (). Radical scavenging activity decreases in the order: red > port > white wines. Thus, the phenolics and polyphenolic compounds are natural antioxidants that enhance the free radical scavenging activity.[Citation21,Citation22] While Galvez et al.[Citation23] showed that there is a correlation between antioxidant capacity and phenolic content, Kahkonen et al.,[Citation24] however, stated that correlation of antioxidant activity with high concentration of phenols is not necessary. Whereas in the present investigation it was observed that there is a correlation between the antioxidant capacity and phenolic content of red wine, white wine and port wine, which are statistically significant (p < 0.001). As wine is the complex mixture of phenolics, antioxidant activity of wines is not a property of single phenolic compound. During maceration, the phenolic compounds from solid parts of the grapes are extracted into wine, which increases the value to be a beneficial man-made product. Radical scavenging activity of the red wine from Spain and Australia was observed to be 82.04 ± 1% and 72 ± 1%, respectively. Similarly, white wines from Spain and California showed 68 ± 2% and 62 ± 1% radical scavenging activity.[Citation16]
Lipid Peroxidation Assay
Reactive oxygen species (ROS) cause lipid peroxidation, which leads to deterioration of food quality and nutritional value due to formation of potential toxic compounds.[Citation13] Antioxidative compounds increase the shelf life and nutritive value of food by their radical scavenging activity.[Citation24] The data in represents inhibitory effect of various selected wine samples on lipid peroxidation in rat liver mitochondria. Among all the selected wine samples, W1 was found to be most effective, which showed 63.47% protection followed by P2 46.49% and R1 29.27%, respectively. The activity of peroxide decreases with the increase in the antioxidant activity,[Citation25] while the absorbance values are much lower with higher antioxidant activities of the samples. Antioxidants are protected once they enter the body by the same process that our body uses to detoxify ingested ethanol. In the liver, two nicotinamide adenine dinucleotide (NAD)-dependent enzymes are present: alcohol dehydrogenase that converts ethanol to acetaldehyde and then aldehyde dehydrogenase converts ethanol to acetaldehyde and then to acetate. These enzymes produce nicotinimide adenine dinucleotide hydrogen (NADH) (reduced) in each step. NAD is then capable of recycling the used antioxidants by reducing them and simultaneously regenerates NAD+, which is required to detoxify more ethanol.[Citation26] Similarly, lipid peroxidation by TBARS of the wines from China showed that the values varied from 31 to 69% for the red wines, 38 to 46% for the white wines, and 51 to 54% for the rose wines.[Citation1]
Table 3 Effect of various wine samples on rat liver mitochondria for lipid peroxidation and percent protection
Detection of Polyphenols and Resveratrol Using HPLC
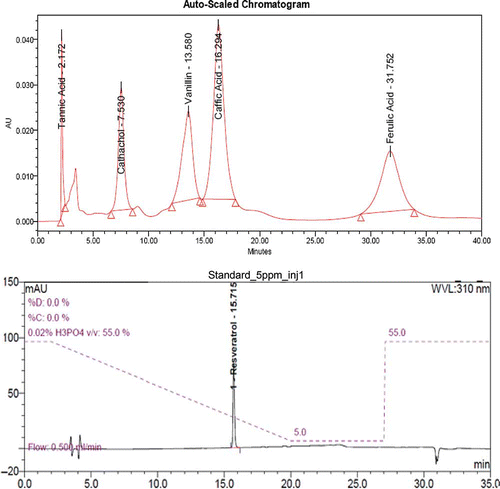
Wine is a very complex mixture, as a rich source of polyphenols, a class of compounds that has gained considerable interest due to research suggesting their health benefits. Combinations of polyphenols in fruits and vegetables can prevent cancer and other diseases.[Citation27] In addition, polyphenols are quality attributes of wine and contribute to color and sensory properties, such as flavor and astringency. Individual phenolic compounds like tannic acid, cathechol, ferulic acid, caeffic acid, vanillin, and resveratrol in wine samples were analyzed and quantified using reverse phase-high performance liquid chromatography (RP-HPLC) and ultra high performance liquid chromatography (UHPLC) (); it was observed that all the wine samples showed the presence of tannic acid (), whereas other polyphenol contents, such as vanillin, catechol, ferullic acid, and resveratrol, were also detected in a lesser concentration compared to that of tannic acid. P2 showed a higher concentration of tannic acid (83.852 ppm), cathechol (11.29 ppm), vanillin (2.56 ppm), and ferullic acid (23.204 ppm) compared to other port wine samples, but resveratrol was not detected in any of the port wines. Similarly, R2 showed a high concentration of tannic acid (72.099 ppm), catechol (5.373 ppm), vanillin (9.607 ppm), and the concentration of resveratrol in R1 was found to be 0.05 ppm, whereas W2 contained tannic acid (47.137 ppm), catechol (0.767 ppm), vanillin (0.113 ppm), caeffic acid (0.538 ppm), and resveratrol (0.09 ppm). Resveratrol was also detected in W3 (0.08 ppm) and W4 (0.07 ppm), but ferullic acid was not detected in any of the red and white wine samples.
Figure 3 HPLC analysis: HPLC chromatogram of standard, separated on C18 column, Hypersil (USA) (Revere phase column 15 cm; particle size 5 μm) using gradient elution–acetic acid and methanol at a total flow rate of 0.75 ml/min. The chromatograms at 280 nm were analyzed and compared. Standard for resveratrol by UHPLC instrument. (Color figure available online.)
From the present investigation it is verified that red wines have higher phenolic content than white and port wines; the same results are obtained for antioxidant activity. The concentration of phenolic compound and antioxidant activity vary considerably in different types of wines, depending on the grape variety and processing techniques. According to the obtained results, Indian wines are acidic in nature and they showed appropriate levels of alcohol and reducing sugars. The concentration of various phenolic compounds including resveratrol was obtained in white wines and red wines but it was present below a detectable level in port wines. Considering the importance of wine, it warrants further investigation for various parameters affecting wine quality, stability, and processing techniques applied in wine making.
ACKNOWLEDGMENTS
The authors are thankful to Mr. Subhash Kudale for his help in RP-HPLC analysis and Dr. Ranjan Mogre and Dr. V. R. Bhate, Analytical Solutions, Navi Mumbai for UHPLC analysis. Thanks are also due to the higher authorities of Padmashree Dr. D. Y. Patil University for providing necessary facilities.
REFERENCES
- Li , H. , Wang , X. , Li , Y. , Li , P. and Wang , H. 2009 . Polyphenolic compounds and antioxidant properties of selected China wines . Food Chemistry , 112 : 454 – 460 .
- Seruga , M. , Novak , I. and Jakobek , L. 2011 . Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods . Food Chemistry , 124 : 1208 – 1216 .
- Di Majo , D. , La Guardia , M. , Giammanco , S. , La Neve , L. and Giammanco , M. 2008 . The antioxidant capacity of red wines in relationship with its polyphenolic constituents . Food Chemistry , 111 : 45 – 49 .
- Gambelli , L. and Santaroni , G.P. 2004 . Polyphenols content in some Italian red wines of different geographical origins . Journal of Food Composition and Analysis , 17 : 613 – 618 .
- Tedesco , I. , Russo , M. , Russo , P. , Iacomino , G. , Russo , G.L. , Carraturo , A. , Faruolo , C. , Moio , L. and Palumbo , R. 2000 . Antioxidant effect of red wine polyphenols on red blood cells . The Journal of Nutritional Biochemistry , 11 ( 2 ) : 114 – 119 .
- Katalinić , V. , Milos , M. , Modun , D. , Musić , I. and Boban , M. 2004 . Antioxidant effectiveness of selected wines in comparison with (+)-catechin . Food Chemistry , 86 ( 4 ) : 593 – 600 .
- Cimino , F. , Sulfaro , V. , Trombetta , D. , Saija , A. and Tomaino , A. 2007 . Radical scavenging capacity of several Italian red wines . Food Chemistry , 103 ( 1 ) : 75 – 81 .
- Roussis , I.G. , Lambropoulos , I. and Soulti , K. 2005 . Scavenging capacities of some wines and wine phenolic extracts . Food Technology and Biotechnology , 43 ( 4 ) : 351 – 358 .
- JBC International . 2011 . Indian Wine Market Report Gore, J., Sundar, K.Washington, DC: JBC International,
- Joslyn , M.A. 1950 . Methods in Food Analysis , 1 – 528 . New York : Academic Press .
- Seo , H.B. , Kim , H.J. , Lee , O.K. , Ha , J.H. , Lee , H.Y. and Jung , K.H. 2009 . Measurement of ethanol concentration using solvent extraction and dichromate oxidation and its application to bioethanol production process . Journal of Industrial Microbiology and Biotechnology , 36 ( 2 ) : 285 – 292 .
- Sengupta , K. , Singh , B.D. and Mustai , C.C. 2000 . A study on the effect of time of harvest of mulberry leaf on silkworm (Bombyx mori L.) . cocoon crop and cocoon quality. Indian Journal of Sericulture , 10 : 1 – 5 .
- Chaturvedi , P.A. , Ghatak , A.A. and Desai , N.S. 2011 . Evaluation of antioxidant activity and polyphenol content in Woodfordia fruticosa from different altitudes . Journal of Plant Biochemistry and Biotechnology , 21 ( 1 ) : 17 – 22 .
- Wang , L. , Xu , H.N. , Yao , H. and Zhang , H. 2012 . Phenolic composition and radical scavenging capacity of Vaccinium Bracteatum Thunb . leaves. International Journal of Food Properties , 15 : 2
- Devasagayam , T.P.A. 1986 . Senescence associated decrease of NADPH-induced lipid peroxidation in rat liver microsomes . FEBS Letters , 205 : 240 – 246 .
- Marchal , R. , Maujean , A. and Bouquelet , S. 1996 . Antioxidant activity and polyphenol content of the South African wines . Journal of Agricultural and Food Chemistry , 44 ( 7 ) : 1716 – 1722 .
- Kruma , Z. , Karklina , D. , Cinkmanis , I. and Rutkovska , O. 2010 . Polyphenolic composition and free radical scavenging activity of red wines available in the Latvian market . Chemine Technologia , 1 ( 54 ) : 55 – 61 .
- Rodrigo , R. , Miranda , A. and Vergara , L. 2011 . Modulation of endogenous antioxidant system by wine polyphenols in human disease . Clinica Chimica Acta , 412 ( 5–6 ) : 410 – 424 .
- Zoecklein , B.W. , Fugelsang , K.C. , Gump , B.H. and Nury , F.S. 1995 . Wine Analysis and Production , New York : Chapman and Halls .
- Paixão , N. , Perestrelo , R. , Marques , J.C. and Câmara , J.S. 2007 . Relationship between antioxidant capacity and total phenolic content of red, rose and white wines . Food Chemistry , 105 ( 1 ) : 204 – 214 .
- Velioglu , Y.S. , Mazza , G. , Goa , L. and Omah , B.D. 1988 . Antioxidant activity and total phenolics in selected fruits, vegetables and grain products . Journal of Agricultural and Food Chemistry , 46 : 4113 – 4117 .
- Kim , B.J. , Kim , J.H. , Kim , H.P. and Heo , M.Y. 1997 . Biological screening of 100 plants extracts for cosmetic use . II-Antioxidative activity and free radical scavenging activity. Journal of Cosmetic Science , 19 : 299 – 307 .
- Galvez , M. , Martin-Codero , C. , Houghton , P.J. and Ayuso , M.J. 2005 . Antioxidant activity of methanol extracts obtained from Plantago species . Journal of Agricultural and Food Chemistry , 53 : 1927 – 1933 .
- Kahkonen , M.P. , Hopia , A.I. , Vuorela , H.J. , Rauha , J.P. , Pihlaja , K. , Kujula , T.S. and Heinonen , M. 1999 . Antioxidant activity of plant extracts containing phenolic compounds . Journal of Agricultural and Food Chemistry , 47 : 5181 – 3962 .
- Bai , X. , Zhang , W. , Chen , W. , Zong , W. , Guo , Z. and Liu , X. 2010 . Anti-hepatotoxic and anti- oxidant effects of extracts from Piper nigrum L . root. African Journal of Biotechnology , 10 ( 2 ) : 267 – 272 .
- Negi , B. and Gargi , D. 2009 . “ Comparative analysis of total phenolic content in sea buckthorn wine and other selected fruit wines ” . In World Academy of Science Engineering and Technology 54 396–399.
- Hee , I.M. , Park , Y.S. , Ham , K.S. , Kang , S.G. , Heo , B.G. , Leontowicz , H. , Leontowicz , M. , Namiesnik , J. , Najman , K. and Gorinstein , S. 2012 . Effects of cooking on the bioactivity of lotus roots and white onions . International Journal of Food Properties , 15 : 49 – 59 .