Abstract
Fish gelatins obtained from perch fish skin pretreated with various solutions containing acetic acid, sodium hydroxide (NaOH) and sodium chloride (NaCl) were successfully characterized for their nanostructure pattern using field emission scanning electron microscopy. Each pretreatment transformed collagen to gelatin with fibril, zigzag cracks, straight rods, and cross-linked rods nanostructure patterns. Pretreatment solutions also affect the gel yield, gel strength, amino acid profile, and functional groups in perch gelatin as analyzed by Fourier transform infrared spectroscopy. Samples pretreated with NaCl, NaOH, and acetic acid solution showed the highest gel yield (22.84%) and gel strength (179.84 g). Fourier transform infrared spectra for perch gelatins also revealed weak C–N amide II and III bond stretches as well as weak C=O bond stretch.
INTRODUCTION
The demand for gelatin for industrial and domestic purposes has been on the increase in recent times. Gelatin quality depends on the gel strength, which equally determines its market value. Global gelatin production is mainly obtained from mammalian raw materials, but interest in fish gelatin has gained momentum due to health concern and/or religious reason.[Citation1] Numerous works have been carried out to improve the functional properties of fish gelatin, and many nano-structural analyses of gelatin have been conducted in order to correlate structural information with physical properties, but the information to date is still not convincing.[Citation2]
Nile perch (Lates niloticus) is a warm water fish species, belonging to the family Centropomidae. Nile perch is one of the most important commercial fish species in East Asia and the fish is mostly filleted for sale.[Citation3] About 55% of the whole fish constitute waste during fillet production, while frames and skins are 6 and 25%, respectively.[Citation4] Extraction of the beneficial component of this “waste” will inevitably lead to a generation of fish residue which can be processed to gelatin.[Citation5]
Acid and alkaline pretreatment as well as extraction conditions affect both composition and distribution of hydrophobic amino acid of fish gelatin, and thereby influenced their physical properties.[ 6, Citation7] Generally, mild acid pretreatment is used for gelatin production due to low concentration of intra- and inter-chain non-reducible crosslink in fish collagen and this process is sufficient to achieve adequate swelling and disruption of the non-covalent intra- and inter-molecular bonds.[Citation8,Citation9]
Alkali, particularly lime, pretreatment of fish skins may reduce protein molecular size through slight hydrolysis of the polar regions and this facilitates lyotropic hydration or swelling of collagen protein by organic acids.[Citation10] The use of hydroxides, such as Ca(OH)2 and NaOH in gelatin extraction, is primarily to remove non-collagenous proteins as well as non-protein substances such as mucopolysaccharides and sulfur-containing compounds.[Citation11,Citation12]
Pretreatment process involving the use of chloride salts are often required in order to remove muscle tissues that adhere to the fish skin. Chloride salt, such as NaCl, facilitates extraction of α- and β-chain polymer, while the application of MgCl2 resulted in less content of dimers of α-chains, trimmers, and greater polymers.[Citation13] Acetic acid, NaOH, and NaCl among others have been applied for the recovery of gelatin from fish skin, and this increased the degreasing and deodourisation of the fish skin which eventually affect the fish gelatin including its nanostructure.[Citation14] All the above-mentioned processes suggest that fish gelatin is affected by various pretreatment methods. In this present study, the effects of pretreatment process involving acetic acid, NaOH, and NaCl, on the gelatin produced from perch (Lates niloticus) skin, were investigated to obtain gelatin with improved functional properties.
MATERIALS AND METHODS
Materials
Pieces of fresh perch fish were obtained from a local fish depot in Gombak, Selangor state, Malaysia, and transported under cool conditions to the Biotechnology Engineering Laboratory, International Islamic University Malaysia, where their skins were peeled, scaled, washed with water (4oC), cut into 2–3-cm squares, and kept at –20oC until use. All reagents used were of analytical grade and were used as supplied without further purification.
Gelatin Extraction
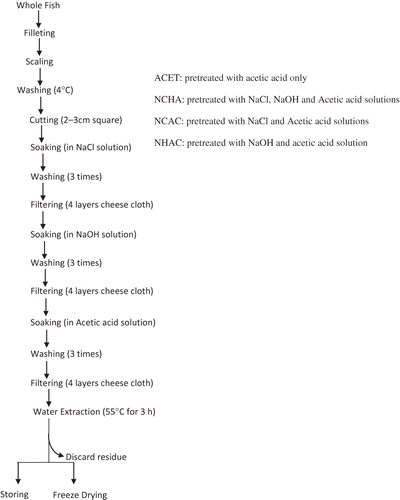
The conditions used for gelatin extraction were as described in the literature.[7, 14 ] Pretreatment solutions were prepared with different molarities as 0.115 M acetic acid, (6.6 ml of 99.7% acetic acid with 993.4 ml of distilled water), 0.2 M NaOH, (8 g NaOH in 1000 ml distilled water), and 0.1 M NaCl, (5.84 g NaCl in 1000 ml distilled water), and kept at 4oC until use. Peeled perch skin (30 g) was carefully weighed and soaked in various pretreatment solutions. A batch was treated with a single solution (acetic acid) while other samples were treated with various combinations of the pretreatment solutions in succession. The treatment time for NaCl, NaOH, and acetic acid were 1440, 84, and 60 min, respectively. After each pretreatment, the skins were adequately washed with 180 ml distilled water, three times, and drained using four layers of cheesecloth before the next pretreatment. Pretreated skins were then soaked in distilled water (1:4 w/v) and heated in 55oC water bath for 3 h. The mixture was then filtered through four layers of cheesecloth and the filtrates were kept at –20oC. Liquid was removed from each filtrate sample (10 ml) by freeze drying (Labconco Corporation, USA). shows the flowchart of gelatin extraction process.
Determination of Gel-Yield
The percentage gel yield was conducted using the method described by Zhou and Regenstein.[Citation15] The soluble protein concentration of the filtrate solution was determined by the Biuret method using bovine serum albumin, BSA (Equitech-Bio Inc., USA) as a standard. The gel yield was calculated using the following equation:
Determination of Gel Strength
Gelatin gel strength was determined following the approach described by Yang et al.[Citation12] The extracted filtrate was diluted with distilled water to a protein concentration of 3.3% in a 55oC water bath for 30 min and then stored in a flat bottom gel jar (31 mm diameter × 25 mm height). After maturity at 4oC for 17 ± 1 h, the gel strength was determined with a Universal TA-XT2 Texture Analyzer (Texture Technologies Corp., USA), by injecting 5 kg load cell at a speed of 1 mm/s at 4oC into the gelatin gel.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Sodium dodecyl sulfate polyacrylamide gel electrophoresis was conducted following the procedure of Gimenez, et al.[Citation16] with slight modification using 4% stacking gel and 9% resolving gel in a Mini Protean II unit (Bio-Rad Laboratories, Hercules, CA, USA) at 25 mA/gel. Protein bands were stained with Coomassie brilliant Blue R250.
Amino Acid Analysis
Amino acid analysis was carried out according to the procedure described by Pedersen et al.[17] Dried gelatin was dissolved in distilled water at concentration of 1 mg/ml, and then 50 ml sample was dried and hydrolysed in vacuum-sealed glass tube at 108oC for 16 h in the presence of constant boiling of 6 N HCl containing 0.1% phenol, using α-amino butanoic acid (AABA) as internal standard. After hydrolysis, sample was again vacuum-dried, dissolved in application buffer and analyzed using a HPLC system (PICO TAG method, Millipore Corp., USA). Amino acid analysis was performed following on-line derivatisation and reversed phase HPLC using a Zorbax 80A C18 column (3.9 mm × 15 cm), with a flow rate of 0.5 ml/min. Retention time for sample spectra was compared with that of standard, and areas of spectra were correlated with concentrations of each amino acid.
Field Emission Scanning Electron Microscopy (FESEM) Imaging
Powdered samples of lyophilized gelatin was mounted on a stub and subjected to prefixing by cold slush nitrogen cryo-fixation for 30 min. Pre-fixed mounted sample was inserted into a sample holder of FESEM (JEOL JIM-5600, Japan) and lightly tightened before the nanostructures were viewed with the aid of a computer imaging software provided by the manufacturer. The operation procedure of Jongjareonrak et al.,[18] with slight modification, was used and the operating parameters were as follow: Acceleration voltage of 15 kV, focus depth of 4, emission current of 10 μA, and SEM column mode.
Fourier Transform Infrared (FTIR) Analysis
The FTIR analysis was conducted according to Muyonga et al.[Citation19] Disc containing dried gelatin (2 mg) mixed with KBr (100 mg) was prepared for each sample and all spectra were obtained using a Bruker infrared spectrometer (Bruker Instruments, USA) from 4000 to 500 cm−1 at data acquisition rate of 2 cm−1 per point. Background was subtracted using the Opus software (Bruker Instruments, USA). Triplicate samples of gelatin were analyzed and the average of the spectra was used for final analysis.
Statistical Analysis
The protein yield was analyzed for significant differences using MATLAB software (version 7.9 [R2009b]) for standard deviation and analysis of variance (ANOVA) and means were separated using Duncan multiple range techniques.
RESULTS AND DISCUSSION
Gelatin Yield
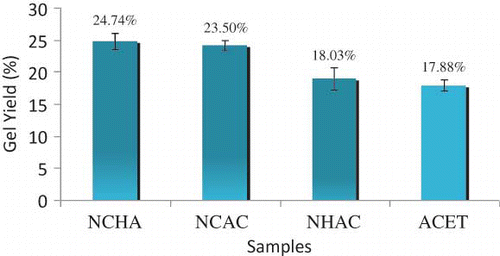
Gelatin was obtained from perch fish skin after pretreatment with as acetic acid, NaOH, and NaCl. Sample ACET was pretreated with acetic acid only while sample NHAC was pretreated with NaOH followed by acetic acid. Similarly, sample NCAC was pretreated with NaCl followed by acetic acid and sample NCHA was pretreated with NaCl followed by NaOH and then acetic acid. The maximum and minimum gel yield were obtained from NCHA (24.74 ± 1.24%) and ACET (17.88 ± 0.89%) respectively. Gelatin yield from the pretreated fish skin sample in decreasing order is NCHA > NCAC > NHAC > ACET ().
The gelatin yield in NCAC sample (24.11 ± 0.78%) showed that additional pretreatment with NaCl increase gelatin yield as compared to acetic acid pretreatment alone (ACET, 17.88 ± 0.89), and this is in agreement with the literature.[Citation13] During pretreatment, electrolytes from base (NaOH) and salt (NaCl) triggered breaking of some bonds in the skin collagen, thus improving the release of collagen during extraction.[Citation20]
Furthermore, the release of collagen into water during extraction might be favored by alkali pretreatment simply due to production of reduced molecular size through partial hydrolysis of the polar regions.[Citation7] Another possibility for the observed increase in gel yield is the release of hydroxyproline during pretreatment with alkali. Proline and hydroxyproline are responsible for the unique secondary structure of collagen as they limit rotation of the polypeptide backbone thus contribute to stability of collagen triple helix.[20] This stable region needs to be unlocked for easy release of collagen into solution during extraction.
The significant difference in percentage gel-yield of the samples with the various pretreatment processes were determined with ANOVA; at p < 0.05. When the means were separated using Duncan Multiple range, NCHA and NCAC were not significant to each other, though they showed significant differences compared to NHAC and ACET. Furthermore, there was no significant difference between NHAC and ACET at p < 0.05. These results suggested that additional pretreatment with alkaline and salt enhance gelatin extraction from the skin of fish since sample NCHA has highest gel-yield. Applications of the selected pretreatment solutions have contributive effects on perch skin conversion to gelatin and release into water during water extraction. It is important to note that a reasonable yield of gelatin is necessary for efficiency of commercial production and economic viability.[6]
Gel Strength
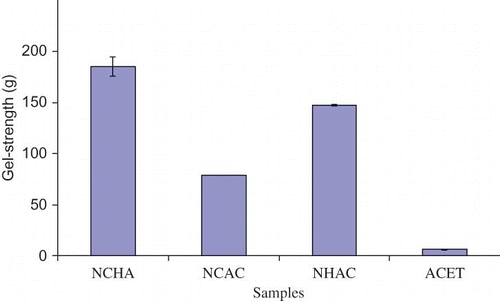
The gel strength obtained for samples were 185.09 ± 9.55 g, 147.80 ± 0.81 g, 78.57 ± 0.09 g, and 91 ± 0.59 g for NCHA, NHAC, NCAC, and ACET respectively (). The trend appears to be similar to the gel yield, but the gel strength of NHAC (147.80 ± 0.81 g) is higher than NCAC (78.57 ± 0.09 g), and this agrees with a report by Zhou and Regenstein[21] that application of alkaline solution favors gelatin with high gel strength. The presence of non-collagenous substance hindered intra-molecular interaction between gelatin molecules during gelation, which made sample ACET had the lowest gel strength. All treatments showed significant differences to one another at p < 0.05. Reduction in gel strength of sample NCAC was due to destabilization of gelatin structure possibly as a result of competition between salt and protein for dehydration water.[Citation22] The presence of NaCl in gelatin solution might result in breaking of both hydrophobic and hydrogen bond, thus, destabilizing gel junctions formation that are needed for high gel strengthening during gelation. This gave alkaline-treated gelatin an edge over salt-treated gelatin in terms of gel strength.[23] When pretreatment include acid, base, and salt (like in sample NCHA), high gel strength was observed.[Citation7]
Amino Acid Profiles
The amino acid composition of all the samples showed the absence of cysteine in the entire sample () because the retention time of a cysteine standard spectrum does not coincide with the retention time of all spectra in the four samples. This supports the fact that collagen have little or no cysteine and this result is comparable to the amino acid profile reported for perch fish.[19] High content of proline in perch fish makes it highly suitable for gelatin production as compared to cold-water fish.[Citation19] Similarly, all samples had high glycine composition which is consistent with the fact that collagen contain one-third glycine. Proline content is second only to glycine in all the samples with values of 14.32, 14.12, 14.19, and 14.44 g for each 100 g sample of NCHA, NCAC, NHAC, and ACET respectively. The hydroxyproline contents were 7.14, 7.01, 7.06, and 7.02 g for 100 g sample of NCHA, NCAC, NHAC, and ACET, respectively. These values were consistent with proline and hydroxylproline showing preference to occupy position next to glycine and the proline + hydrolxyproline content is consistent with these two imino acids content in warm water fish.[19, Citation20]
Table 1 Amino acid composition of gelatin (g of amino acid/100 g of sample)
Generally, all the samples comprise of similar amino acid composition, except for lysine. NCHA and NCAC samples contain higher values of lysine at 2.51 and 2.53 g, respectively compare to NHAC (1.17 g) and ACET (0.96 g). High degree of hydroxylated lysine was found in highly insoluble collagens with a high cross-linking degree.[Citation8] It was reported that aldehydes formation from the oxidative de-amination of the NH2 group of lysine were responsible for covalent cross-linking reaction.[Citation24] Higher value of lysine in NCAC and NCHA was simply due to additional effect of NaCl to release more lysine by affecting the aforementioned covalent cross-links. Similarly, this accounted for low extraction yield obtained for NHAC and ACET, and appears to be coherent with the suggestion that retention of stable cross-linkages accounted for low gelatin yield of the extraction process.[25]
SDS-PAGE
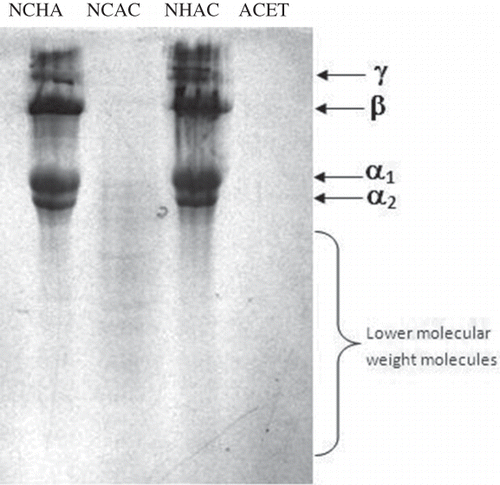
SDS-PAGE for the three samples, NCHA, NCAC, and NHAC, showed four distinct protein bands for gelatin namely alpha (α1 and α2), beta (β), and gamma (γ) (). Some low molecular weight proteins were also present in sample NCAC and some traces of other proteins were also observed for samples NHAC and NCHA. α, β, and γ protein bands were not observed in ACET sample and this result correlated well with the result of Gimenez et al.[Citation16] where gelatin obtained with acetic acid pretreatment alone lacks α, β, and γ protein bands, except for the presence of a 100 kD protein band.
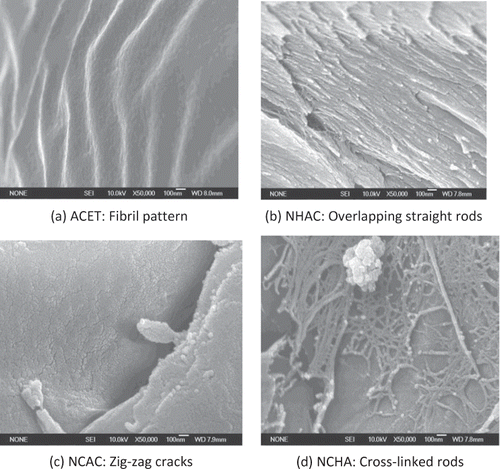
Nanostructure
Dried gelatins obtained from various pretreatments were analyzed for nano structure characteristics (). H+ from an acid caused collagen fibrils to swell by electrostatic swelling and lyotropic hydration, thus, created accessibility for water molecules.[Citation26] The presence of fibrils in ACET () suggests that some bonds were not affected by 0.115 M acetic acid since the precursor collagen material also contained fibrils.[Citation27,Citation28] Fibril pattern had been associated with partial formation or retention of collagen helical structure.[Citation7,Citation29] On the other hand, additional effects of 0.2 M NaOH and 0.1 M NaCl in NCHA caused the collagen rod to open up () forming the familiar Affensen's collagen tubular patterns. It is apparent that ACET was least denatured while that of NCHA was highly denatured.
Pretreatments with alkali and salt affect collagen structure differently and can lead to the formation of distinct surface pattern. Fine gelatin surface had been observed when alkali and acid were used for pretreatment as compared to alkali pretreatment alone.[Citation6] Pretreatment of sample NHAC with 0.115 M acetic acid 0.2 M NaOH caused tubular collagen structures to separate from each other leading to dismantling of rods from each other, similar to separated rods observed in acid and alkali pretreated gelatin.[Citation6] In the presence of NaOH, a slight swelling that occurred in collagen sample allows for water absorption as well as paving the way for any non-collagenous substances initially entrapped within this region to be released,[Citation30] making migration of non-collagenous substance from the interior region of skin possible, and changing the colorless pretreatment solution into light brown. In addition, the removal of non-collagenous materials and rod formation created spaces for water percolation during rehydration. This explained the pore formation in the nano structure of gelatin.[6]
Addition of salt can alter the interaction of protein molecules.[Citation22] Salt electrolytes influenced the biophysical properties such as swelling, solubility, gelatin, viscosity, and water binding capacity, of a protein at different ionic strength and pH values.[Citation31] This could be achieved by either binding directly to the peptide backbone of collagen and affect collagen folding indirectly by interacting with structurally bonding-water molecules.[Citation31]
The zigzag cracks in depicted that some bonds were broken from collagen primary structure. Lyotropic hydration occurred when ions of neutral salts disrupted non-ionic bonds such as hydrogen bonds of collagen. So, salt electrolytes, acting as lyotropic agent, consequently altered water structure around collagen molecules, interrupted internal hydrogen bonds, or interacted with internal hydrophobic bonds by direct bindings at same sites of protein chain. NaCl favors extraction of α- and β-chain polymer, which was a sign of good quality gelatin.[13] A reduced molecular size would favor intermolecular association and would hence facilitate better stabilization of the collagen helix, thus enhancing rheological properties.[Citation32]
Table 2 Conspicuous peaks of spectrum of each sample and their associated bonds
The cross link rods observed in for NCHA was an indication that NaCl and NaOH with acetic acid was able to create nano sized protein tubes. This particular pretreatment protocol is another method of producing protein nonmaterial and production of gelatin with increased functionalities. Protein nanoparticles provide added advantages since they are biodegradable, metabolizable and can also be easily amenable for surface modification and covalent attachment of drugs and ligands.[Citation33] High surface area in porous NCHA can enhance force of attraction between molecules during gelation and provided wider surface for attachment of bioactive components. Since drug compounds can be entrapped into the matrix of carrier,[34] NCHA with meshes of protein tubes will possibly allow easy penetration of bioactive materials to be embedded. Likewise, its usage as a micro carrier in tissue culturing might be enhanced since more surface area will be available for cell attachment.[34]
FTIR Spectra
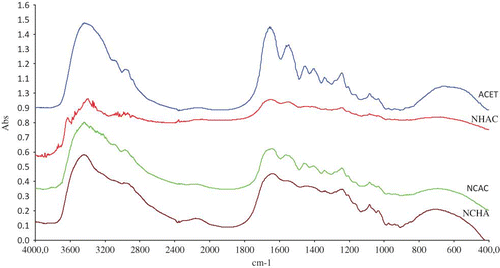
Functional group analysis was performed using FTIR spectroscopy, and the position of absorption bands in the spectra and their intensity were used as a guide for interpretation (). All the samples showed spectra () with apparent patterns in four regions located at 3600–2300 cm−1 (amide A), 1656–1644 cm−1 (amide I), 1560–1335 cm−1 (amide II), and 1240–670 cm−1 (amide III), that these apparent patterns are similar with those reported in the literature.[Citation35] Low intensity absorption bands observed for amide I, amide II, and amide III suggest greater disorder associated with loss of triple helix state of collagen.[Citation36,Citation19] This pattern is consistent with the observation that additional pretreatment with base and salt contribute to more denaturation of collagen. There was reduction in the absorption band for amide II and IIIC–N stretch bonds in NHCA, NCHA, and NCAC compared to ACET. This corroborated the fact that NaOH and NaCl broke some of C–N bonds of the skin protein structure, due to hydrolysis as stated earlier. Likewise, a decrease in absorption band intensity occurred at frequency between 1690–1600 cm−1 (for C=O bond). Other regions affected are weak absorption bands for CH2 bending and CH2 wagging of proline as well as diminished absorption bands in the 1000–1100 cm−1 frequency due to the lack of carbohydrate in the gelatin samples.
The above observations and the nano-structure analysis earlier suggest that a simple relationship can be made. ACET, that was pretreated with acetic acid, still maintain fibril patterns () is vividly reflected in its FTIR spectra where absorption bands are strong for C–N and C=O bonds. In addition, further degradation of collagen by application of NaCl and NaOH pretreatment as depicted by nano-structures is also supported by FTIR spectra since NHCA, NCHA, and NCAC exhibit low absorption band intensity.
CONCLUSION
Pretreatment of perch skin with NaCl, NaOH, and acetic acid solutions showed effective opening up of collagen structure and enhance release of non-collagenous proteins and increase in yield. Nano-structural examination of gelatin revealed effects of different pretreatment solutions and enhance our understanding gelatin processing mechanism and requirements.
ACKNOWLEDGEMENTS
The authors extend their unreserved appreciation to the Research Management Centre and International Institute for Halal Research and Training (INHART), International Islamic University Malaysia, for financial support.
Notes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ljfp.
REFERENCES
- Karim , A.A. and Rajeev , B. 2009 . Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins . Food Hydrocolloids , 23 : 563 – 576 .
- Weiss , G.R. and Terech , P. 2006 . Molecular gels materials with self-assembled fibrillar networks , 255 Springer:Dordrecht the Netherlands .
- Usydus , Z. and Functional , Szlinder-Richert, J. International Journal of Food Properties 2011 . Properties of Fish and Fish Products: A Review , DOI:10.1080/10942912.2010.503356.
- Wasswa , J. , Tang , J. and Gu , X. 2008 . Functional properties of grass carp (Ctenopharyngodon idella), Nile Perch (Lates niloticus), and Nile Tilapia (Oreochromis niloticus) Skin Hydrolysates . International Journal of Food Properties , 11 ( 2 ) : 339 – 350 .
- Prinyawiwatkul , W. , Suvanich , V. , Harrison , R.W. , King , J.M. , Sathivel , S. , Pacheco , K. , Rout , S. , Nadarajah , K. and Sonti , S. 2002 . “ Value-added from crawfish and catfish ” . In Louisiana Agriculture 20 – 21 .
- Yang , H. , Wang , Y. and Joe , R.M. 2008 . Characterization of fish gelatin at nanoscale using atomic force microscopy . Food Biophysics , 3 : 269 – 272 .
- Montero , P. and Gomez-Guillen , M.C. 2000 . Extracting conditions for megrim (Lepidorhombusboscii) skin collagen affect functional properties of the resulting gelatin . Journal of Food Science , 65 : 434 – 438 .
- Montero , P. , Borderıas , J. , Turnay , J. and Lizarbe , M.A. 1990 . Characterization of hake (Merluccius merluccius L.) . and trout (Salmo irideus Gibb.) collagen. Journal of Agricultural and Food Chemistry , 38 : 604 – 609 .
- Norland , R.E. 1990 . “ Fish gelatin ” . In Advances in Fisheries Technology and Biotechnology for Increased Profitability , Edited by: Voight , M.N. and Botta , J.K. 325 – 333 . Technomic Publishing Co : Lancaster .
- Johnston-Banks , F.A. 1990 . Food Gels , Edited by: Harris , P. 233 – 289 . Elsevier Applied Science Publishers : London . Gelatin
- Yoshimura , K. , Terashima , M. , Hozan , D. and Shirai , K. 2000 . Preparation and dynamic viscoelasticity characterization of alkali-solubilized collagen from shark skin . Journal of Agricultural and Food Chemistry , 48 : 685 – 690 .
- Yang , H. , Wang , Y. , Jiang , M. , Oh , J.H. , Herring , J. and Zhou , P. 2007 . 2-step optimization of the extraction and subsequent physical properties of Channel Catfish (Ictalurus punctatus) skin gelatin . Journal of Food Science , 72 ( 4 ) : 188 – 195 .
- Giménez , B. , Gómez-Guillén , M.C. and Montero , P. 2005b . The role of salt washing of fish skins in chemical and rheological properties of gelatin extracted . Food Hydrocolloids , 19 ( 6 ) : 951 – 957 .
- Yang , H. and Wang , Y. 2009 . Effects of concentration on nanostructural images and physical properties of gelatin from channel catfish skins . Food Hydrocolloids , 23 : 577 – 584 .
- Zhou , P. and Regenstein , J.M. 2004 . Optimization of extraction condition for Pollock skin gelatin . Journal of Food Science , 69 : 393 – 398 .
- Gimenez , B. , Turnay , J. , Lizarbe , M.A. , Montero , P. and Gomez-Guillen , M.C. 2005a . Use of lactic acid for extraction of fish skin gelatin . Food Hydrocolloids , 19 : 941 – 950 .
- Pedersen , G.M. , Gilberg , A. , Steiro , K. and Olsen , R.O. 2003 . Histone-like proteins from Atlantic cod milt: Stimulatory effect on Atlantic salmon leucocytes in vivo and in vitro . Comparative Biochemistry and Physiology Part B , 134 : 407 – 416 .
- Jongjareonrak , A. , Benjakul , S. , Visessanguan , W. and Tanaka , M. 2006 . Skin gelatin from bigeye snapper and brownstripe red snapper: Chemical compositions and effect of microbial transglutaminase on gel properties . Food Hydrocolloids , 20 : 1216 – 1222 .
- Muyonga , J.H. , Col , C.G.B. and Duodu , K.G. 2004 . Extraction and physico-chemical characterisation of Nile perch (Latesniloticus) skin and bone gelatin . Food Hydrocolloids , 18 : 581 – 592 .
- Schrieber , R. and Gareis , H. 2007 . “ Gelatine handbook ” . In Weinhem: Wiley-VCH GmbH and Co.: 120 – 125 .
- Zhou , P. and Regenstein , J.M. 2005 . Effects of alkaline and acid pretreatments on Alaska pollock skin gelatin extraction . Journal of Food Science , 70 : 392
- Elysee-Collen , B. and Lencki , R.W. 1996 . Effect of ethanol, ammonium sulfate, and temperature on the phase behaviour of type B gelatin . Journal of Agricultural and Food Chemistry , 44 : 1651 – 1657 .
- Choi , S.S. and Regenstein , J.M. 2000 . Physicochemical and sensory characteristics of fish gelatin . Journal of Food Science , 65 ( 2 ) : 194 – 199 .
- Asghar , A. and Henrickson , R.L. 1982 . Chemical, biochemical, functional, and nutritional characteristics of collagen in food systems . Advances in Food Research , 28 : 232 – 372 .
- Gomez-Guillen , M.C. , Turnay , J. , Fernandez-Diaz , M.D. , Ulmo , N. , Lizarbe , M.A. and Montero , P. 2002 . Structural and physical properties of gelatin extracted from different marine species: A comparative study . Food Hydrocolloids , 16 : 25 – 34 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Gustavson , K. 1956 . The chemistry and reactivity of collagen , 102 – 202 . Academic Press: New York .
- Mackle , A.R. , Gunning , A.P. , Ridout , M.J. and Morris , V.J. 1998 . Gelatin of gelatin observation in the bulk and at the air-water interface . Biopolymers , 46 : 245 – 252 .
- Jiang , D. and Wang , Y. 2010 . Physical properties and internal microstructures of films made from catfish skin gelatin and triacetin mixtures . Food Hydrocolloids , 24 : 105 – 110 .
- Badii , F. and Howell , N.K. 2006 . Fish gelatin: Structure, gelling properties, and interaction with egg albumen proteins . Food Hydrocolloids , 20 : 630 – 640 .
- Cho , S.M. , Gu , Y.S. and Kim , S.B. 2005 . Extracting optimization and physical properties of yellow fin tuna (Thunnusalbacares) skin gelatin compared to mammalian gelatins . Food Hydrocolloids , 19 ( 2 ) : 221 – 229 .
- Hermansson , A.M. 1975 . Functional properties of proteins for food: Flow properties . Journal of Texture Studies , 5 : 425 – 439 .
- Ledward , D.A. 1986 . “ Gelation of gelatin ” . In Functional properties of food macromolecules , Edited by: Mitchell , J.R. and Ledward , D.A. 171 – 201 . Elsevier Applied Science Publishers: London .
- Ezpeleta , I. , Irache , J.M. , Stainmesse , S. , Chabenat , C. , Gueguen , J. , Popineau , Y. and Orecchioni , A.M. 1996 . “ Gliadin nanoparticles for the controlled release of all trans-retinoic acid ” . In International Journal of Pharmaceutics 191 – 200 . 131
- Langer , K. and Wiley-VCH VerlagBmbH , Challa Kuma . 2006 . Peptides Nanoparticles: Biological and Pharmaceutical Nanomaterials , 1st Edited by: KGaA: Weinhem , Co .
- Hashim , D.M. , Che Man , Y.B. , Norakasha , R. , Shuhaimi , M. , Salmah , Y. and Syahariza , Z.A. 2010 . Potential use of Fourier transform infrared spectroscopy for differentiation of bovine and porcine gelatins . Food Chemistry , 118 ( 3 ) : 856 – 860 .