Abstract
The aim of this research was to determine the total phenolic and flavonoid contents as well as to measure antioxidant activity of 24 different commercial beers consumed in Serbia. The major phenolic acids (gallic acid, protocatechuic acid, 4-hydroxybenzoic acid, 2,5-dihydroxybenzoic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, sinapic acid, salicylic), (+)-catechin, and (-)-epicatechin were also determined by high pressure liquid chromatography method using a photodiode array detector. Gallic acid, ferulic acid, and protocatechuic acid are the most abundant phenolic acids in all samples, followed by (+)-catechin. The total phenolic content was determined using the Folin-Ciocalteu assay. The total flavonoids were measured using spectrophotometrics as the aluminum chloride assay. The results showed that the highest total phenolic and flavonoid contents were established in dark and light beer samples. 2,2-Diphenyl-1-picrylhidrazyl radical scavenging activity, 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) radical cation scavenging activity, and ferric reducing/antioxidant power were used to assess the antioxidant potential of beers. These assays, based on different chemical mechanisms, were selected to take into account the wide variety and range of action of antioxidant compounds present in selected beer samples. All beers showed antioxidant power, but a wide range of antioxidant capacities was observed. Statistical differences between ferric reducing-antioxidant power and the other two antioxidant capacity assays were confirmed. This study will be useful for the appraisal of phenolic profile and antioxidant activities of various beers, and it will also be of interest for people who like drinking this beverage.
INTRODUCTION
Beer has been consumed for more than 6000 years, from the time when it was first made by happenstance in the middle age of ancient times.[Citation1] Ever since, it has become a staple part of the diet in many cultures. Beer has a higher nutritional value than other alcoholic beverages because of its minerals, organic acids, vitamins, proteins, and hops.[Citation2] Furthermore, it has not only comprised a valuable addition to the table, but has served various medicinal roles. Little is known about the influence of moderate consumption of beer on health. Clinical and statistical evidence and laboratory studies have shown that active substances in beer could influence the immune system, block cancer formation, protect against coronary disease, and even prolong life.[Citation3 − Citation5] Recent results indicate that polyphenols from beer might produce the health beneficial effects. According to Gerhauser et al.,[Citation6] phenolic compounds from beer display good antioxidant activity, especially versus the highly reactive hydroxyl radicals involved in lipid peroxidation processes. A summary of the antioxidant potential is also given by this author. Additionally, beer may play an active role in preventing Alzheimer's disease and other related disorders.[Citation7]
Several methods were developed for analysis of physical and physicochemical properties of beer.[Citation8] Also, various methods were described in the literature for measuring the total antioxidant capacity (TAC): 2,2-diphenyl-1-picrylhidrazyl (DPPH) radical scavenging activity,[Citation9] 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation scavenging activity,[Citation10] superoxide anion radical scavenging activity,[Citation11] total radical-trapping antioxidant parameter (TRAP),[Citation12] ferric reducing/antioxidant power (FRAP),[Citation13] metal chelating activity,[Citation14] reducing power,[Citation15] oxygen radical absorbance capacity (ORAC),[Citation16] cupric reducing antioxidant capacity (CUPRAC).[Citation16] Because different antioxidant compounds may act in vivo through different mechanisms, no single method can fully evaluate the TAC of beer. In the present study, three antioxidative tests were performed to assess the TAC of beer and obtain data useful for determining the potential intake of antioxidants in beer consumed in Serbia. Beer was chosen because it is one of the most popular beverages in Serbia.
In Serbia, famous for vintage wines, beer took a long time to conquer the market and today, on the basis of annual consumption of some 80 liters per person,[Citation17] is around the European average. Serbia produces some 5 million hectoliters of beer annually;[Citation17] almost all the quantities are placed on the domestic and neighboring markets. One-fifth of the total consumption of drinks goes to beer. Beer is obtained through fermentation of malted barley and brewer's yeast, water, and hops. By the way, every other consumed bottle of beer in Serbia has been produced in the Apatin brewery, which is a member of StarBev Company, a regional leader in beer production. The beer industry in Serbia was among the first to find its place in the transition process. Foreign companies invested significant funds, so the beer industry became one of the most profitable branches of business. To compare different beer types, total and individual phenolics, total flavonoid content, and total antioxidant activity were measured in 24 different commercial beers consumed in Serbia.
MATERIALS AND METHODS
Chemicals
The 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was purchased from Acros Organics (New Jersey, USA). ABTS, DPPH, 2,4,6-tri(2-pyridyl)-S-tirazine (TPTZ), gallic acid, protocatechuic acid, 4-hydroxybenzoic acid, 2,5-dihydroxybenzoic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, sinapic acid, salicylic acid, (+)-catechin, (-)-epicatechin, and quercetin were purchased from Sigma Aldrich (Steineheim, Germany). Folin Ciocalteu's phenol reagent, potassium peroxodisulfate, ammonium iron(II) sulfate hexahydrate, sodium hydroxide, sodium acetate, sodium nitrite, sodium carbonate, sodium sulphate, aluminum chloride hexahydrate, acetic acid, formic acid, and acetonitrile high pressure liquid chromatography (HPLC grade) were purchased from Merck® (KGaA, Darmstadt, Germany). Ethanol (96% by vol.) and methanol (HPLC grade) were from J.T. Baker.
Beers
Beers were purchased at local markets, stored in a refrigerator at 4°C, and analyzed immediately upon opening to prevent loss of phenols by oxidation. The detailed characteristics of these beers were presented in .
Table 1 Characteristics of commercial beers
Instruments
An Agilent 8453 UV/Vis spectrophotometer was used for absorbance measurements and spectra recording, using an optical or quartz cuvettes of 1 cm optical path. The pH measurements were made with Hanna Instruments pH-meter equipped with glass electrode.
A model 1200 Agilent Technologies was used for HPLC analysis. The analytical column was C18 (Zorbax Eclipse XDB-C18, 5 μm, 4.6 × 150 mm).
Total Phenolic (TP) Content
The TP content of the beers was measured spectrophotometrically at 760 nm after the reaction with Folin-Ciocalteu (FC) phenol reagent, according to the method described by Singleton et al.[Citation18] and Stratil et al.[Citation19] The measurement was compared to a calibration line of prepared gallic acid (GA) solution, and the results were expressed as milligrams of gallic acid equivalents (GAE) per liter of beer (mg GAE/L). All measurements were performed in triplicate.
Total Flavonoid (TF) Content
The TF content in selected beer samples was determined according to the aluminum chloride spectrophotometric method described by Zhishen et al.,[Citation20] with minor modification. The solution of beer (0.25 mL) was added to a 10-mL volumetric flask containing 3 mL of ethanol-water (50:50 v/v) solution. Then 0.3 mL of 5% NaNO2 was added. After incubation at room temperature for 5 min, 1.5 mL of 2% aluminum chloride hexahydrate (AlCl3·6H2O) was added. Again, the flask was kept at room temperature for 5 min and then 2 mL of 1 mol L−1 sodium hydroxide (NaOH) was added. The flask was filled with ethanol-water (50:50 by vol.) to the mark. Absorbance of the reaction mixture was measured at 360 nm against an ethanol-water blank on a spectrophotometer. Quercetin was chosen as a standard and the results were expressed in milligram quercetin equivalents per liter of beer (mg QE/L). The levels of total flavonoid contents in beer were determined in triplicate.
Antioxidative Assays
For the DPPH method,[Citation9] which is slightly modified, a solution of DPPH (1 × 10−4 mol L−1) was prepared in methanol. Initially, 5.0 mL of this solution and 100 μL of beer were mixed in 10-mL volumetric flask and filled with methanol-water (50:50 by vol.) to the mark. The discoloration of the DPPH radical was measured at 520 nm, 30 min after the reaction started. The Trolox calibration curve was plotted as a function of the decrease in absorbance (ΔA = Ablank – A) of DPPH radical scavenging activity. The final results were expressed as millimoles of Trolox equivalents (TE) per liter of beer (mmol TE/L).
The ABTS radical scavenging activity was measured using the method of Re et al.[Citation10] and Arts et al.,[Citation21] with some modification. ABTS was dissolved in methanol to a concentration of 7 × 10−3 mol L−1. The ABTS radical cation was produced by reacting ABTS stock solution with 2.4 × 10−3 mol L−1 potassium persulfate and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. The ABTS radical cation solution was diluted with methanol to obtain the absorbance of 0.70 ± 0.02 at 734 nm. An aliquot of each beer (100 μL) was mixed with 3.9 mL of diluted ABTS radical cation solution. After reaction at room temperature for 6 min, the reduction in absorbance at 734 nm was measured. The Trolox calibration curve was plotted as a function of the decrease in absorbance (ΔA = Ablank – A) of ABTS radical cation scavenging activity. The final results were expressed as millimoles of Trolox equivalents (TE) per liter of beer (mmol TE/L).
FRAP assay measures the sample capacity to reduce a substrate. The FRAP was assessed according to Benzie and Strain.[Citation13] The method is based on the electron transfer of a reduction of the Fe3+-TPTZ complex to the ferrous form at low pH (pH = 3.6). This reduction is monitored by measuring the absorption change at 595 nm. Briefly, 2.1 mL of working FRAP reagent prepared daily was mixed with 100 μL of the beer sample in a 10-mL volumetric flask and filled with water to the mark. The absorbance at 595 nm was recorded after 5 min incubation at 37°C. FRAP values were expressed as mmol of Fe2+ equivalents (FE) per liter of beer (mmol FE/L).
Extraction of Phenolic Compounds
Samples (50 ml) were extracted three times with diethyl ether (30 ml) and then with ethyl acetate (30 ml). After each extraction, samples were centrifuged (3000× g, 10 min) and supernatants collected. The organic fractions were combined, dried for 30 min with anhydrous sodium sulphate, filtered through a Whatman filter, and evaporated to dryness under reduced pressure at 35°C. The residue was re-dissolved in 2 ml of methanol and then filtered through a 0.45-μm membrane filter (Millipore).[Citation22]
Individual Phenolic Compounds in Beer
HPLC analysis was performed using an Agilent chromatograph equipped with an autosampler and photodiode-array detector (1200 Series). Separation was performed with a Zorbax Eclipse C18 (XDB-C18, 5 μm, 4.6 × 150 mm) column at 30°C. Elution was carried out by using a gradient procedure with a mobile phase containing solvent A (acetonitrile) and solvent B (0.1% formic acid in water) as follows: 0 min, 10% A; 15 min 10% A; 35 min 30% A. The solvent flow rate was 0.8 ml/min, and the injection volume was 20 μl. Detection was performed by scanning from 260 to 400 nm.[Citation22] The individual phenolic compounds were separated within 30 min. Identification was carried out by comparing the retention times and spectral data with those of standards. Quantitative determination of individual phenolic compounds in beer was calculated using calibration lines. Results were expressed as milligrams per liter of beer (mg/L).
Statistical Analysis
Data are presented as the mean ± standard deviation (SD) for triplicate determinations. Statistical analysis was performed by a paired Student t test, using a statistical package running on a computer (Statistica 8.0, StatSoft, Inc., Tulsa, OK, USA). A probability of p < 0.05 was considered to be statistically significant.[Citation23]
RESULTS AND DISCUSSION
Twenty-four beer samples of the three beer types (lager, dark, and alcohol free) selected in this study were analyzed.
TP and TF Contents in the Selected Beer Samples
Beer is one of the major sources of phenolic compounds. Their presence in beer is contributed to its flavor, color, and sensory properties. Also, phenolic compounds may be a major factor in assuring the antioxidant potential of the diet and may contribute to maintaining the endogenous redox balance in humans.[Citation24] Beer samples that were rich in phenolic antioxidants showed higher quality, more stable sensory properties, such as flavor and aroma, foam stability, and longer shelf life when compared to beer with lower antioxidant activity.[Citation25 − Citation27] Therefore, determination of total phenolics in 24 beer samples was examined and the results are presented in .
Table 2 Total phenolic and flavonoid contents of beer samples
The total phenolic content significantly varied depending on the beer type, ranging from 328.22 mg GAE/L for Jelen Cool to 545.32 mg GAE/L for Staropramen. The average concentration of total phenolic content in lager, dark, and alcohol-free beers was 420.32, 490.26, and 340.40 mg GAE/L, respectively. The total phenolic content was significantly higher in dark and lager beers than in alcohol-free beers. The results obtained by Folin-Ciocalteu assay were similar with those obtained from Shahidi and Naczk[Citation28] (270–600 mg GAE/L), Piazzon et al.[Citation29] (366–622 mg GAE/L), and Lugasi[Citation30] (average concentration of 376 and 473 mg GAE/L for alcohol-free and dark beers, respectively) and lower than those obtained by Zhao et al.[Citation31] (152.01–339.12 mg GAE/L) and Obruča et al.[Citation32] (156.49–201.15 mg GAE/L). This might be due to the redox-active substances, such as proteins,[Citation33] which are presented in beer in the concentration range of 3–5 g per liter of beer.[Citation2] Therefore, separation and identification of individual phenolic compounds are of importance to reveal the real differences in phenolic profiles present in beers. Similarly to the phenols content, the total flavonoid content () in the selected beer samples showed significant differences depending on beer type, ranging from 103.85 mg QE/L in Amstel to 208.58 mg QE/L in Nikšićko. The results were higher by those from Obruča et al.[Citation32] (53.49–76.80 mg/L).
Individual Phenolic Compounds
Because the color formation of the Folin-Ciocalteau reaction is based on chemical reduction of the reagent, this reaction might not only reflect the levels of phenolic compounds, but also the contents of Maillard reaction products (MRPs) and other interferences with reducing activity.[Citation33] Therefore, separation and identification of individual phenolic compounds are of importance to give information about the real differences in phenolic profiles present in beers.
Twelve phenolic compounds, including gallic acid, protocatechuic acid, 4-hydroxybenzoic acid, 2,5-dihydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, sinapic acid, salicylic acid, (+)-catechin, and (-)-epicatechin, were identified in 24 beer samples. The results are summarized in . The chromatographic profile for the phenolic acids in a selected beer sample is given in . In all beer samples, gallic acid (1.13–1.96 mg/L), ferulic acid (0.85–2.16 mg/L), protocatechuic acid (1.04–2.04 mg/L), and (+)-catechin (0.57–1.27 mg/L) are the major phenolic compounds, followed by p-coumaric acid (0.11–0.68 mg/L), sinapic acid (0.13–0.38 mg/L), (-)-epicatechin (0.08–0.39 mg/L), vanillic acid (0.06–0.039 mg/L), caffeic acid (0.07–0.57 mg/L), 2,5-dihydroxybenzoic acid (0.021–0.63 mg/L), and 4-hydroxybenzoic acid (0.014–0.13 mg/L). The results obtained are in agreement with data from the literature.[Citation22, Citation29, Citation31, Citation34 − Citation36]
Figure 1 HPLC chromatograms recorded at 320 nm of individual phenolic compounds in selected beer sample. Peaks identification: 1: gallic acid; 2: protocatechuic acid; 3: 4-hydroxybenzoic acid; 4: 2,5-dihydroxybenzoic acid; 5: (+) catechin; 6: vanillic acid; 7: caffeic acid; 8: (-)-epicatechin; 9: p-coumaric acid; 10: ferulic acid; 11: sinapic acid; 12: salicylic acid.
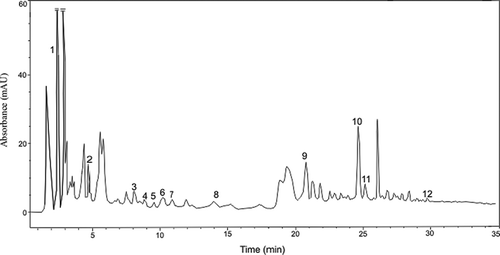
Table 3a Individual phenolic content in 24 commercial beers.Footnote a
Table 3b Individual phenolic content in 24 commercial beersa
Table 4 DPPH radical scavenging activity, ABTS radical cation activity, and ferric-ion reducing antioxidant parameter-FRAP of beer samples
The concentration of individual phenolic compounds is lower in the alcohol-free beers than in the standard beers. The lower values for the alcohol-free beers have been attributed to losses produced by the dealcoholization processes employed. Several processes are currently employed commercially for the dealcoholization of alcoholic beverages, including vacuum-evaporation, membrane pervaporation, and reverse osmosis. Dealcoholization removes from the beverage not only volatile low molecular weight components, such as water, alcohol, flavor, and fragrance components, and dissolved gases, such as carbon dioxide and sulfur dioxide, but may also remove certain low-molecular-weight, nonvolatile, nonionic solutes, such as organic acids and simple sugars.[Citation22] The differences in total phenols content determined by Folin-Ciocalteu and HPLC methods are the result of the non-specific of Folin-Ciocalteu method. Results obtained indicated that individual phenolic contents could reflect the antioxidant activity of beer more objectively than total phenols content.
DPPH Radical Scavenging Activity
The DPPH radical scavenging activities of light, dark, and alcohol-free beer samples ranged from 0.39–0.65, 0.35–0.83, and 0.26–0.34 mmol TE/L, respectively (). Significant differences in DPPH radical scavenging activities were evident depending on beer type, with the lowest levels in alcohol-free beers and the highest levels in dark beers. Pils Plus showed the lowest DPPH radical scavenging activity, whereas Tuborg had the highest activity with respect to light beers; Guinness showed the lowest and Nikšićko had the highest activity for dark beer; and among alcohol-free beers, Jelen Cool showed low and Union high DPPH activity. Obtained results were in agreement with those obtained by Zhao et al.[Citation31] (0.24–0.70 mmol TE/L; of 34 beer samples only 4 have DPPH radical scavenging activity higher than 0.70 mmol TE/L) and Lugasi.[Citation30] All results suggest that raw material and brewing process might have significant influence on the DPPH radical scavenging activity of beer.[Citation37, Citation38]
Polyphenols content and antioxidant activity increased in the order: alcohol-free < lager < dark, so that the polyphenols content and the antioxidative activity of dark beer were found to be 1.5–2 times higher with respect to alcohol-free beers. Vinson et al.[Citation39] reported higher polyphenols content, measured with the Folin-Ciocalteu method, in dark beer with respect to light and alcohol-free beers. Recently, both polyphenols content and antioxidant activity have been reported to be lower in alcohol-free beers with respect to strong and dark beers.[Citation40] Alcohol-free beers are usually brewed with lower original wort extract and inhibition of alcohol formation, or as normal alcoholic beers, with the alcohol removal at the last step, whereas dark are brewed from wort with higher extract content. Moreover, the brewing process itself may influence the final polyphenols content and antioxidant activity of beers.[Citation37, Citation38]
ABTS Radical Cation Scavenging Activity
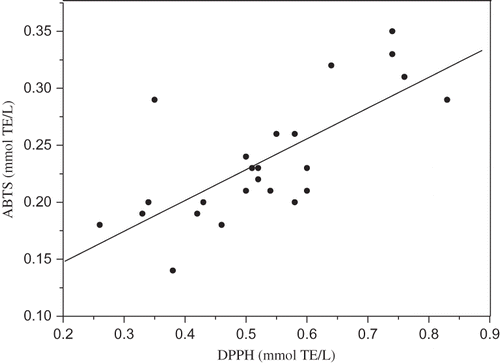
Twenty-four beer samples were also measured and compared for their free radical scavenging activity against the ABTS radical cation (). The range of ABTS values for light, dark, and alcohol-free beers are 0.20–0.32, 0.26–0.35, and 0.18–0.20 mmol TE/L, respectively. ABTS antioxidant activity increased in the order: alcohol free < lager < dark, which was consistent with the result from DPPH radical scavenging activity assay. Similarly to the DPPH radical scavenging activities, ABTS radical cation scavenging activity of beers showed significant differences depending on beer type, with the lowest levels in alcohol free beers and the highest levels in dark beers. In addition, TE values obtained by the ABTS assay were consistently lower than those obtained by the DPPH assay. This observation may result from the different reaction kinetics between phenol and ABTS radical cation and DPPH radical. This might lead to the different results from two methods.[Citation41] The same items were found in other studies.[Citation42, Citation43] It was also run that the ABTS assay on a subset of beer samples representative of the three different types is parallel with the DPPH assay (). A significant correlation was evident between ABTS and DPPH values.
Ferric Reducing/Antioxidant Power (FRAP) Assay
The values of total antioxidant activity of beer evaluated by the FRAP assay are higher than those obtained by DPPH and ABTS assays (). Similar to the DPPH radical scavenging activities and ABTS radical cation scavenging activity, FRAP activity of beers showed significant differences depending on beer type. In examining results of the FRAP assay, dark beer samples (629.82–831.20 mmol FE/L) had the greatest antioxidant capacity, followed by light (22.99–762.57 mmol FE/L) and alcohol-free beers (87.11–572.03 mmol FE/L). Similar activities were also found in beers analyzed by Tafulo et al.[Citation44] Higher results were found by Piazzon et al.[Citation29] These results also indicate that raw material and brewing process might have considerable influence of ferric reducing-antioxidant power. About 80% of phenolics present in beer are derived from barley malt. The high antioxidant activity of dark beers is due to its high level of MRPs because the crystal malts with a high level of MRPs are used as raw material during the dark beer brewing. Therefore, the measurement of phenolic profiles by HPLC method could give more information about their chemical characteristics and antioxidant activities. The strong correlation between FRAP values and TP of beers and the lack of correlation between FRAP values and the content of free phenolic acids () indicate that ester-bound phenolic acids in beer retain antioxidant activity and denote higher ferric reducing ability. From it can also be seen that the caffeic, vanillic, sinapic, and ferulic acids are the major contributors to the antioxidant activity of beers. Also, a correlation between FRAP and ABTS (), and FRAP and DPPH () assays was demonstrated by linear regression analysis. The correlation between assays was found to be 0.62 (p < 0.001) and 0.53 (p < 0.007), respectively.
Figure 3 Correlation between antioxidant values measured with FRAP and ABTS assay (r = 0.62, p < 0.001).
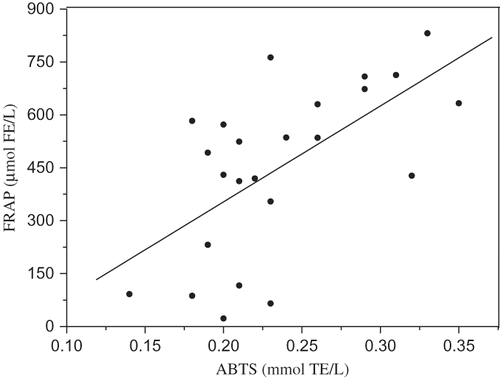
Figure 4 Correlation between antioxidant values measured with FRAP and DPPH assay (r = 0.53, p < 0.007).
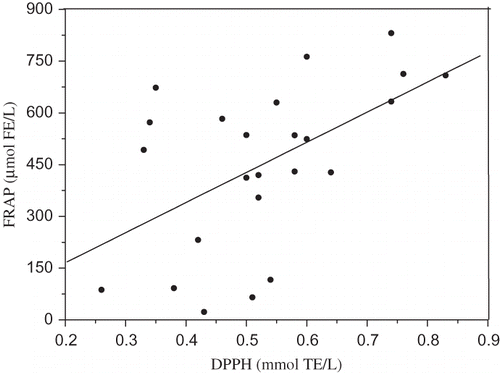
Table 5 Relation between antioxidant activity (FRAP values) and free phenolics acids content
The post-hoc test showed only two homogeneous sub-groups: one with DPPH and ABTS, and the other with FRAP. This confirmed the statistical difference between FRAP and the other two (DPPH and ABTS) assays.
CONCLUSION
In summary, the total phenols and flavonoid content were determined and total antioxidant activities of 24 beer samples were measured by three different methods. Also, different phenolic compounds responsible for beer antioxidant activity were characterized and determined. The results obtained showed that there is considerable variation in total phenolic profiles and antioxidant activities of commercial beers across different brands. Generally, beers with less alcohol have lower levels of phenols than regular light and dark beers, attributed to differences in the duration of the fermentation process. Both light and dark beers have high antioxidant activity. Since the activities and mechanisms of antioxidants present in beer might depend on the composition and conditions of the test system, no single method can fully evaluate the total antioxidant capacity of beers. Results showed that the two radical scavenging tests (DPPH and ABTS) are compatible due to their similar mechanism of radical scavenging. In contrast, correlation between DPPH and FRAP, and ABTS and FRAP are not significant because they follow different mechanisms. When the content of phenolic compounds in the beer samples was considered, gallic acid, protocatechuic acid, ferulic acid, and (+)-catechin are found in major amounts in all samples. All compounds determined, although present in beer at low levels, might contribute to antioxidant activity of beer. In addition, the results of chemical analysis of phenolic compounds in commercial beers could be used as one of the quality indicators for beer processing and marketing. Also, these results could be important to the Serbian beer industry and international beer traders in profiling the Serbian beer market and the diverse demands of its consumers.
ACKNOWLEDGMENT
This research was supported by grant numbers 31060 and 172047 from the Serbian Ministry of Science and Environmental Protection. The authors are grateful for the financial support provided by this Ministry.
REFERENCES
- Bamforth , C.W. 2003 . Beer: Tap into the Art and Science of Brewing , 3 – 10 . Oxford University Press : New York .
- Bamforth , C.W. 2002 . Nutrition aspect of beer: A review . Nutrition Research , 22 ( 1–2 ) : 227 – 237 .
- Bamforth , C.W. 2004 . Beer: Health and Nutrition , 124 – 152 . Blackwell Science Ltd : Oxford . England
- Gerhauser , C. , Alt , A.P. , Klimo , K. , Knauft , J. , Frank , N. and Becker , H. 2002 . Isolation and potential cancer chemopreventive activities of phenolic compounds of beer . Phytochemistry Reviews , 1 ( 3 ) : 369 – 377 .
- Sohrabvandi , S. , Mortazavian , A.M. and Rezaei , K. 2012 . Health-related aspects of beer: A review . International Journal of Food Properties , 15 ( 2 ) : 350 – 373 .
- Gerhauser , C. , Alt , A.P. , Gamal-Eldeen , A. , Neumann , I. , Frank , N. , Chimiel , H. , Bartsch , H. and Becker , H. 2001 . Antioxidant and radical scavenging potential of phenolic constituents of beer . Proceedings of the American Association for Cancer Research , 42 : 103 – 108 .
- Gonzalez-Munoz , M.J. , Pena , A. and Meseguer , I. 2008 . Role of beer as a possible protective factor in preventing Alzheimer's disease . Food and Chemical Toxicology , 46 ( 1 ) : 49 – 56 .
- Sohrabvandi , S , Mousavi , S.M. , Razavi , S.H. and Mortazavian , A.M. 2010 . Application of advanced instrumental techniques for analysis of physical and physicochemical properties of beer: a review . International Journal of Food Properties , 13 ( 4 ) : 744 – 759 .
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of a free radical method to evaluate antioxidant activity . Lebensmittel-Wissenschaft und Technolgie , 28 ( 1 ) : 25 – 30 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolonization assay. Free Radical Biology and Medicine , 26 ( 9–10 ) : 1231 – 1237 .
- Takao , T. , Kitatani , F. , Watanabe , N. , Yagi , A. and Sakata , K. 1994 . A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish . Bioscience, Biotechnology and Biochemistry , 58 ( 10 ) : 1780 – 1783 .
- Ghiselli , A. , Serafini , M. , Maiani , G. , Azzini , E. and Ferro-Luzi , A. 1995 . A fluorescence-based method for measuring total plasma antioxidant capability . Free Radical Biology and Medicine , 18 ( 1 ) : 29 – 36 .
- Benzie , I.F.F. and Strain , J.J. 1999 . Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration . Methods in Enzymology , 299 : 15 – 27 .
- Dinis , T.C.P. , Madeira , V.M.C. and Almeidam , L.M. 1994 . Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and peroxyl radicals scavengers . Archives of Biochemistry and Biophysics , 315 ( 1 ) : 161 – 169 .
- Zhao , H. , Dong , J. , Lu , J. , Chen , J. , Li , Y. , Shan , L. , Lin , Y. , Fan , W. and Gu , G. 2006 . Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.) . Journal of Agricultural and Food Chemistry , 54 ( 19 ) : 7277 – 7286 .
- Huang , D. , Ou , B. and Prior , R.L. 2005 . The chemistry behind antioxidant capacity assays . Journal of Agricultural and Food Chemistry , 53 ( 6 ) : 1841 – 1856 .
- Euromonitor International. Beer in Serbia. 2011 http://www.euromonitor.com/beer-in-serbia/report (http://www.euromonitor.com/beer-in-serbia/report)
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent . Methods in Enzymology , 299 : 152 – 178 .
- Stratil , P. , Klejdus , B. and Kuban , V. 2006 . Determination of total content of phenolic compounds and their antioxidant activity in vegetables-evaluation of spectrophotometric methods . Journal of Agricultural and Food Chemistry , 54 ( 3 ) : 607 – 616 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoids content in mulberry and scavenging effect on superoxide radicals . Food Chemistry , 64 ( 4 ) : 555 – 559 .
- Arts , M.J.T.J. , Haenen , G.R.M.M. , Voss , H.P. and Bast , A. 2004 . Antioxidant capacity of reaction products limits the applicability of the trolox equivalent antioxidant capacity (TEAC) assay . Food and Chemical Toxicology , 42 ( 1 ) : 45 – 49 .
- Callemien , D. and Structure , Collin, S. 2010 . organoleptic properties, quantification methods, and stability of phenolic compounds in beer—A review . Food Reviews International , 26 ( 1 ) : 1 – 84 .
- Miller , J.N. and Miller , J.C. 2005 . “ Pearson Education Limited: London ” . In Statistics and Chemometrics for Analytical Chemistry 1 – 165 . England
- Scalbert , A. and Williamson , G. 2000 . Dietary intake and bioavailability of polyphenols . Journal of Nutrition , 130 ( 8 ) : 2073S – 2085S .
- McMurrough , I. , Madigan , D. and Kelly , R.J. 1996 . The role of flavonoid polyphenols in beer stabulity . Journal of the American Society of Brewing Chemists , 54 ( 3 ) : 141 – 148 .
- Woffenden , H.M. , Ames , J.M. and Chandra , S. 2001 . Relationships between antioxidant activity, color, and flavour compounds of crystal malt extracts . Journal of Agricultural and Food Chemistry , 49 ( 11 ) : 5524 – 5530 .
- Guido , L.F. , Curto , A.F. , Boivin , P. , Benismail , N. , Goncalves , C.R. and Barros , A.A. 2007 . Correlation of malt quality parameters and beer flavor stability: Multivariate analysis . Journal of Agricultural and Food Chemistry , 55 ( 3 ) : 728 – 733 .
- Shahidi , F. and Naczk , M. 1995 . Phenolic compounds of beverages , 128 – 136 . Lancaster : Food Phenolics, Sources, Chemistry, Effects, Applications; Technoming Publishing Co. . InPA
- Piazzon , A. , Forte , M. and Nardini , M. 2010 . Characterization of phenolics content and antioxidant activity of different beer types . Journal of Agricultural and Food Chemistry , 58 ( 19 ) : 10677 – 10683 .
- Lugasi , A. 2003 . Polyphenol content and antioxidant properties of beer . Acta Alimentaria , 32 : 181 – 192 .
- Zhao , H. , Chen , W. , Lu , J. and Zhao , M. 2010 . Phenolic profiles and antioxidant activities of commercial beers . Food Chemistry , 119 ( 3 ) : 1150 – 1158 .
- Obruča , S. , Marova , I. , Parilova , K. , Muller , L. , Zdrahal , Z. and Mikulikova , R. 2009 . A contribution to analysis of Czech Beer authenticity . Czech Journal of Food Sciences , 27 ( 1 ) : S323 – S326 .
- Davalos , A. , Gomez-Cordoves , C. and Bartolome , B. 2003 . Commercial dietary antioxidant supplements assayed for their antioxidant activity by different methodologies . Journal of Agricultural and Food Chemistry , 51 ( 9 ) : 2512 – 2519 .
- Nardini , M. and Ghiselli , A. 2004 . Determination of free and bound phenolic acids in beer . Food Chemistry , 84 ( 1 ) : 137 – 143 .
- Floridi , S. , Montanari , L. , Marconi , O. and Fantozzi , P. 2003 . Determination of free and phenolic acids in wort and beer by coulometric array detection . Journal of Agricultural and Food Chemistry , 51 ( 6 ) : 1548 – 1554 .
- Bartolome , B. , Pena-Neira , A. and Gomez-Cordoves , C. 2000 . Phenolics and related substances in alcohol-free beers . European Food Research and Technology , 210 ( 6 ) : 419 – 423 .
- Vanbeneden , N. , Van Roey , T. , Willems , F. , Delvaux , F. and Delvaux , R.F. 2008 . Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing . Food Chemistry , 111 ( 1 ) : 83 – 91 .
- Krofta , K. , Mikyska , A. and Haskova , D. 2008 . Antioxidant characteristics of hops and hop products . Journal of the Institute of Brewing , 114 ( 2 ) : 160 – 166 .
- Vinson , J.A. , Mandarano , M. , Hrist , M. , Trevithick , J.R. and Bose , P. 2003 . Phenol antioxidant quantity in foods: Beers and the effect of two types of beer on an animal model of atherosclerosis . Journal of Agricultural and Food Chemistry , 51 ( 18 ) : 5528 – 5533 .
- Gorjanovic , S. , Novakovic , M. , Potkonjak , N. , Leskosek-Cukalovic , I. and Suznjevic , D. 2010 . Application of a novel antioxidative assay in beer analysis and brewing process monitoring . Journal of Agricultural and Food Chemistry , 58 ( 2 ) : 744 – 751 .
- Campos , A.M. and Lissi , E.A. 1997 . Kinetics of the reaction between 2,2-azinobis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) derived radical cation and phenols . International Journal of Chemical Kinetics , 29 ( 3 ) : 219 – 224 .
- Pellegrini , N. , Serafini , M. , Colombi , B. , Del Rio , D. , Salvatore , S. , Bianchi , M. and Brighenti , F. 2003 . Total antioxidant capacity of plant foods beverages and oils consumed in Italy assessed by three different in vitro assays . Journal of Nutrition , 133 ( 9 ) : 2812 – 2819 .
- Zhao , H. , Fan , W. , Dong , J. , Lu , J. , Chen , J. , Shan , L. , Lin , Y. and Kong , W. 2008 . Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties . Food Chemistry , 107 ( 1 ) : 296 – 304 .
- Tafulo , P.A.R. , Querios , R.B. , Delerue-Matos , C.M. and Sales , M.G.F. 2010 . Control and comparison of the antioxidant capacity of beers . Food Research International , 43 ( 6 ) : 1702 – 1709 .