Abstract
This study investigated the antioxidant and anti-inflammatory properties of the fractionated tea extract, a lower-polymerized polyphenol-rich extract ultrafiltrated from oolong tea. The amounts of total polyphenols, flavonoids and condensed tannin were enriched in fractionated tea extract compared to oolong tea. Furthermore, fractionated tea extract was a stronger scavenger of nitric oxide and 2,2-diphenyl-1-picryhydrazyl radicals than oolong tea. When lipopolysaccharide-treated RAW264.7 macrophages were co-incubated with both tea extracts (500 μg/mL) or (-)-epigallocatechin gallate (100 μg/mL) for 16 h, all samples (oolong tea, fractionated tea extract, and (-)-epigallocatechin gallate) suppressed nitric oxide production by 60, 48, and 46%, respectively, and the suppression was due to nitric oxide-scavenging. However, only fractionated tea extract lowered the proportion of phospholipid arachidonic acid and type II-cyclooxygenase expression, thereby decreasing PGE2 synthesis by 29%. In conclusion, fractionated tea extract was rich in potent antioxidant substances capable of inhibiting the production of pro-inflammatory mediators.
INTRODUCTION
Oxidative stress refers to the imbalance between the production of free radicals and the biological systems that protect against them. Ample evidence shows that oxidative stress can damage and destroy cells, and contribute to the pathology of various diseases, such as atherosclerosis and Alzheimer's disease.Citation[1] To reduce the damages caused by free radicals, dietary supplementation of antioxidants has been considered as one of the strategies, in addition to the endogenous antioxidative enzymes. For example, Nabavi and coworkersCitation[2–Citation Citation4] demonstrated that the protective effects of plant antioxidants against chemical-induced oxidative stress in rats.
Like other natural antioxidants, tea polyphenols, which are a mixture of phenolic compounds identified originally in extracts of tea leaves, have been demonstrated to possess beneficial effects, including the protection of humans against chronic degenerative diseases such as cardiovascular diseases and cancer.Citation[5–Citation Citation7] These beneficial effects could be due in part to bioactive compounds in tea, such as (-)-epigallocatechin gallate (EGCG) and theaflavin (TF), which scavenge reactive oxygen species, thereby exhibiting their antioxidantCitation[8] and anti-inflammatory activitiesCitation[9,Citation10] known to modulate various cellular signaling processes.Citation[7,Citation11] For example, TF-like compounds inhibited nitric oxide (NO) production and arachidonic acid (AA) release in lipopolysacchardie- (LPS-) activated murine macrophages.Citation[12] Hou and colleagues have also shown that tea polyphenols block mitogen-activated protein kinase- (MAPK-) mediated inflammatory processes, including type II cyclooxygenase (COX-2) expression.Citation[13]
The kinds and amounts of tea polyphenols are greatly influenced by a variety of factors, including climate, season, horticultural practices, the type and age of the plant species, and also by processing procedures.Citation[14–Citation Citation Citation Citation18] During the process of fermentation, polyphenols in the green tea are enzymatically oxidized and subsequently polymerized. Consequently, the total catechin content may be reduced and new polymerized products are formed, such as theasinensins and TFs in partially fermented tea, oolong tea (OT) or TFs, thearubigins (TR) and theabrownins in fully fermented tea (black tea).Citation[19] While consuming most all varieties of tea beverages is beneficial for health, it seems that OT contains more potent antioxidant capacitiesCitation[20] and anti-inflammatory activitiesCitation[14] than most green tea and black tea in cell culture and animal models. We hypothesized that the stronger and more diverse biological effects of OT relative to other teas might be associated with low-polymerized and galloyl moiety-rich polyphenols (e.g., EGCG, theasinensins, TFs, etc.) produced during light fermentation of OT.Citation[7,Citation13]
Since tea is a relatively inexpensive source of polyphenols, the food industry is interested in optimizing the processes used to extract polyphenols from tea leaves. Copeland and coworkers developed a method combining caffeine precipitation and solvent partition to extract EGCG from a decaffeinated aqueous brew of commercial green tea.Citation[21] Using a series of nanofiltration membranes and organic solvents, Nwuha was able to effectively and efficiently, separated and concentrated the bioactive components in green tea.Citation[22] However, due to food safety concerns, extraction procedures that employ one or more organic solvents may not be suitable for the production of nutraceuticals. Recently, an improved technique for isolating tea polyphenols and caffeine from green tea infusion using ultrafiltration membranes and resin adsorption was developed.Citation[23] We hypothesize, therefore, that membrane technology might provide an alternative and acceptable method to concentrate and fractionate tea polyphenols with different degrees of polymerization.
The anti-inflammatory effects of tea polyphenols have been intensely investigated; however, most test samples were either pure compounds fractionated by chromatography, or crude extracts prepared by extraction with water or some organic solvent (e.g., ethanol). In the present study, fractionated polyphenols from OT were collected using ultrafiltration membranes, and analyzed and compared antioxidant properties of the fractionated extract with those of the original tea infusion (OT). Furthermore, we compared the ability of the fractionated tea extract (FTs) to modulate the production of pro-inflammatory mediators, including NO, prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS) and COX-2 in lipopolysaccharide- (LPS-) stimulated murine RAW264.7 macrophages. This study provides new knowledge regarding the modulatory effects of medium-polymerized polyphenols derived from Taiwan-grown OT on the production of inflammatory mediators by LPS-activated, cultured macrophages.
MATERIALS AND METHODS
Materials and Reagents
Taiwan OT (Chin-Shin-Dapang cultivar) was provided by the Taiwan Tea Experimental Station (Taoyuan, Taiwan). One thousand grams of tea were extracted with 8 L of distilled water at 90°C for 20 min. The tea infusion was filtered through filter paper (Whatman No. 1, Piscataway, NJ, USA), and the filtrate was then collected and freeze-dried. The OT filtrate was defined as OT. To collect fractionated tea polyphenols with a low-degree polymerization, a self-assembled equipment with two cross-flow polymeric membranes (GE Water & Process Technologies Co.; Trevose, PA, USA), such as Desal GE type for ultrafiltration (Cut offs molecular weight: 150–300 Da) and Desal DL Type for nanofiltration (Cut offs molecular weight: 1000 Da) were used sequentially. The initial tea extract was fractionated by the first membrane to remove low molecular-weight substances (MW < 150), and then concentrated by second membrane to exclude MW>1000 molecules. Thus, the ultrafiltrate of the tea extract contained a mixture of lower-polymerized polyphenols with molecular weights that ranged from 150–1000 Da; this ultrafiltrate was designated the FT.
EGCG (95%), Folin-Ciocalteu reagent, BCIP/NBT, gallic acid, caffeic acid, epicatechin, LPS (from Escherichia coli O26:B6), triheptadecanoin, DMSO, cell proliferation kit (MTT), Tween-20, and protease inhibitors were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The PGE2 assay kit was obtained from Cayman Chemicals (Ann Arbor, MI, USA). The GLC standard RL-461 was from Nu-Chek-Prep, Inc. (Elysian, MN, USA). Dulbecco's modified Eagles medium (DMEM), fetal bovine serum (FBS) and phosphate-buffered saline (PBS) were supplied by Gibco (Carlsbad, CA, USA). All reagent-grade organic solvents were purchased from Burdick & Jackson (Muskegon, MI, USA).
Measurement of Phytochemicals
The total content of phenolic compounds present in the tea extract was determined by the Folin-Ciocalteu colorimetric assay as reported previously.Citation[24] The standard curve was prepared using gallic acid and results are expressed in terms of milligrams of gallic acid equivalents per gram of extract-solid (GAE/g). The total content of flavonoid compounds was measured as described by Zhishen et al.Citation[25] The amount of condensed tannin was determined as described by Julkunen-Titto.Citation[24] Results for both flavonoids and condensed tannin are expressed as milligrams catechin equivalents per gram of dry weight (CE/g). The proanthocyanidin content was determined by spectrophotometry at 550 nm, on the basis of a colorimetric reaction with 10% NH4Fe(SO4)2, as described by Luximon-Ramma et al.Citation[26] The proanthocyanidins content was expressed in terms of milligrams of cyanidin chloride equivalents per gram of extract-solid (CCE/g).
Contents of particular catechins were determined by high-performance liquid chromatography with a Thermo scientific ODS Hypersil C18 reverse phase column (250 mm × 4.6 mm; i.d., 5 μm) and an UV-VIS detector (Shimadzu Co., Kyoto, Japan). The mobile phase contained 1% acetic acid (solvent A) and acetonitrile (solvent B), with a linear gradient starting with A/B (90:10), changing to A/B (70:30) over a period of 60 min with a flow rate of 1 mL/min. The effluent was monitored at 280 nm to identify gallic acid, caffeic acid, EGCG, and catechins.
Evaluation of Antioxidant Activity
The 2,2-diphenyl-1-picryhydrazyl (DPPH) radical-scavenging capacity of the extracts was determined as previously described.Citation[14] The total antioxidant capacity of the tea polyphenol extracts was determined using a commercial kit (Randox Laboratories Ltd., Crumlin, UK). This assay is based on 2,2'-azinobis(3-ethylbenzothiazoline sulfonate) (ABTS) reacting with metmyoglobin and hydrogen peroxide to produce the radical cation ABTS+. ABTS+ which has a stable blue-green color that can be measured at 600 nm. Total antioxidant capacity was calculated relative to Trolox, which was used as a standard under the same conditions; results are expressed as mmol/g Trolox equivalent antioxidant activity (TEAC). The scavenging effects of the extracts on NO were measured according to the method of Dirsch et al.Citation[27] The reducing power of that extracts was determined according to the method of Oyaizu.Citation[28]
Cell Culture and Culture Conditions
Murine RAW 264.7 macrophages were obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). This macrophage line has been shown to be a suitable model for studying the effects of a wide range of substances on many components of the anti-inflammatory response. They were incubated in DMEM supplemented with 10% heated-inactivated FBS and maintained in a 5% CO2 humidified environment at 37°C. In order to monitor cell viability after treatment, the MTT method and trypan blue dye exclusion were used.
Determination of NO Production
To test the effectiveness of tea extracts on NO production, RAW264.7 cells were seeded at a density of 1 × 106/mL per well in a 96-well culture plate, and allowed to adhere overnight at 37°C. Then, the adherent cells were rinsed three times with PBS and incubated with medium containing LPS (0.1 μg/mL) alone (control), or with LPS and tea extract (500 μg/mL) or EGCG (100 μg/mL). These concentrations of tea extract and EGCG have been shown to exert strong anti-inflammatory effects in cultured, LPS-activated macrophages.Citation[14,Citation29] After incubation for 16 h, the medium was collected and the levels of nitrite were analyzed as an indicator of NO production using the Griess reaction.Citation[30]
Determination of PGE2 Production
Macrophage RAW264.7 cells were seeded at a density of 5 × 105 cells/well in 24-well plates with DMEM containing 10% FBS and allowed to adhere overnight. The cells were then stimulated with 0.1 μg/mL LPS and/or tea extract or EGCG for 16 h. Finally, the cell-free supernatants were collected and the concentrations of PGE2 measured according to the manufacturer's instructions using an enzyme immunoassay kit.Citation[31]
Detection of iNOS and COX-2 Expression by Western Blotting
Confluent RAW264.7 macrophages (5 × 106/mL) were incubated with LPS and/or various tea samples or EGCG for 16 h. After harvesting, total cellular protein was extracted and the protein concentration was determined using the BCA Protein Assay Reagent Kit (Pierce, Rockford, IL, USA). Heat-denatured proteins were separated by 10% (w/v) sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane. After nonspecific blocking and rinsing, the membranes were probed with either 1:500 dilution of anti-mouse iNOS antibody or 1:500 dilution of anti-mouse COX-2 antibody (BD Biosciences, Franklin Lakes, NJ, USA). Next, the membranes were washed with PBS-T [10% Tween 20 (v/v)], and then reacted with a 1:5000 dilution of anti-mouse immunoglobulin-alkaline phosphatase (Sigma, St. Louis, MO, USA). After further washing with PBS-T, the targeted proteins were visualized with BCIP/NBT (Sigma, St. Louis, MO, USA).Citation[31]
Lipid Extraction and Fatty Acid Analysis
To examine whether the reduction in PGE2 synthesis was due to a decrease in the level of the precursor of PGE2, namely AA, cells at a density of 1 × 106/mL were seeded in 100 mm culture dishes, and allowed to adhere overnight at 37°C. Cells were then incubated in DMEM containing 10% FBS (control) and/or supplemented with EGCG or tea extract for 16 h. After incubation, cells were collected and harvested by centrifugation. The pellet was washed three times with sterile PBS. Total cellular lipids were then extracted according to the modified Folch's method.Citation[32] Briefly, cell pellets were extracted with 20 mL chloroform:methanol (2:1,v/v) at room temperature for 1 h. The extracted lipids in the lower phase (chloroform) were separated from the aqueous phase by shaking with 4 mL of 0.9% (w/v) NaCl solution. The lower chloroform phase was evaporated at 40°C with the aid of a stream of nitrogen.
The phospholipid fraction was then separated by thin-layer chromatography (TLC) using a developing solvent mixture consisting of hexane/diethyl ether/acetic acid (80:20:1, v/v/v). Fatty acids of the phospholipid fraction were methylated and the fatty acid methyl esters (FAME) were analyzed by gas chromatography (GC) using an Agilent 6890 system equipped with a fused-silica capillary column (Omegawax; 30 m × 0.32 mm, i.d., film thickness 0.25 μm, Supelco, Bellefonte, PA, USA) and a flame-ionization detector (FID). To quantify the levels of fatty acid, a known amount of an internal standard (triheptadecanoin) was added to each FAME sample.
Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) and Duncan's multiple range test using SPSS software (SPSS for Windows 17.0; SPSS Inc., Chicago, IL, USA). Differences between means were considered significant at the p ≤ 0.05 levels.
RESULTS
Quantitation of Total Phenolics, Total Flavonoids, Condensed Tannins, and Proanthocyanidins
Table 1 reports the equivalent amounts of total phenols, total flavonoids, condensed tannins, and proanthocyanidins in both kinds of tea extracts. FTs contained higher amounts of phenolic compounds (129 ± 2 vs. 105 ± 1 mg GAE/g), total flavonoids (16.3 ± 0.3 vs. 11.9 ± 0.4 mg CE/g), and condensed tannins (14.1 ± 0.3 vs. 8.2 ± 1.3 mg CE/g) than OT. However, a larger amount of proanthocyanidins was found in the OT extract (6.7 ± 0.2 vs. 2.6 ± 0.3 mg CCE/g). Results of HPLC analysis showed that the contents of some lower-polymerized polyphenols in FT were enriched as compared to OT, including gallic acid (6.2 fold), caffeic acid (2.4 fold), EC (7.8 fold). ().
Table 1 Contents of total phenolics, total flavonoids, condensed tannin, and proanthocyanidins in oolong tea filtrates and fractionated tea extracts
Table 2 Contents of gallic acid, caffeic acid, epicatechins (EC), and (-)-epigallocatechin gallate (ECGC) in TMT, MMT, and HMT
Determination of Antioxidant Activity
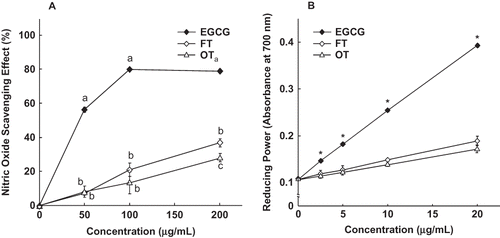
The DPPH radical, TEAC, and the NO radical scavenging assays were used to determine if the process of membrane filtration changed the antioxidant capacities of tea extracts. Results in show how much of the various extracts were required to scavenge 50% of the DPPH radical, which is reported as the half-maximal inhibitory concentration (IC50). EGCG was the most potent (17.9 ± 0.7 μg/mL), followed by FT (41.2 ± μg/mL) and OT (57.2 ± 6.0 μg/mL). When the TEAC assay was used, EGCG was again found to contain the most antioxidant capacity (2.50 ± 0.09 mmol Trolox equivalents per gram of sample) among samples tested, followed by OT (2.41 ± 0.10) and FT (2.36 ± 0.09) (). However, these differences were not statistically significant.
Table 3 2,2-diphenyl-1-picryhydrazyl- (DPPH-) radical-scavenging activity and antioxidant capacity of EGCG, OT, and FT
The NO radical-scavenging activities of EGCG and the various tea extracts are shown in EGCG was especially effective in quenching NO radicals and the scavenging effect reached a plateau when the concentration reached 100 μg/mL. The other two tea extracts exhibited a dose-dependent effect on scavenging NO radicals, but were not as potent as EGCG. Results in show that the reducing power of EGCG and the two tea extracts increased in a concentration-dependent manner. Both tea extracts contained significantly lower reducing power as compared to EGCG.
Effect of the Tea Extracts on Synthesis of NO and PGE2
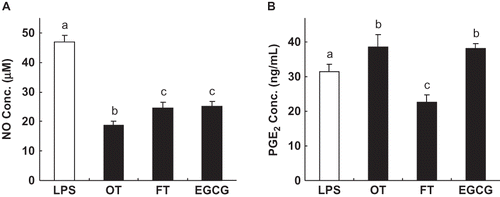
Previous studies have demonstrated that tea polyphenols have anti-inflammatory properties.Citation[6,Citation29] We therefore tested to determine if both tea extracts could modulate the production of pro-inflammatory NO and PGE2 by LPS-stimulated cultured macrophages. The concentration of EGCG (100 μg/mL) and the tea extracts (500 μg/mL) used in the present study were not cytotoxic to RAW264.7 cells (data not shown). The results in show that all tea samples significantly reduced the concentration of NO: OT suppressed NO production by 60% compared to the control, followed by FT (48%), and EGCG (46%). The synthesis of PGE2 was also significantly suppressed by FT. However, OT and EGCG slightly increased the PGE2 concentration ().
Figure 2 Effect of EGCG and the tea extracts on NO production (a) and prostaglandin E2 (PGE2) synthesis (b) in lipopolysaccharide- (LPS-) activated RAW264.7 cells. Adherent RAW264.7 cells were incubated with medium containing LPS (0.1 μg/mL) alone (control), or with LPS and tea extract (500 μg/mL) or EGCG (100 μg/mL) for 16 h. Each lane represents the mean ± SD of three independent experiments. In each category, the means with different letters are significantly different from each other at p < 0.05.
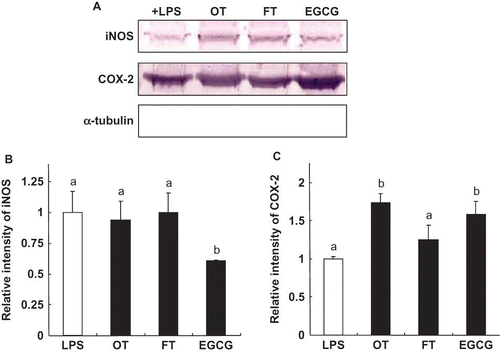
Effect of Tea Extracts on Expression of iNOS and COX-2 Proteins
To determine whether the reduction of NO and PGE2 was associated with the modification of gene expression at the translation level, we measured the iNOS and COX-2 expression. The over-expression of iNOS stimulated by LPS was significantly suppressed by EGCG (39%); however, OT and FT did not significantly affect iNOS protein expression (). The two tea extracts and EGCG enhanced COX-2 expression (); specifically the increases were OT (73%), EGCG (59%), and FT (27%), respectively.
Figure 3 Effect of EGCG and tea extracts on the expression of iNOS, and COX-2 in RAW264.7 cells (a). The relative density of the bands, which were assessed after Western blotting (b and c). Adherent RAW264.7 cells were incubated with medium containing LPS (0.1 μg/mL) alone (control), or with LPS and tea extract (500 μg/mL) or EGCG (100 μg/mL) for 16 h. The induction of NO synthase and cyclooxygenase-2 expression was determined by Western blotting. α-tubulin was used as a loading control. Each value represents the mean ± SD of three independent experiments. The values with different letters are significantly different from each other at p < 0.05. (Color figure available online.)
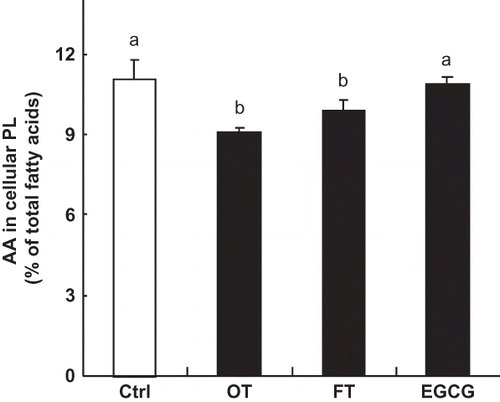
Effect of Tea Extracts on the Proportions of AA
In addition to regulating COX-2 expression, PGE2 synthesis might be affected by altering the proportion of AA in cellular phospholipids since AA is the major precursor of pro-inflammatory eicosanoids. To determine if tea extracts could alter n-6 polyunsaturated fatty acid (PUFA) metabolism so as to reduce the proportion of AA in the macrophages, we examined the fatty acid composition of cellular phospholipids in macrophages exposed to the various tea extracts. Results in show that both tea extracts (OT and FT) significantly decreased the proportions of AA by 18 and 10%, respectively. However, no such reduction was observed when cells were treated with EGCG.
Figure 4 Effect of EGCG and tea extracts on the level of AA. RAW264.7 cells were incubated with only medium or medium containing EGCG (20 μg/mL) or tea extracts (400 μg/mL) for 16 h. Each value represents the mean ± SD of three independent experiments. The values with different letters are significantly different from each other at p < 0.05.
DISCUSSION
In this study, the tea extracts FT which was produced by ultrafiltration was shown to be rich in lower-polymerized phenolic compounds and had comparable or greater antioxidant activities as compared to OT. FT also exerted inhibitory effects on the production of pro-inflammatory NO and PGE2 by LPS-stimulated macrophages. Results in show that the amounts of total phenolic substances, total flavonoids and condensed tannin, but not proanthocyanidins were significantly higher in FT than in OT. Similarly, the amounts of gallic acid, caffeic acid, and epicatechin were greater in FT than OT (data not shown). Therefore, we conclude that the FT contained mostly condensed polyphenols with molecular weights in the 150 to 1000 Da range and which consisted of gallic acid, caffeic acid, epicatechin, EGCG, and several other unidentified single or oligomeric catechins, such as TF. When compared using either the DPPH or NO radical quenching assays, the gallic acid-rich and oligomeric polyphenols-rich FT was a more potent scavenger than OT ( and ). This difference could be accounted for by the larger amounts of condensed polyphenols (e.g., epicatechin) and medium-polymerized polyphenols (e.g., TF) in fractionated FT relative to OT. Furthermore, TF has been reported to exert more potent DPPH-radicals than TR, a highly-polymerized group of catechins in OT.Citation[16] Our findings are in accord with results in previous reports which showed that the levels of the galloyl moiety in the monomeric or oligomeric catechins and the degree of polymerization of tea polyphenols may be critical in determining their antioxidant capacities,Citation[16,Citation33] but not be related in some simple manner to the amounts of phenolic compounds that are present.
In this study, it was found that NO production was significantly inhibited by two of the extracts (), however, this effect did not involve the suppression of iNOS expression (). These results support an earlier finding that polyphenols from green and black tea did not alter iNOS expression in a rat modelCitation[34] but this was inconsistent with results from our previous report, which demonstrated that extracts from two batches of different fermented teas inhibited iNOS expression.Citation[14] In the present study, the inhibition of NO production was approximately 80% when cells were treated with 500 μg/mL of tea extract;Citation[34] but only 40% when the same concentration of OT was used (). These differences might be attributed to differences in the plant species, the fermentation process or the processing techniques, which may have affected the content, character and polymerization of tea polyphenols. In addition, since no inhibition of iNOS expression by tea extracts was found, the potent NO radical-scavenging activities of tea extracts might play an important role in reducing the production of NO. Modulation of iNOS activity has been suggested as one of mechanisms that might lower NO production;Citation[35] however, this possibility was not investigated in the present study. As for the standard EGCG, the NO suppression we observed could be due to the combinatorial effect of the NO-scavenging activity and inhibition on iNOS expression.
The tea extracts differentially modulated the synthesis of PGE2. FT significantly decreased PGE2 synthesis (); however this suppression was not due an effect on COX-2 expression (), but rather, in part at least, to the lowering the proportion of AA in membranes of the macrophages (), which is the precursor of the pro-inflammatory 2- and 4-series eicosanoids. A similar decrease in the proportion of AA was also found in OT-treated cells (); however, there was significant induction of COX-2 expression by OT, which might attenuate the effect of lowering the proportion of AA and blunting in the increase in PGE2. Furthermore, it is notable that OT reduced the percentage of AA in macrophage phospholipids to a greater extent than FT or EGCG, which had no effect on the proportion of AA. This finding suggests that certain components present in the original tea extract (i.e., OT), but absent from FT, are effective in reducing the AA percentage in macrophage membranes.
It is noteworthy that FT, but not OT, inhibited PGE2 synthesis (). This observation led us to inquire with regard to which components in FT might be responsible for this inhibition. EGCG, one of major catechins found in unfermented and partially fermented teas (>40%),Citation[14] slightly enhanced PGE2 production and COX-2 expression ( and ). This finding might partially account for the increasing concentrations of PGE2 in macrophages preincubated with OT, since this particular tea extract contained 44% more EGCG than FT (). Our results are in accordance with earlier reports using RAW264.7 macrophages and A549 cell lines,Citation[9,Citation10,Citation36] and indicate that EGCG is not the active component in FT that reduces the level of PGE2 in LPS-stimulated macrophages. Gallic acid and TF are enriched in FT, and these compounds have been demonstrated to lower eicosanoid synthesis by suppressing both the COX-2 and lipoxygenase pathways.Citation[37,Citation38] Thus, these anti-inflammatory catechins (gallic acid and TF) in FT may decrease PGE2 synthesis through the similar mechanism to attenuate up-regulation of COX-2 expression triggered by EGCG.Citation[39]
CONCLUSION
OT subjected to ultrafiltration (FT) was rich in lower-polymerized polyphenols and exerted superior antioxidant capacity compared to the original tea infusion (OT). The antioxidative capacity of a particular tea extract depends not only on its content of total phenolic compounds, but also on the degree of polymerization of tea polyphenols in the extract. Both tea samples significantly inhibited NO production, and this effect was partly attributable to NO-scavenging ability. Moreover, the concentration of PGE2 varied when cells were treated with both tea extracts, and these differences could be attributed to the combined effects of the decrease in AA and differential changes in COX-2 expression. It was also shown that the degree of polymerization of the oxidized catechins in the tea mixture might be responsible for this difference. Since the characteristics of the FTs were modified in various ways, this ultrafiltration technique can be applied to enrich certain types of tea polyphenols in a purposive manner, which should allow for the preparation of new nutraceuticals and healthier beverages in the future.
ACKNOWLEDGMENTS
The authors sincerely thank Dr. Robert H. Glew for helpful comments and for editing the manuscript. This work was support in part by research grant (NSC 96-2313-B-264-001) from the National Science Council, Taiwan. The authors also appreciated Dr. Chwei-Feng Chiou (Taiwan Tea Experiment Station) for providing the OTs for this study.
REFERENCES
- Valko , M. , Leibfritz , D. , Moncol , J. , Cronin , M.T.D. , Mazur , M. and Telser , J. 2007 . Free radicals and antioxidants in normal physiological functions and human disease . International Journal of Biochemistry & Cell Biology , 39 : 44 – 84 .
- Nabavi , S.F. , Moghaddam , A.H. , Eslami , S. and Nabavi , S.M. 2012 . Protective effects of curcumin against sodium fluoride-induced toxicity in rat kidneys . Biological Trace Element Research , 145 : 369 – 374 .
- Nabavi , S.F. , Nabavi , S.M. , Abolhasani , F. , Moghaddam , A.H. and Eslami , S. 2012 . Cytoprotective effects of curcumin on sodium fluoride-induced intoxication in rat erythrocytes . Bullutin Environmental Contamination and Toxicology , 88 : 486 – 490 .
- Nabavi , S.M. , Nabavi , S.F. , Eslami , S. and Moghaddam , A.H. 2012 . In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue . Food Chemistry , 132 : 931 – 935 .
- Suzuki , J. , Ogawa , M. , Maejima , Y. , Isobe , K. , Tanaka , H. , Sagesaka , Y.M. and Isobe , M. 2007 . Tea catechins attenuate chronic ventricular remodeling after myocardial ischemia in rats . Journal of Molecular and Cellular Cardiology , 42 : 432 – 440 .
- Tipoe , G.L. , Leung , T.M. , Hung , M.W. and Fung , M.L. 2007 . Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection . Cardiovascular and Hematological Disorders Drug Targets , 7 : 135 – 144 .
- Hou , Z. , Lambert , J.D. , Chin , K.V. and Yang , C.S. 2004 . Effects of tea polyphenols on signal transduction pathways related to cancer chemoprevention . Mutation Research , 555 : 3 – 19 .
- Tsai , B.H. , Ho , S.C. , Kan , N.B. , Liu , C.C. and Lin , C.C. 2005 . The effect of drinking oolong tea on the oxidative stress of athletes at test and post-exercise . Journal of Food Science , 70 : 581 – 585 .
- Park , J.W. , Choi , Y.J. , Suh , S.I. and Kwon , T.K. 2001 . Involvement of ERK and protein tyrosine phosphatase signaling pathway in EGCG-induced cyclooxygenase-2 expression in RAW264.7 cells . Biochemical and Biophysical Research Communications , 286 : 721 – 725 .
- Moon , Y. , Lee , M. and Yang , H. 2007 . Involvement of early growth response gene 1 in the modulation of microsomal prostaglandin E synthase 1 by epigallocatechin gallate in A549 human pulmonary epithelial cells . Biochemical Pharmacology , 73 : 125 – 135 .
- Kobuchi , H. , Virgili , F. and Packer , L. 1999 . Assay of inducible form of nitric oxide synthase activity: Effect of flavonoids and plant extracts . Methods in Enzymology , 301 : 504 – 513 .
- Sang , S. , Lambert , J.D. , Tian , S. , Hong , J. , Hou , Z. , Ryu , J.H. , Stark , R.E. , Rosen , R.T. , Huang , M.T. , Yang , C.S. and Ho , C.T. 2004 . Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities . Bioorganic and Medicinal Chemistry , 12 : 459 – 467 .
- Hou , D.X. , Masuzaki , S. , Tanigawa , S. , Hashimoto , F. , Chen , J. , Sogo , T. and Fujii , M. 2010 . Oolong tea theasinensins attenuate cyclooxygenase-2 expression in lipopolysaccharide (LPS)-activated mouse macrophages: Structure-activity relationship and molecular mechanisms . Journal of Agricultural and Food Chemistry , 58 : 12735 – 12743 .
- Lin , C.C. , Lu , M.J. , Chen , S.J. and Ho , S.C. 2006 . Heavy fermentation impacts NO-suppressing activity of tea in LPS-activated RAW264.7 macrophages . Food Chemistry , 98 : 483 – 489 .
- Kim , E.S. , Liang , Y.R. , Jin , J. , Sun , Q.F. , Lu , J.L. , Du , Y.Y. and Lin , C. 2007 . Impact of heating on chemical compositions of green tea liquor . Food Chemistry , 103 : 1263 – 1267 .
- Yang , Z. , Tu , Y. , Xia , H. , Jie , G. , Chen , X. and He , P. 2007 . Suppression of free-radicals and protection against H2O2-induced oxidative damage in HPF-1 cell by oxidized phenolic compounds present in black tea . Food Chemistry , 105 : 1349 – 1356 .
- Pekal , A. , Drozdz , P. and Pyrzynska , K. 2012 . Comparison of the antioxidant properties of commonly consumed commercial teas . International Journal of Food Properties , 15 : 1101 – 1109 .
- Imrana , A. , Butta , M.S. and Sharifa , M.K. 2012 . Phytochemical density of some promising commercial tea brands . International Journal of Food Properties , 15 : 99 – 108 .
- Neilson , A.P. and Ferruzzi , M.G. 2011 . Influence of formulation and processing on absorption and metabolism of flavan-3-ols from tea and cocoa . Annual Review of Food Science and Technology , 2 : 125 – 151 .
- Lin , C.C. and Liang , J.H. 2002 . Antioxidant activity of various tea extracts as affected by their fermentation degree . Food Science and Agricultural Chemistry , 4 : 122 – 127 .
- Copland , E.L. , Clifford , M.N. and Williams , C.M. 1998 . Preparation of (−)-epigallocatechin gallate from commercial green tea by caffeine precipitation and solvent partition . Food Chemistry. , 61 : 81 – 87 .
- Nwuha , V. 2000 . Novel studies on membrane extraction of bioactive components of green tea in organic solvents: Part I . Journal of Food Engineering , 44 : 233 – 238 .
- Li , P. , Wang , Y. , Ma , R. and Zhang , X. 2005 . Separation of tea polyphenol from green tea leaves by a combined CATUFM-adsorption resin process . Journal of Food Engineering , 67 : 253 – 260 .
- Julkunen-Titto , R. 1985 . Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics . Journal of Agricultural and Food Chemistry , 33 : 213 – 217 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoids contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Luximon-Ramma , A. , Bahorun , T. , Soobrattee , M.A. and Aruoma , O.I. 2002 . Antioxidant activities of phenolic, proanthocyanidins, and flavonoid components in extracts of . Cassia fistula. Journal of Agricultural and Food Chemistry , 50 : 5042 – 5047 .
- Dirsch , V.M. , Stuppner , H. and Vollmar , A.M. 1998 . The griess assay: Suitable for a bio-guided fractionation of anti-inflammatory plant extracts . Planta Medica , 64 : 423 – 426 .
- Oyaizu , M. 1986 . Studies on products of browning reaction prepared from glucoseamine . Japanese Journal of Nutrition , 44 : 307 – 314 .
- Yang , F. , de Villiers , W.J.S , McClain , C.J. and Varilek , G.W. 1998 . Green tea polyphenols block endotoxin-induced tumor necrosis factor production and lethality in a murine model . Journal of Nutrition , 128 : 2334 – 2340 .
- Green , L.C. , Wagner , D.A. , Glogowski , J. , Skipper , P.L. , Wishnok , J.S. and Tannenbaum , S.R. 1982 . Analysis of nitrate, nitritie and [15N]nitrate in biological fluids . Analytical Biochemistry , 126 : 131 – 138 .
- Huang , Y.S. , Huang , W.C. , Li , C.W. and Chuang , L.T. 2011 . Eicosadienoic acid differentially modulates production of pro-inflammatory modulators in murine macrophages . Molecular and Cellular Biochemistry , 358 : 85 – 94 .
- Folch , J. , Lees , M. and Sloane-Stanley , G.H. 1957 . A simple method for the isolation and purification of total lipids from animal tissues . Journal of Biological Chemistry , 226 : 497 – 509 .
- Shiraki , M. , Hara , Y. , Osawa , T. , Kumon , H. , Nakayama , T. and Kawakishi , S. 1994 . Antioxidantive and antimutagenic effects of theaflavins from black tea . Mutation Research , 323 : 29 – 34 .
- Luceri , C. , Caderni , G. , Sanna , A. and Dolara , P. 2002 . Red wine and black tea polyphenols modulate the expression of cyclooxygenase-2, inducible nitric oxide synthase and glutathione-related enzymes in azoxymethane-induced F344 rat colon tumors . Journal of Nutrition , 132 : 1376 – 1379 .
- Sheu , F. and Yen , G.C. 2001 . Modulation of nitric oxide production by foodstuffs . Food Science and Agricultural Chemistry , 3 : 42 – 58 .
- Liang , Y.C. , Huang , Y.T. , Tsai , S.H. , Lin-Shiau , S.Y. , Chen , C.F. and Lin , J.K. 1999 . Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages . Carcinogenesis , 20 : 1945 – 1952 .
- Lee , S.J. , Lee , I.S. and Mar , W. 2003 . Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 activity by 1,2,3,4,6-penta-O-galloyl-β-D-glucose in murine macrophage cells . Archives of Pharmacal Research , 26 : 832 – 839 .
- Huang , M.T. , Liu , Y. , Ramji , D. , Lo , C.Y. , Ghai , G. , Dushenkov , S. and Ho , C.T. 2006 . Inhibitory effects of black tea theaflavin derivatives on 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and arachidonic acid metabolism in mouse ears . Molecular Nutrition and Food Research , 50 : 115 – 122 .
- Murakami , A. , Takahashi , D. , Hagihara , K. , Koshimizu , K. and Ohigashi , H. 2003 . Combinatorial effects of nonsteroidal anti-inflammatory drugs and food constituents on production of prostaglandin E2 and tumor necrosis factor-α in RAW264.7 murine macrophages . Bioscience, Biotechnology and Biochemistry , 67 : 1056 – 1062 .