Abstract
Emulsions undergo an oxidation process because of the presence of water. The undesired phenomenon of autoxidation needs to be prevented as the lipidic fraction in the emulsion undergoes degradation. The surface active compounds so formed in the process disturb the stability of the emulsion. Accordingly, the characteristics of the emulsions are altered. This autoxidation process can be prevented by means of antioxidant. In the present study, a well known hydrophilic antioxidant, ascorbic acid, was examined at various concentrations to stabilize water-in-oil emulsion. The outcome showed that the concentration of a compound played an important role in determining its antioxidant or pro-oxidant effect.
INTRODUCTION
Emulsion-based delivery systems have been widely used in the food industry to protect active ingredients against extreme conditions, to enhance their stability, to maintain their effectiveness, and to mask bad odors and bitter tastes.[Citation1] A good emulsion formulation should retain its sensorial, chemical, and physical characteristics over time.[Citation2] The lipid oxidation in water-in-oil emulsion takes place in the continuous phase, i.e., oil. During storage, the setting in of chemical reactions that occur in the droplet interior, interfacial region, or continuous phase may change the properties of the system. Newly formed surface-active molecules in the continuous phase may exchange for those adsorbed to the droplet surface, thus changing the composition and properties of the interfacial layer. In this context, the oxidation of the lipidic fraction of an emulsion system is one of the causes of emulsion quality deterioration. Some lipid oxidation products are fairly surface active and, therefore, they can modify the composition of the interface.[Citation3]
Though water exerts a strong influence on the rate of chemical deterioration of foods, especially on lipid peroxidation, it has been suggested that lipid oxidation in water-in-oil (w/o) will occur at a rate similar to that of oils because the surface of the lipid phase is exposed directly to air.[Citation4] Saturated lipids are considerably more stable to lipid oxidation than unsaturated lipids, i.e., essential fatty acids (EFAs).[Citation5] Hence, the rate of oxidation of w/o emulsion formulated using oil containing EFAs is more than that prepared using oil containing saturated fatty acids. Consequently, a suitable antioxidant needs to be added to protect such w/o emulsions from oxidative deterioration. Some antioxidants are effective at low concentration and behaved as a pro-oxidant at a high concentration.[Citation6]
Though antioxidants are useful to prevent lipid oxidation, their activity may vary widely depending upon the composition of the system. Partitioning of antioxidants, hydrogen bonding, interphase transport, surface accessibility, and interaction of emulsifier with antioxidants are considered to be important parameters that determine antioxidant activity in lipid-containing systems.[Citation7] Thus, potent antioxidants can undergo oxidation forming reactive substances and subsequently act as pro-oxidants depending on the systems.[Citation8] Moreover, the type of emulsifier influences the distribution of antioxidant in emulsion[Citation9] and thereby decreases lipid oxidation in droplets.[Citation10]
Among natural antioxidants, ascorbic acid is used without restriction in foods because of its potent antioxidant activity and its nutritional value as vitamin C. Besides possessing antioxidant potential, it has owed medicinal benefits. It is one of the important water soluble vitamins that is easily absorbed but not stored in the body. Hence, ascorbic acid has to be regularly supplemented through diet or tablets to maintain ascorbic acid pool in the body. A deficiency of ascorbic acid leads to scurvy. Ascorbic acid plays an important role in the maintenance of collagen, which represents about one-third of the total body protein. Ascorbic acid is known to enhance the availability and absorption of iron from non-heme iron sources. Ascorbic acid supplementation is found to facilitate the dietary absorption of iron.[Citation11]
Similarly, well known natural antioxidants, such as kalonji seeds and curcuminoids, possess therapeutic applications. The seeds of kalonji are known to have a number of therapeutic uses,[Citation12] such as anticarcinogenic, antibacterial, anti-inflammatory, analgesic, and antipyretic. Likewise, curcumin, a major component of curcuminoids, has medicinal properties against various diseases, such as anorexia, coughs, diabetes, hepatic disorders, rheumatism, and Alzheimer disease.[Citation13]
The present work aims to study the effect of ascorbic acid on the oxidative stability of w/o emulsion formulated using refined sunflower oil (RSFO) as a source of EFAs. Polyglycerol polyricinoleate (PGPR) and tween 80 were used for the emulsification process. The optimum quantity of ascorbic acid needed to inhibit the oxidative deterioration of the emulsion was evaluated. The work is further extended to evaluate the effect of ascorbic acid in synergism with kalonji seeds ethanol extract (KEE) and curcuminoids. The results showed that the antioxidant is effective only at a particular proportion.
MATERIALS AND METHODS
Materials
RSFO was received as a gift sample from M/s Cargill India Pvt. Ltd. (New Delhi, India). The oil contained 428 ppm residual tocopherol and did not contain any synthetic antioxidants. Polyglycerol polyricinoleate (PGPR) was procured from Fine Organics (India). Tween 80 was procured from Croda Chemicals India Pvt. Ltd. Curcuminoids powder was obtained from Kancor India Ltd. (Angamaly South, India). Kalonji seeds were procured from a local vendor. All other chemical reagents and solvents were obtained from S.D. Fine-Chem Limited (Mumbai, India).
Extraction of Kalonji Seeds
Kalonji seeds were crushed and extracted using ethanol in a Soxhlet extractor with a minimum of 14 siphons. The crude KEE was obtained after removal of the solvent using a rotary vacuum evaporator.
Preparation of Emulsions
Water-in-oil emulsions were prepared by dropwise addition of a 15-ml water phase containing 1% (w/v) Tween 80 to 85 g RSFO containing 18% (w/v) PGPR. Emulsification was done using a homogenizer (Komal Scientific, India) at 3000 rpm for 30 min. The concentration of ascorbic acid in the water phase was varied (5, 10, 25, 50, and 100 ppm) to find its effect on the oxidative stability. Curcuminoids (50 ppm) and KEE (2% w/v) were added to the oil phase (RSFO) and emulsions containing various combinations of hydrophilic and lipophilic antioxidants were prepared.
Study of Antioxidant Activity
The oxidative stability of emulsions was checked at 35°C for 10 days at regular intervals of 2 days by determining peroxide value as per the AOCS Official Method.[Citation14] This temperature was selected so as to accelerate the oxidation without changing the structure of the emulsion. Also, induction period (IP) at 110°C was measured using Rancimat (Rancimat 679, Metrohm Ltd., Switzerland) with air flow at 20 L/h. The antioxidant activities of natural antioxidants were compared with control sample and tert-Butylhydroquinone (TBHQ (200 ppm based fat content)) under the same conditions. The relative antioxidant activities were compared using oxidative factor (OXF) for antioxidants based on mean peroxide value of triplicate experiments using the following formula:[Citation15]
Protection coefficient (Pc) was calculated as the ratio of IP with antioxidant to control sample,[Citation16]
RESULTS AND DISCUSSION
Effect of Ascorbic Acid on Oxidative Stability of Emulsion
The oil oxidation, i.e., autoxidation is a radical chain process involving initiation (radical formation), propagation (oxygen absorption to from peroxides), and termination (formation stable products) reaction. The radical formation is catalyzed by metal ions. Certain antioxidants like TBHQ work by deactivating these radicals, whereas certain antioxidants like lecithin work by chelating metal ions. In either of the cases, there is an induction period in which antioxidants continue to inhibit radical formation without much increase in peroxide formation. After the induction period, radicals continue to absorb oxygen to form peroxides. The rate of peroxide formation mainly depends on oxygen supply. Hence, higher induction period implies better radical inhibition power of an antioxidant.
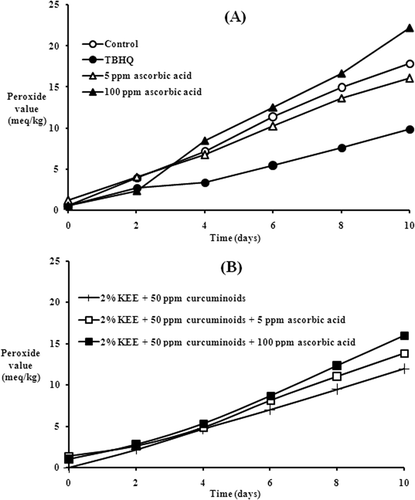
The effect of various concentrations of ascorbic acid on the oxidative stability of the emulsions is shown in . The data indicated that as the concentration increased, the rate of peroxide formation was increased. But at the same time, induction period was also increased. By evaluating the antioxidant activity of ascorbic acid for various concentrations, a 5 ppm concentration was found to be better in terms of peroxide formation, and antioxidant activity was decreased with an increase in concentration. In the case of emulsions, the presence of the aqueous phase generally decreases the activity of antioxidants because hydrogen-bonded complexes are ineffective hydrogen donors to lipid radicals.[Citation17] Due to the hydroperoxides being more polar than the starting lipid, the peroxy radicals tend to direct to the aqueous phase of the emulsion where they get scavenged by ascorbate anion AscH−[Citation18] according to the reaction,
TABLE 1 Effect of ascorbic acid on oxidative stability of emulsions at 35°C
or their ability to reduce hydroperoxides to more stable compounds that do not continue the radical chain reaction:[Citation19,Citation20]
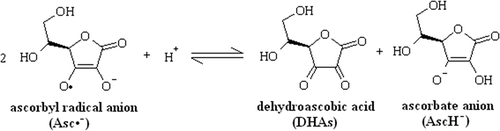
Two ascorbyl radical anions (Asc•–) can react to form a dimer and further undergo a reversible disproportionation reaction () to form stable dehydroascorbic acid (DHAs) and ascorbate anion (AscH−).[Citation21] This would regenerate ascorbic acid in its active form.[Citation22,Citation23] At a higher concentration of ascorbic acid, an excess amount of ascorbate anion (AscH−) was present to deactivate radicals forming ascorbyl radical anion (Asc•–). Thus, at higher concentrations, the induction period was more as compared to lower concentrations. The ascorbyl radical anion was further degraded reversibly to form dehydroascorbic acid (DHAs) and ascorbate anion (AscH−) in acidic medium. As the concentration of dehydroascorbic acid (DHAs) increased, the rate of degradation of ascorbyl radical anion decreased resulting in higher concentration of ascorbyl radical anion, which led to higher peroxide formation. At a higher concentration of ascorbic acid, more dehydroascorbic acid (DHAs) was formed and more ascorbyl radical anions were present in the system. Hence, the rate of peroxide formation was also more in this case (). At a lower concentration, the amount of ascorbic acid was insufficient to scavenge radicals resulting in a lower induction period. But at the same time, the disproportionation reaction () of ascorbyl radical anion is mainly in forward direction thereby facilitating the removal radicals for the system to form stable compounds. This reduced the peroxide formation.
Effect of Ascorbic Acid in Synergism with Lipophilic Antioxidants
As discussed in a previous paper,[Citation24] KEE and curcuminoids as such and in synergism with each other showed antioxidant activity in w/o emulsions. Based on the OXF (), the emulsion containing 50 ppm curcuminoids with 100 ppm ascorbic acid also showed marginal pro-oxidant activity, whereas the emulsion containing 2% KEE, 50 ppm curcuminoids, and 100 ppm ascorbic acid gave a slight antioxidant effect that was lower than the emulsion containing TBHQ. The Pc indicated that the stability of emulsion was increased by addition of 50 ppm curcuminoids to 100 ppm ascorbic acid that was enhanced by further addition of 2% KEE. The results were consistent with that of OXF. It is to be noted that even though OXF are lower for TBHQ, induction periods were higher for emulsions containing 100 ppm ascorbic acid along with lipophilic antioxidants, i.e., KEE and curcuminoids.
TABLE 2 Effect of ascorbic acid on antioxidant activity of KEE and curcuminoids in emulsions at 35°C
As is consistent with the previous discussion, a lower concentration (5 ppm) of ascorbic acid showed better antioxidant activity than a higher concentration (100 ppm) even in the presence of lipophilic antioxidants (, ). Also, the addition of 5 ppm ascorbic did not influence the activity of lipophilic antioxidants as seen by OXF but there was a marginal increase in Pc with the addition of 5 ppm ascorbic acid.
CONCLUSIONS
The antioxidant activity is concentration dependent. Ascorbic acid imparts antioxidant activity at a much lower concentration (5 ppm) in w/o emulsion. The lipophilic antioxidants were more active in w/o emulsions to increase oxidative stability of emulsions than lipophobic antioxidants. The study has given an approach for the development functional food using essential fatty acids. The optimisation of the concentration of the antioxidant is crucial in developing a functional food.
ACKNOWLEDGMENTS
This study was financially supported by Technical Education Quality Improvement Programme (TEQIP), Government of India and World Bank.
REFERENCES
- Madene, A.; Jacquot, M.; Scher, J.; Desorby, S. Flavour encapsulation and controlled release—A review. International Journal of Food Science and Technology 2006, 41, 1–21.
- Mosca, M.; Ceglie, A.; Ambrosone, L. Biocompatible water-in-oil emulsion as a model to study ascorbic acid effect on lipid oxidation. Journal of Physical Chemistry B 2008, 112, 4635–4641.
- McClements, D.J. Food Emulsions: Principles, Practice, and Techniques; CRC Press: Boca Raton, FL, 2005.
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. Journal of Food Science 2000, 65, 1270–1282.
- Knothe, G.; Kenar, J.A.; Gunstone, F.D. Chemical properties. In: The Lipid Handbook; Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J.; Eds.; CRC Press: Boca Raton, FL, 2007; 535–541.
- Przybylski, R.; Lee, Y.C.; Eskin, N.A.M. Antioxidant and radical scavenging activities of buckwheat seed components. Journal of the American Oil Chemists’ Society 1998, 75, 1595–1601.
- Schwarz, K.; Huang, S.W.; German, J.B.; Tiersch, B.; Hartmann, J.; Frankel, E.N. Antioxidant activities of antioxidants are affected by colloidal properties of oil-in-water and water-in-oil emulsions and bulk oils. Journal of Agricultural and Food Chemistry 2000, 48, 4874–4882.
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Parajo, J.C. Review: Natural antioxidants from residual sources. Food Chemistry 2001, 72, 145–171.
- Yuji, H.; Weiss, J.; Villeneuve, P.; Giraldo, L.J.L.; Figureo-Espinoza, M.C.; Decker, E. Ability of surface-active antioxidants to inhibit lipid oxidation in oil-in-water emulsion. Journal of Agricultural and Food Chemistry 2007, 55, 11052–11056.
- Silvestre, M.P.C.; Chaiyasit, W.; Brannan, R.C.; McClements, D.J.; Decker, E.A. Ability of surfactant headgroup size to alter lipid and antioxidant oxidation in oil-in-water emulsions. Journal of Agricultural and Food Chemistry 2000, 48, 2057–2061.
- Naidu, K.A. Review Vitamin C in human health and disease is still a mystery? An overview. Journal of Nutrition 2003, 2, 7.
- Machmudah, S.; Shiramizu, Y.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of Nigella sativa L. using supercritical CO2: A study of antioxidant activity of the extract. Separation Science and Technology 2005, 40, 1267–1257.
- Wang, X.; Jiang, Y.; Wang, Y.-W.; Huang, M.-T.; Ho, C.-T.; Huang, Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chemistry 2008, 108, 419–424.
- American Oil Chemists’ Society. Method: Cd 8-53. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th Ed.; Firestone, D.; Ed.; AOCS Press: Champaign, IL, 1994.
- Ghada, A.; Vassiliki, O. Antioxidant properties and composition of Majorana syriaca extracts. European Journal of Lipid Science and Technology 2007, 109, 247–255.
- Michalowska, A.G.; Korczak, J.; Regula, J. Use of plant extracts in summer and winter season butter oxidative stability improvement. Asia Pacific Journal of Clinical Nutrition 2007, 16, 85–88.
- Roginsky, V.A. Effectiveness of lipid- and water-soluble phenolic antioxidants in the autoxidation of polyunsaturated fatty acids esters in microheterogeneous solutions. Biologicheskie Membrany 1990, 4, 437–452.
- Mosca, M.; Ceglie, A.; Ambrosone, L. Biocompatible water-in-oil emulsion as a model to study ascorbic acid effect on lipid oxidation. Journal of Physical Chemistry B 2008, 112, 4635–4641.
- Pokorny, J.; Yanishlieva, N.; Gordon, M. Antioxidant in Food-Practical Applications; Woodhead Publishing Ltd: Abington Hall, Abington, Cambridge, 2001.
- Frankel, E.N. Lipid Oxidation; The Oily Press: Dundee, UK, 1998.
- Bielski, B.H.J.; Allen, A.O.; Schwarz, H.A. Mechanism of disproportionation of ascorbate radicals. Journal of the American Chemical Society 1981, 103, 3516–3518.
- Gallarate, M.; Carlotti, E.; Trotta, M.; Bovo, S. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. International Journal of Pharmaceutics 1999, 188, 233–241.
- Bors, W.; Buettner, G.R. The vitamin C radical and its reactions. In: Vitamin C in Health and Disease; Packer, L.; Fuchs, J.; Eds.; Marcel Dekker, Inc.: New York, 1997; 25–94.
- Rege, S.A.; Momin, S.A.; Bhowmick, D.N.; Pratap, A.P. Stabilization of emulsion and butter like products containing essential fatty acids using kalonji seeds extract and curcuminoids. Journal of Oleo Science 2012, 61, 11–16.