Abstract
This work describes the effect of a repetitive cooking-chilling process on resistant starch content in crackers prepared from a mixture of fish and starch, which are popularly known in Malaysia as “keropok.” Three fish cracker formulations were prepared using tapioca, wheat, and sago starch. Up to four cycles of repetitive cooking-chilling increased the resistant starch content in all products; however, the hardness of chilled samples decreased, and their moisture content increased. For the fried samples, the texture became harder, the color turned darker, and linear expansion was reduced. The dried fish cracker samples prepared with sago starch yielded the highest resistant starch content. The results demonstrated that four cycles of repetitive cooking-chilling were able to enhance resistant starch in fish crackers.
INTRODUCTION
The fish cracker or “keropok” is a low-density snack prepared from the mixture of fish and starch with other ingredients, including water, salt, and sugar. It has been classified as a third-generation snack[Citation1] and is very popular in Southeast Asian countries. In Malaysia, this snack was included in a list from 2009 of 100 local foods designated as contributors to intangible Malaysian heritage.[Citation2] The fish cracker is an important source of protein in places where it is considered a favorite snack, particularly the eastern coastal area of Malaysia. Although it is recognized as a protein and carbohydrate source, the other nutritional benefits of fish crackers are seldom discussed.[Citation3] Considering the selective behavior of modern consumers and their demand for food that not only tastes good but is also healthy and nutritious,[Citation4] the development of healthier fish crackers represents an advantageous approach. One possible way to make this traditional snack healthier is to convert the starch in fish crackers into resistant starch (RS). It has been shown that changes in food preparation and processing can increase the enzyme resistance of starch.[Citation5] RS is defined as the sum of starch and starch degradation products that remain unabsorbed in the small intestine of healthy individuals.[Citation6] It has the advantages of dietary fibers, which influence the digestive tract, microbial flora, blood cholesterol level, and the glycemic index as well as assist in the control of diabetes; unlike traditional sources of fiber, however, RS does not heavily impact the sensory properties of food.[Citation4] Our preliminary analysis indicated that dried fish crackers (laboratory and commercial samples) contained RS in the range of 0.3 to 3.0%. These values are comparable to those in other snack foods, including corn chips (0.7%), potato chips (3.5%), wheat crackers (0.4%), pretzels (1.0%), and rice crunch crackers (0.4%).[Citation7] The major difference with these other products is that fish crackers are a high-protein snack.
As previously mentioned, modifications of processing methods may be used to enhance the RS content in fish crackers. Repeating the heating and storage to increase RS content was first introduced by Berry,[Citation8] who claimed that the use of this technique in wheat starch produced an RS content greatly exceeding that in untreated starch. The same technique was later applied by other researchers[Citation9 − Citation11] who similarly observed increased RS content in various starch samples. However, the samples used in these studies were pure starch powders, and this technique has never been applied to food samples. As the conventional fish cracker processing method involves steaming (heating) and chilling (storage), this technique could be incorporated into the process of fish cracker production. Considering the high percentage of starch in the formulation of this snack, the steaming and chilling steps could be repeated several times to promote RS production.
In addition to analyzing the effect of this repetitive heating-storage technique on RS content, the effects on the quality characteristics of fish crackers should also be monitored. Most studies on fish crackers have focused on the linear expansion, taste, crunchiness, and color of the final fried products,[Citation3,Citation12] while other studies[Citation13 − Citation15] have evaluated the quality characteristics of the intermediate products, especially after the cooking, chilling, and drying processes. The type of starch used in the cracker formulation is also an important consideration because it can influence the RS formation and other quality characteristics. Tapioca starch is normally used for the production of fish crackers because of its highly expandable characteristics during frying, which are attributable to its high amylopectin content and subsequently low amylose/amylopectin ratio (17:83). Sago starch and wheat starch, on the other hand, are less preferred because they have higher amylose/amylopectin ratios, 27:73 for sago and 25:75 for wheat, and are less expandable.[Citation16] Expandability is important because consumers prefer fish crackers with the highest linear expansion.[Citation17] A high RS yield, however, requires a high amylose starch,[Citation8] making comparisons between starches critical for the production of a fish cracker with a high RS content and high linear expansion after frying.
The objectives of this study were (a) to increase the RS content in fish crackers through repetitive cooking and chilling (RCC) and (b) to compare crackers prepared using tapioca, wheat and sago starches by evaluating the quality of chilled, dried, and fried products after exposure to RCC.
MATERIALS AND METHODS
Materials
Fresh sardines (Sardina pilchardus) were purchased from the wet market in Serdang, Malaysia; and salt, sugar, and monosodium glutamate (MSG) were purchased from a grocery store. Tapioca starch (Manihot esculentus) was obtained from BFEE Bakery Ingredients Sdn. Bhd., Kuala Lumpur, Malaysia; wheat starch (Triticum aestivum) was purchased from Mackessen (M) Sdn. Bhd., Shah Alam, Malaysia, and sago starch (Metroxylon sagu) was purchased from Songiing Holding Sdn. Bhd., Sibu, Malaysia. Filtered ice water was obtained from the laboratory.
Preparation of Fish Crackers
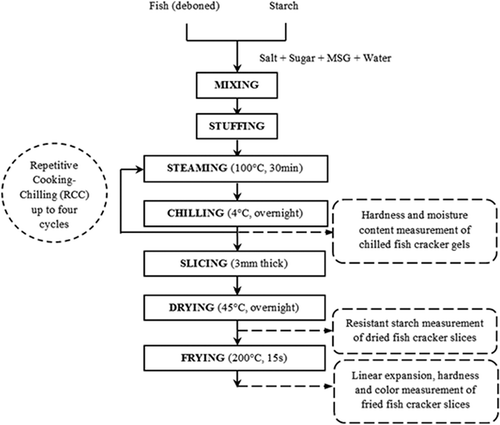
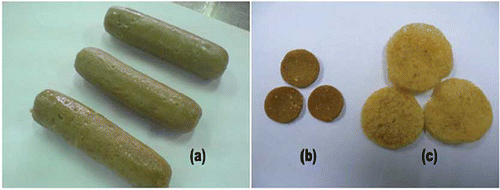
The fish crackers were prepared using a modified version of the procedure previously described by Siaw et al.[Citation18] The preparation methods are illustrated in . The fish cracker formulation was as follows: 1:1 fish to starch ratio (1 kg:1 kg), 2% salt, 1% sugar, 0.1% MSG, and 30% ice water (% weight based on the total weight of starch and wet fish). First, the fresh sardines were manually beheaded, degutted, and deboned using a knife to obtain fresh flesh after the fish were transported from the local wet market to the laboratory. The fish flesh was ground using a meat grinder (National, Japan) then transferred into a silent cutter (Khin Shang Hoo Iron Works, Taiwan) before being mixed with salt, sugar, and MSG. Ice water and starch were added until the mixture formed a homogenous dough. Three types of starch, tapioca, wheat, and sago were used to produce three different formulations. This mixing process took approximately 20 min. A sausage stuffer (F-Dick, Germany) was used to stuff the dough-like mixture into circular cellulose casings (2.5 cm diameter and 12 cm length). Both ends of the stuffed casings were tied with cotton strings, and the stuffed rods were steamed for 30 min at 100°C in a steamer (Eagle Wares, Malaysia). After cooking, the steamed gels were immediately immersed in ice water to prevent shrinkage and to facilitate separation from the casings. The gels were then chilled overnight in a cold room at 4°C to complete one cooking-chilling cycle. This single cooking-chilling cycle is a conventional practice in the fish cracker industry, and it was used as a reference in this study. The cooking-chilling cycle was repeated up to four times, according to the method described by Berry.[Citation8] The chilled gels were manually cut into 3 mm slices, which were oven dried at 45°C overnight until the final moisture content was 8–10%. The dried gels were deep fried in palm oil at 200°C for 15 s until fully expanded. Samples were taken after chilling (in gel form, ), after drying (in dried slice form, ), and after frying (in expanded cracker form, ) to obtain measurements. The protein contents for single cooking-chilling fish crackers with a similar formulation of tapioca, wheat, and sago starches were found at 15.6, 24.9, and 15.7% respectively at a standard fat content of 0.4%.[Citation19]
Measurement of RS in Dried Fish Crackers
Protein removal was performed prior to RS analysis,[Citation20] and the RS Assay Analysis (Megazyme International Ireland Ltd. Co., Ireland)[Citation21] was used for the RS content determination. The method includes incubation with α-amylase (37°C, 16 h) to hydrolyze the digestible starch, solubilization of precipitate using 2 M KOH, incubation with amyloglucosidase (50°C, 30 min) and quantification of glucose using a glucose oxidase/peroxidase reagent.
Measurement of Instrumental Hardness and Moisture Content in Chilled Fish Crackers
The chilled gels were maintained at room temperature (32°C) for 2 h prior to analysis[Citation13] and cut into cylinders (25 mm diameter and 25 mm length). The hardness test was performed using a texture analyzer (Stable Micro System, TA.XT.Plus, UK). Samples were placed on a heavy-duty platform, and force was applied onto the sample using a compression probe (P/36R) 35 mm in diameter at a constant speed of 2 mm/s. The compression tests were set at a distance of 50% deformation. Force-deformation data were recorded to determine the textural characteristics of the sample. The hardness force was obtained from the absolute value of the peak height, and the hardness measurements were performed in quadruplicate.
The moisture content was determined using an oven drying method.[Citation22] The chilled gels were divided into two sections, corresponding to the center and periphery of the cylindrical rods, and each section was used to determine the moisture content of the gel. The center portion was 15 mm in diameter. A balance of the center portion was used to determine the peripheral portion, and three samples were used for each measurement.
Measurement of Linear Expansion, Hardness, and Color in Fried Fish Crackers
The percent linear expansion of the fried fish cracker sample was determined according to Yu et al.[Citation23] Three parallel lines were drawn on the dried fish crackers using a marker. The length of each line was measured before and after frying. The calculation for linear expansion is as follows:
Textural analysis of the fried fish cracker sample was performed using a texture analyzer (Stable Micro System, TA-XT2i, UK). Samples were placed on a fabricated hollow cylindrical base (25 mm inner diameter, 1.5 mm thickness, stainless steel). Force was applied using a 5 mm spherical compression probe (P/0.25S) at a constant speed of 3 mm/s until the sample cracked. The maximum force of break was used as an indicator of sample hardness. The average value was calculated from 25 measurements. The color of the fried samples was measured using a color reader (Konica Minolta, CR-10, Japan). Ten measurements were used to determine lightness (L*), redness (a*), and yellowness (b*) and the average value was recorded for each sample.
Data Analysis
All tests were conducted in triplicate, and statistical analysis was performed using Microsoft Excel 2007 (Vista Edition, Microsoft Corporation, USA) and Statistical Analysis System (SAS) software (Version 9.2, SAS Institute, Inc., USA).[Citation24] A significance level of α = 0.05 was used throughout the analysis, and p-values larger than α (p > 0.05) indicated that the difference between treatments was not significant. When a significant difference was found from the ANOVA results, the treatments were compared using a t-test.
RESULTS AND DISCUSSION
Effect of RCC Treatment on RS Content of Dried Samples
The effect of one to four RCC cycles on the RS yield in dried fish crackers is shown in . RS content increased as the number of RCC cycles increased, which can be explained by the self-association and crystallization of amylose during the process of retrogradation after gelatinization.[Citation25,Citation26] Subsequent heating causes the crystallites to lose order and facilitates further gelatinization and crystallite ordering when the retrogradation process is repeated. The repeated occurrence of this phenomenon in subsequent cycles increases the extent of starch gelatinization with each successive cooking cycle and promotes retrogradation upon cooling.[Citation10] There is possibility of protein and lipid interactions with starch in the samples during the treatment that can affect the RS yields.[Citation25,Citation27] Some studies speculated that protein and lipid could be bound to starch during retrogradation causing less amylose is available to form enzyme-resistant double helices.[Citation28,Citation29] Thus, reduces RS yield during RCC treatment.
Table 1 Effect of RCC treatment on resistant starch content of dried fish cracker slices
The increased RS content in this study is consistent with the findings from other studies that RS forms after repeated cycles of heating and storage.[Citation8,Citation9,Citation30] Under the RCC treatment, the highest level of RS (3.81%) was obtained using the formulation containing sago starch and four repeated cycles of cooking and chilling. Separate experiment (data not shown) demonstrated that when this cracker was subjected to frying, the RS content was reduced to 3.71%. Indeed, this is still exceeds the range of RS (0–3.5%) in various chips, snacks and crackers.[Citation7] This approach for fish cracker production therefore provides consumers an alternative snack that is a good protein source and has an appreciably higher RS content.
When the starch sources were compared, the ranking of the RS content in the dried samples exposed to RCC treatment was as follows: Sago starch > wheat starch > tapioca starch. Of the three starches, sago starch contains the highest amylose content, which helps explain the results.[Citation31] A previous study investigating the relationship between amylose and RS content[Citation8] showed that autoclaved amylomaize starch (50% amylose) had a higher RS content than autoclaved wheat starch (25% amylose). With regards to the increase in RS content, the fish crackers prepared using tapioca starch exhibited the highest RS content increase (104.23%) between RCC cycles one and four, followed by wheat starch (67%) and sago starch (24.1%). This trend was similar with results reported by Berry[Citation8] which has shown after three cycles of heating and storing, RS content in treated wheat starch increased ˜200% compared to amylomaize starch with only ˜8.57%. However, he did not clearly discuss the observation made. Possible explanation is that most of the amylose in starch with higher amylose content was utilized to form crystalline structure during the first cycle of RCC while starch with higher amylopectin experienced higher increment in further RCC treatment due to fragmentation of amylopectin branches to formed linear chain for RS formation. Unfortunately, this was not proven from this work.
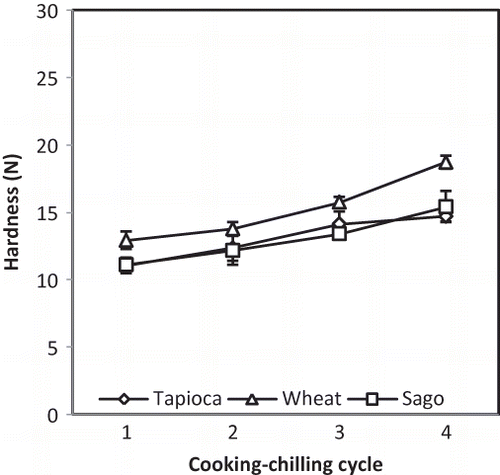
Effect of RCC Treatment on the Hardness and Moisture Content of Chilled Samples
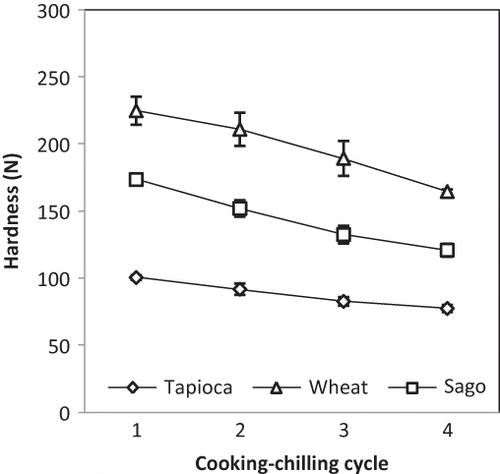
shows the effect of RCC treatment on the hardness of fish cracker gels. The hardness value of all samples decreased with increasing cycle number and was likely attributable to the degradation of starch granules as the processing was repeated. Additional cooking-chilling cycles caused the starch in the sample to undergo further rounds of gelatinization and retrogradation, possibly causing increased granule rupture and allowing soluble materials to leach out. These phenomena would in turn affect the overall hardness value of the sample structure. Several studies have focused on the effect of severe heat-moisture treatment on starch structures. By prolonging the duration of steaming to be more than 30 min at 100°C, a study by Kyaw et al.[Citation32] reported that the compressive force of the fish cracker gel gradually decreased, while the proportion of fragmented starch granules increased. This prolonged heat-moisture treatment of starch gels and the decrease in hardness values are associated with the leaching out of soluble materials from the starch granules, which also diluted the gels and made them less rigid.[Citation33]
Table 2 Effect of RCC treatment on moisture content of chilled fish cracker gels
The previous observations concerning decreased hardness value are further supported by the findings of the present study that RCC treatment increased the moisture content of the fish cracker gels (). For all samples, additional cooking-chilling cycles caused the moisture content to increase in both the central and peripheral parts. Higher moisture content resulted in softer and less firm fish crackers. Comparing the different starches used in the fish cracker gels, wheat starch and sago starch had higher hardness values than tapioca starch (). Accordingly, wheat starch and sago starch have a higher amylose content, which promotes a higher degree of retrogradation.[Citation33] The moisture contents of the three formulations were largely similar, and all samples had the same rate of increase corresponding to additional cooking-chilling cycles in both the central and peripheral parts ().
Effect of RCC Treatment on the Linear Expansion, Hardness, and Color of Fried Samples
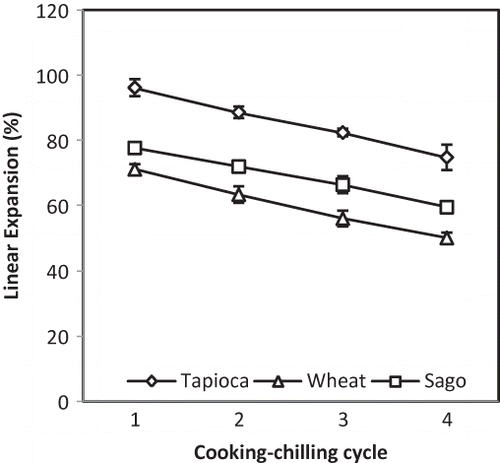
As shown in , the linear expansion of all samples decreased as the number of cooking-chilling cycles increased. The most likely explanation is that the hydrogen bonding involved in RS formation improved the strength of the crackers and made them more resistant to breaking. In addition, maximal swelling and rupture of the starch granules caused by extensive heating in excess water through repetitions of the cooking-chilling process may have caused the expansion to decrease. The linear expansion of fish crackers is strongly associated with starch swelling power because expansion results from the vaporization of water within the starch matrix during frying.[Citation14] After full swelling occurs at the respective gelatinization temperature, further heating causes starch to rupture and disperse, resulting in a decrease of swelling power.[Citation33] Furthermore, ruptured starch granules allow water to be released from the starch structure, and upon frying, the reduced impact force of the water vapor in the starch matrix results in a decreased linear expansion.[Citation14] The varied expansion properties of the different starches reflect differences in their amylose and amylopectin contents.[Citation3,Citation23] For example, the linear expansion of fish crackers is positively correlated to amylopectin content.[Citation3] Among the three starches included in the present study, tapioca starch has the highest amylopectin content; accordingly, the samples prepared using tapioca starch exhibited the highest %LE (96.07% for the first cycle). The percentage was reduced to 74.72% after cycle four. In contrast, the sago starch samples decreased to 59.45% after cycle four, which is consistent with the higher amylose fraction in sago starch. Due to the restriction of protein-starch complexes in wheat starch[Citation14,Citation16,Citation19] and its low amylopectin content, the wheat starch fish cracker samples had the lowest linear expansion values. As previously mentioned, linear expansion is the main attribute contributing to customer acceptance of fish crackers. Siaw et al.[Citation18] found that a linear expansion of 77% was the minimum level for acceptability. In this study, it is only suitable for tapioca based fish crackers up to the third RCC cycle. However, better linear expansion of fish crackers can be achieved by reducing the fish to starch ratio in the fish cracker formulation.[Citation3, Citation17] Nevertheless, improvement on its healthy benefits should be taken into consideration for customer acceptances. In addition, a wide range of linear expansion (38–145%) has been observed in commercial fish crackers.[Citation12]
shows the increase in hardness values for all fried samples under RCC treatment. These values are reflected in the decrease of linear expansion in , where hardness and expansion is inversely proportional.[Citation34] The relationship between linear expansion and hardness values is similar for all samples, which is mostly related to the RS content. Samples with a high RS content generally produced less expandable and harder fish crackers, but the type of botanical starch used was also a contributing factor. Although the sago starch samples had a higher RS content, they had better linear expansion compared to the wheat starch samples. As previously mentioned, the choice of a suitable starch is critical for the production of samples with a high RS content without compromising the quality characteristics important for the consumer.
As shown in , the lightness value (L*) for color measurement significantly decreased for all samples with sago starch fish cracker had the highest percentage of differences for L when the number of cooking-chilling cycles were increased from one to four cycles. In other words, samples that underwent more cooking-chilling cycles tended to be darker in color. There are many factors contributing to the color of fried crackers, including the ratio of fish to starch in the formulation, the type of starch and additives used, cracker thickness, the species of fish used and, due to the presence of sugar, the Maillard reaction.[Citation12] In the present study, the decrease in linear expansion appeared to be the major factor contributing to changes in the L* value. Products tend to be less bright due to less expansion during puffing which associates with their compact structures.[Citation35] When the linear expansion and L* values of each sample were compared, samples with low %LE tended to be darker in color (low L* value). No significant changes (p < 0.05) in a* (redness) or b* (yellowness) were observed for the varying number of cooking-chilling cycles; however, the a* values exhibited a slight increase. The different starches affected the final fish cracker colors. Tapioca starch and sago starch produced fish crackers with high L* values and low a* and b* values. In contrast, wheat starch fish crackers had lower L* values and higher a* and b* values. To date, there are no reports assessing consumer preference for specific color values of fish crackers, but some researchers have observed that lighter colored fish crackers are preferred.[Citation17]
Table 3 Effect of RCC treatment on color measurement of fried fish crackers
CONCLUSIONS
The findings of the present study demonstrated that up to four cycles of RCC increased RS content in fish crackers. However, the repetition of cycles also resulted in some minor drawbacks of the quality characteristics of chilled and fried samples. The hardness of chilled samples decreased, while their moisture content increased. The fried samples exhibited harder texture, darker color, and reduced linear expansion. Fish crackers prepared using sago starch had yielded the highest RS content under the RCC treatment. The results suggest that four cycles of the cooking-chilling process can enhance the RS content in fish crackers compared to the single-cycle process (conventional method); however, some minor drawbacks affecting the quality characteristics of fish crackers were also observed.
REFERENCES
- Suknark , K. , McWatters , K.H. and Phillips , R.D. 1998 . Acceptance by American and Asian consumers of extruded fish and peanut snack products . Journal of Food Science , 63 ( 4 ) : 721 – 725 .
- Anon. Makanan Warisan. 2013(accessed October 22, 2013). https://www.heritage.gov.my/v2/index.php?option=com_content&view=article&id=159&Itemid=254 (http://https://www.heritage.gov.my/v2/index.php?option=com_content&view=article&id=159&Itemid=254)
- Taewee , T.K. 2011 . Cracker “Keropok”: A review on factors influencing expansion . International Food Research Journal , 18 ( 3 ) : 825 – 836 .
- Fuentes-Zaragoza , E. , Riquelme-Navarrete , M.J. , Sánchez-Zapata , E. and Pérez-Álvarez , J.A. 2010 . Resistant starch as functional ingredient: A review . Food Research International , 43 ( 4 ) : 931 – 942 .
- Dhital , S. , Katawal , S.B. and Shrestha , A.K. 2010 . Formation of resistant starch during processing and storage of instant noodles . International Journal of Food Properties , 13 ( 3 ) : 454 – 463 .
- Eerlingen , R.C. and Delcour , J.A. 1995 . Formation, analysis, structure, and properties of type III enzyme resistant starch . Journal of Cereal Science , 22 : 129 – 138 .
- Murphy , M.M. , Douglass , J.S. and Birkett , A. 2008 . Resistant starch intakes in the United States . Journal of the American Dietetic Association , 108 ( 1 ) : 67 – 78 .
- Berry , C.S. 1986 . Resistant starch: Formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during the determination of dietary fibre . Journal of Cereal Science , 4 ( 4 ) : 301 – 314 .
- Shin-Kyung , L. , Sae-Hun , M. and Mal-Shick , S. 1997 . Effect of heating conditions on the resistant starch formation . Agricultural Chemistry and Biotechnology , 40 : 220 – 224 .
- Gormley , R. and Walshe , T. 1999 . Effects of boiling, warm holding, mashing, and cooling on the levels of enzyme-resistant potato starch . International Journal of Food Science and Technology , 34 : 281 – 286 .
- Aparicio-Saguilán , A. , Sáyago-Ayerdi , S.G. , Vargas-Torres , A. , Tovar , J. , Ascencio-Otero , T.E. and Bello-Pérez , L.A. 2007 . Slowly digestible cookies prepared from resistant starch-rich lintnerized banana starch . Journal of Food Composition and Analysis , 20 ( 3–4 ) : 175 – 181 .
- Huda , N. , Leng , A.L. and Yee , C.X. 2010 . Herpandi . Chemical compostion, colour, and linear expansion properties of Malaysian commercial fish cracker (keropok). Asian Journal of Food and Agro-Industry , 3 ( 5 ) : 473 – 482 .
- Cheow , C.S. , Kyaw , Z.Y. , Howel , N.K. and Dzulkifly , M.H. 2004 . Relationship between physiochemical properties of starches and expansion of fish cracker “keropok” . Journal of Food Quality , 27 ( 1 ) : 1 – 12 .
- Kyaw , Z.Y. , Cheow , C.S. , Yu , S.Y. and Dzulkifly , M.H. 2001 . The effect of pressure cooking on the microstructure and expansion of fish cracker (“keropok”) . Journal of Food Quality , 24 : 181 – 194 .
- Cheow , C.S. , Yu , S.Y. and Howel , N.K. 1999 . Effect of salt, sugar, and monosodium glutamate on the viscoelastic properties of fish cracker (“keropok”) gel . Journal of Food Processing and Preservation , 23 : 21 – 37 .
- Pomeranz , Y. 1991 . Functional Properties of Food Components , 2nd , 26 – 33 . San Diego : Academic Press . EdInc
- Huda , N. , Boni , I. and Noryati , I. 2009 . The effect of different ratios of Dory fish to tapioca flour on the linear expansion, oil absorption, colour, and hardness of fish crackers . International Food Research Journal , 16 : 159 – 165 .
- Siaw , C.L. , Idrus , A.Z. and Yu , S.Y. 1985 . Intermediate technology for fish cracker (Keropok) production . Journal of Food Technology , 20 : 17 – 21 .
- Yu , S.Y. 1991 . Acceptability of fish crackers (keropok) made from different type of flour . Asean Food Journal , 6 ( 3 ) : 114 – 116 .
- Goñi , I. , García-Diza , L. , Mańas , E. and Saura-Calixto , F. 1996 . Analysis of previous resistant starch: A method for foods and food products . Food Chemistry , 56 ( 4 ) : 445 – 449 .
- Anon. Megazyme resistant starch assay procedure. K-RSTAR 08/11. Megazyme International Ireland 2011, 1–16accessed July 3, 2010 http://secure.megazyme.com/downloads/en/data/K-RSTAR.pdf (http://secure.megazyme.com/downloads/en/data/K-RSTAR.pdf)
- AOAC . 2000 . “ Official Methods of Analysis ” . In MN, American Association of Cereal Chemist , 17th Ed , Washington, DC .
- Yu , S.Y. , Mitchell , J.R. and Abdullah , A. 1981 . Production and acceptability testing of fish crackers (“keropok”) prepared by extrusion method . Journal of Food Technology , 16 : 51 – 58 .
- Inc , S.I. 2009 . Base SAS 9.2 Procedures Guide; SAS Institute Inc.: Cary NC
- Sajilata , M.G. , Singhal , R.S. and Kulkarni , P.R. 2006 . Resistant starch-A review . Comprehensive Reviews in Food Science and Food Safety , 5 : 1 – 17 .
- Haralampu , S.G. 2000 . Resistant starch-A review of the physical properties and biological impact of RS3 . Carbohydrate Polymers , 41 : 285 – 292 .
- Koksel , H. , Basman , A. , Kahraman , K. and Ozturk , S. 2007 . Effect of acid modification and heat treatments on resistant starch formation and functional properties of corn starch . International Journal of Food Properties , 10 ( 4 ) : 691 – 702 .
- Escarpa , A. , Gonzalez , M.C. , Morales , M.D. and Saura-Calixto , F. 1997 . An approach to the influence of nutrients and other food constituents on resistant starch formation . Food Chemistry , 60 ( 4 ) : 527 – 532 .
- Bird , A.R. , Lopez-Rubio , A. , Shrestha , A.K. , Gidley , M.J. , Stefan , K. , Ian , T.N. and Johan , B.U. 2009 . “ Resistant starch in vitro and in vivo: Factors determining yield, structure, and physiological relevance ” . In Modern Biopolymer Science , 449 – 510 . Academic Press : San Diego . InChapter 14
- Sievert , D. and Pomeranz , Y. 1989 . Enzyme-resistant starch I . Characterization and evaluation by enzymatic, thermoanalytical, and microscopic methods. Cereal Chemistry , 66 ( 4 ) : 342 – 347 .
- Brumovsky , L.A. , Brumovsky , J.O. , Fretes , M.R. and Peralta , J.M. 2009 . Quantification of resistant starch in several starch sources treated thermally . International Journal of Food Properties , 12 ( 3 ) : 451 – 460 .
- Kyaw , Z.Y. , Yu , S.Y. , Cheow , C.S. and Dzulkifly , M.H. 1999 . The effect of steaming time on linear expansion of fish crackers (“keropok”) . Journal of the Science of Food and Agriculture , 79 : 1340 – 1344 .
- Mohd Adzahan , N. , Dzulkifly , M.H. and Russly , A.R. 2010 . Effect of heat treatment on the physico-chemical properties of starch from different botanical sources . International Food Research Journal , 17 : 127 – 135 .
- Yu , S.Y. and Low , S.L. 1992 . Utilization of pre-gelatinized tapioca starch in the manufacturer of a snack food, fish cracker (“keropok”) . International Journal of Food Science and Technology , 27 : 593 – 596 .
- Altan , A. , McCarthy , K.L. and Maskan , M. 2008 . Evaluation of snack foods from barley-tomato pomace blends by extrusion processing . Journal of Food Engineering , 84 ( 2 ) : 231 – 242 .