Abstract
Whey protein concentrate hydrolysates, capable of inhibiting the activity of angiotensin-converting enzyme, were prepared in the present study. Several parameters, such as type of enzyme, enzyme:substrate ratio, and use of ultrafiltration were evaluated. The angiotensin-converting enzyme inhibitory activity was observed only after the enzymatic treatment and high rates of inhibition were obtained, ranging from 17.29 to 91.88%. Most hydrolysates obtained with pancreatin showed higher angiotensin-converting enzyme inhibitory activity than those obtained with papain both in the absence and presence of ultrafiltration. The smallest enzyme:substrate ratio for pancreatin resulted in better angiotensin-converting enzyme inhibitory activity values. The use of ultrafiltration proved to be disadvantageous for inhibitory activity of hydrolysates obtained with papain.
INTRODUCTION
Hypertension is a serious public health problem and is one of the most important risk factors for various cardiovascular diseases. Hypertension is considered responsible for approximately 40% of deaths from stroke and 25% of deaths from coronary heart disease both in Brazil and worldwide. When occuring in co-morbidity with diabetes, hypertension accounts for a 50% rate of renal failure.[Citation1]
The angiotensin-converting enzyme (ACE) plays an important role in blood pressure regulation; catalyzes the conversion of angiotensin I into a potent vasoconstrictor, angiotensin II, and inactivates bradykinin, an important vasodilator.[Citation2] Several authors have demonstrated the inhibitory effects of peptides and protein hydrolysates on ACE. These inhibitors can be obtained through the enzymatic hydrolysis of various protein sources, such as milk and dairy products,[Citation3 − Citation5] fish,[Citation6,Citation7] mushrooms,[Citation8] canola,[Citation9] mustard,[Citation10] soybeans,[Citation11 − Citation14] beans,[Citation15] maize,[Citation16] and wheat.[Citation17]
The peptides from food have been found to be physiologically active or bioactive, either in a direct manner through their presence in the undisturbed food itself, or after their release from the respective host proteins by hydrolysis in vivo or in vitro. Bioactives have many beneficial properties in the organism, such as antithrombotic, anticarcinogenic, hypocholesterolemic, antimicrobial, antioxidant, antiulcerative, antihypertensive, immunostimulating, and opioid properties.[Citation18]
Whey proteins have a globular structure, corresponding to approximately 20% of the protein content of cow's milk and the main fractions represented by the α-lactalbumin and β-lactoglobulin. Considerable interest has been directed to whey proteins as a source of bioactive peptides, due to their high levels of essential amino acids.[Citation19] However, the usefulness of whey in its natural form is limited because it is perishable, and its components are highly diluted. As an alternative, a whey derivative can be used, such as whey protein concentrate (WPC), which consists of a product originated from the separation of whey proteins using a membrane system and is composed of 35 to 80% protein.[Citation20] WPC is an ingredient that is widely used in many food products, due to the excellent functional properties of its proteins. Thus, the goals of the present study were to prepare enzymatic hydrolysates from WPC, to evaluate their ACE inhibitory activities of the isolates, and to test the effects of enzyme type, E:S ratio and ultrafiltration (UF) on this process.
MATERIALS AND METHODS
Materials
Powdered WPC, consisting of 34.23% protein (Kerrylac 750), was furnished by Kerry do Brasil Ltda (Três Corações, Minas Gerais, Brazil). Pancreatin (Corolase PP), EC 3.4.21.4, 34.71 U.mL−1 and papain (Corolase L10), EC 3.4.22.2, 31.56 U.mL−1 were supplied by AB Enzymes® (Barueri, São Paulo, Brazil). ACE from rabbit lung, 0.25 units mg−1 of protein, EC 3.4.15.1, Hippuryl-histidyl-leucine (HHL) and hippuric acid were purchased from Sigma (St. Louis, MO, USA). HPLC-grade trifluoroacetic acid was purchased from Vetec (Duque de Caxias, Rio de Janeiro, Brazil). HPLC-grade acetonitrile was acquired from J. T. Backer (Phillipsburg, NJ, USA). Polyvinylidene fluoride membranes for the filtration of samples (0.22 μm) and solvents (0.45 μm) and the tangential flow system with a 10 kDa cut-off membrane were purchased from Millipore (São Paulo, Brazil). All other reagents used in this study were of analytical grade.
The reversed-phase high performance liquid chromatography (RP-HPLC) system consisted of a GraceSmart chromatography column RP-18, 150 × 46 mm, 5 μm, 120 Å (Grace Davison, Deerfield, IL, USA), a quaternary pump and a UV-VIS detector (HP series 1100, Waldbronn, Germany) coupled to a computer with analytical software (HP chemstation, Avondale, USA). The water used for this technique was purified on a purification system (Aries Vaponics, Rockland, USA).
Methods
Preparation of enzymatic hydrolysates from WPC
Sixteen hydrolysates were prepared by varying the following parameters: Type of proteolytic enzyme, enzyme:substrate ratio (E:S), and use of UF. The conditions used for preparing these hydrolysates are shown in . Solutions of WPC (10%, w/v) were prepared with distilled water; the concentration of protein in each solution was 3.42%, and the pH was adjusted to 7.0 with a 3 mol L−1 NaOH solution. The solutions were heated in a Vaseline bath under constant agitation by a magnetic stirrer (752A model, Fisatom, São Paulo, SP, Brazil) at the optimum temperature for each enzyme (50°C for pancreatin and 55°C for papain), followed by the addition of these enzymes to obtain the desired E:S ratio (w/w). The total hydrolysis time was 5 h, and after this time, the enzymes were inactivated by heating in a water bath at 75°C for 15 s. The samples were subsequently lyophilized (Freeze Dry System/Freezone 4.5, model 77500, Labconco, Kansas City, MO, USA).
Table 1 Parameters used in the preparation of hydrolysates from WPC
UF of protein hydrolysates
Some samples of protein hydrolysates () were submitted to UF and diafiltration using a volume of water equivalent to ten times the initial volume. A system of tangential flow (40 mL min−1) with a 10 kDa cut-off membrane coupled to a peristaltic pump was used. The filtrated samples were subsequently lyophilized.
Evaluation of the ACE-inhibitory activity of the protein hydrolysates in vitro
Evaluation of the ACE-inhibitory activity of the WPC hydrolysates was performed according to the method developed by Wu et al.[Citation21] using RP-HPLC. Initially, a volume of 12.5 μL of the substrate HHL (2.17 mM) was mixed with 50 μL of hydrolyzed WPC (10 mg mL−1), both prepared with 100 mM borate buffer, pH 8.3, containing 300 mM NaCl, and the mixture was incubated at 37°C for 10 min. A volume of 200 μL of ACE (4 mU), prepared in the same buffer, was subjected to a similar treatment. The two solutions were subsequently mixed, and after incubation at 37°C for 30 min, the reaction was stopped by the addition of 125 μL of HCl (1 mol L−1). Next, the mixture was filtered through a 0.22 μm membrane for analysis by RP-HPLC.
For this analysis, a GraceSmart RP-18 column was used, and the hippuric acid and HHL were detected at 228 nm. The elution flow was of 0.5 mL min−1 with a two-solvent system: (A) 0.05% trifluoroacetic acid (TFA) in water and (B) 0.05% TFA in acetonitrile. The solvent B was used as following: for the first 10 min, in a gradient from 5 to 60%; during the following 2 min it was kept at 60% and for an additional 1 min at 5%. This procedure was followed by isocratic elution for 4 min at the constant flow rate of 0.5 mL min−1, using the solvent A. The ACE inhibitory activity was expressed in two ways, i.e., as a percentage of inhibition and IC50 value, which is defined as the concentration of WPC hydrolysate (mg mL−1) necessary to reduce the activity of this enzyme at 50%.
Evaluation of the effects of some parameters
The effects of certain parameters (type of enzyme, E:S ratio, and UF) on the ACE-inhibitory activity of WPC hydrolysates were also evaluated. Two enzymes (pancreatin and papain) were used for preparing these hydrolysates with E:S ratios of 0.5:100, 1:100, 2:100, and 3:100. For each of these cases, certain samples were submitted to UF, and others were not.
Statistical Analysis
All experiments and analyses were performed in triplicate. The data were subjected to analysis of variance. In order to evaluate the differences between the mean values of either IC50 or ACE-inhibitory activity of the WPC hydrolysates, the Duncan test (p ≤ 0.05) was used.[Citation22]
RESULTS AND DISCUSSION
Among the 16 hydrolysates evaluated (), seven showed ACE-inhibitory activity (ACE-IA) > 80% (H1, H2, H3, H5, H6, H7, and H8), and four showed ACE-IA > 70% (H4, H13, H14, and H16). Three hydrolysates (H9, H11, and H15) showed moderate inhibitory activity (40–60%), and only two samples (H10 and H12) showed low ability to inhibit ACE (<20%). It is also noteworthy that the highest results for the ACE-IA were obtained when pancreatin was used at E:S ratios of 0.5:100 (90.73% in the presence of UF and 91.88% in the absence of UF) and 3:100 (89.96% in the presence of UF). Additionally, there was no significant difference between these results. Moreover, no ACE-IA was found for WPC, indicating that enzymatic treatment, which gives rise to molecules of small sizes, is required for the manifestation of this bioactive property. A similar phenomenon had already been reported in the literature by some authors.[Citation4,Citation23,Citation24] The association of peptide size and inhibitory activity has been discussed by several authors. Costa et al.[Citation3] demonstrated that ACE-IA is more common in peptides of low molecular weight, especially those with fewer than 12 amino acid residues. However, according to Otte et al.,[Citation5] the most potent peptides obtained by enzymatic hydrolysis of α-lactalbumin and β-casein showed molecular masses of approximately 1000 Da and 2000 Da, respectively, suggesting that even peptides containing more than 12 amino acid residues could show considerable ACE-IA.
Table 2 ACE-inhibitory activity of hydrolysates from WPC
Costa et al.[Citation3] stated that even when the inhibitory activity is present in vitro, a decrease in blood pressure may not occur in vivo, as the peptide must be absorbed intact. This finding could indicate that hydrolysates with high di- and tripeptide contents could be more bioactive because, as demonstrated by Frenhani and Burini,[Citation25] dietary protein is absorbed in the form of these small peptides, as well as free amino acids.
Only two studies on the ACE-inhibitory activity of enzymatic hydrolysates of WPC were found in the literature. Mullally et al.,[Citation23] using five different proteases, obtained hydrolysates with ACE-IA values that ranged from 60.8 to 88.6%, which values are close to those found for several hydrolysates in the present study. Guo et al.[Citation24] employed only one enzyme to hydrolyze WPC (a protease from L. helveticus) and used different temperatures, pHs, E:Ss, and reaction times; these researchers obtained hydrolysates with ACE-IA values that ranged from 15 to 63%, which values are close to those of five hydrolysates in this study that showed moderate or low ACE-IA.
The fact that the whey protein hydrolysates prepared in this study had lower ACE inhibitory activity than the synthetic antihypertensive Captopril (IC50 = 0.006 μmol L−1) does not hinder the application of whey protein-derived peptides in the treatment/prevention of hypertension. In fact, it is expected that milk protein-derived ACE inhibitory peptides, unlike Captopril, would have no undesirable side-effects.[Citation23]
Effects of Some Parameters on ACE-Inhibitory Activity
The effects of enzyme type, E:S, and the use of UF were evaluated for two different purposes: Production of hydrolysates with high ACE-inhibitory activity and reduction of costs for scaling-up the process (use of low E:S ratio and absence of UF).
Effect of the type of enzyme
The influence of this parameter on the ACE-inhibitory activity of protein hydrolysates can be seen in . To keep the other parameters constant, analysis of the data must consider eight groups (four for each type of treatment): with and without UF for E:S values of 0.5:100, 1:100, 2:100, and 3:100. Four of these groups are shown in each part (a and b) of . Pancreatin was more advantageous than papain for all cases, and this effect was more evident when the lowest values of E:S (0.5:100, 90.73% for pancreatin and 17.92% for papain; and 1:100, 79.28% for pancreatin and 17.29% for papain) and UF were used. Certainly, significant differences between these results were observed. The choice of the enzyme for protein hydrolysis can affect the ACE-inhibitory activity of hydrolysates, because the presence of certain amino acids at the C- or N-terminal positions influences this property.[Citation3,Citation26] The most potent inhibitors consist of peptides containing either dicarboxylic or branched amino acid residues, such as valine and isoleucine, at the N-terminal position. Furthermore, the presence of hydrophobic amino acids, such as tryptophan, tyrosine, phenylalanine or proline, at the C-terminal portion is necessary.[Citation3] These reports explain, at least in part, the superior performance of pancreatin, because the action of chymotrypsin, one of the enzymes found in this enzymatic complex, is associated with the breaking of peptide bonds at the C-terminal position of aromatic amino acids, releasing peptides with ACE-inhibitory activity. In contrast, papain, which shows only endopeptidase activity and acts in the cleavage of substrates containing amino acid residues of lysine, arginine or valine, produces less potent peptides.[Citation27]
Figure 1 Effect of enzyme type on the ACE-inhibitory activity of enzymatic hydrolysates from whey protein concentrate. ACE = angiotensin-converting enzyme; ACE-IA = ACE-inhibitory activity; PANC = pancreatin; PAP = papain. Values of enzyme:substrate ratio (E:S) = 0.5:100, 1:100, 2:100 and 3:100. Values are means with their standard errors depicted by vertical bars. Different letters are significantly different (p < 0.05) for the same group of different hydrolysates.
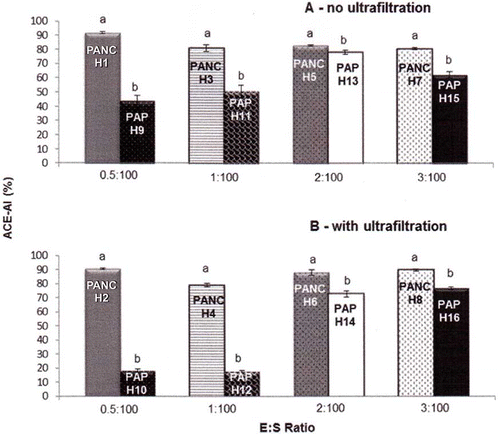
The influence of the type of enzyme on the ACE-inhibitory activity of protein hydrolysates was previously studied by other researchers. Because only one study used WPC as a substrate, the results obtained here were compared with those obtained using other milk-protein sources. With regard to WPC, Mullally et al.[Citation23] evaluated the effects of five enzymes and showed that the most efficient enzymes were trypsin (88.6%) and chymotrypsin (87.7%), followed by corolase PP (78.2%) which is a pancreatin, PTN 3.0S (60.8%), and, finally, elastase (35.5%) (PTN is a commercial enzyme preparation enriched with trypsin).
The use of four enzymes (trypsin, chymotrypsin, thermolysin, and proteinase K) for the hydrolysis of β-lactoglobulin showed that thermolysin was the most efficient and chymotrypsin the least efficient enzyme in obtaining protein hydrolysates with ACE-inhibitory activity (91 and 27%, respectively).[Citation26] Costa et al.[Citation3] evaluating the effects of alcalase, chymotrypsin, and proteomix on the ACE-inhibitory activity of WPI enzymatic hydrolysates, observed that the best result, with an IC50 of 0.40 mg mL−1, was obtained using chymotrypsin, due to the release of aromatic amino acids at the C-terminal position.
In another study, the use of six proteolytic enzymes (alcalase, flavourzyme, neutrase, papain, pepsin,and trypsin) variably affected the ACE-inhibitory activity of hydrolysates from casein and yak milk, and the best results were obtained using neutrase or papain, yielding ACE-IA values around 80%, which were not explained by the authors.[Citation4]
Effect of the E:S ratio
shows the influence of E:S (0.5:100, 1:100, 2:100, and 3:100) on the ACE-inhibitory activity of enzymatic hydrolysates of WPC. To keep the other parameters constant, analysis involved four groups (two each for pancreatin and papain) with application or not of UF. These two groups are presented in each part (a and b) in . As shown in this figure, for pancreatin the values of E:S ratios of 0.5:100 (91.88%), without UF, as well as of 0.5:100 (90.73%), 2:100 (88.35%), and 3:100 (89.96%), with UF, produced the highest ACE-inhibitory activities. For papain, the highest ACE-inhibitory activities were obtained with E:S of 2:100 (78.17%) and 3:100 (76.86%), without and with UF, respectively. It can be inferred that the use of lower E:S ratio can be more advantageous depending on the conditions used to prepare protein hydrolysates.
Figure 2 Effect of E:S ratio on the ACE-inhibitory activity of enzymatic hydrolysates from whey protein concentrate. ACE = angiotensin-converting enzyme; ACE-IA = ACE-inhibitory activity; values of enzyme:substrate ratio: 0.5:100, 1:100, 2:100, and 3:100. These results represent the average of triplicates. Values are means with their standard errors depicted by vertical bars. Different letters are significantly different (p < 0.05) for the same group of different hydrolysates.
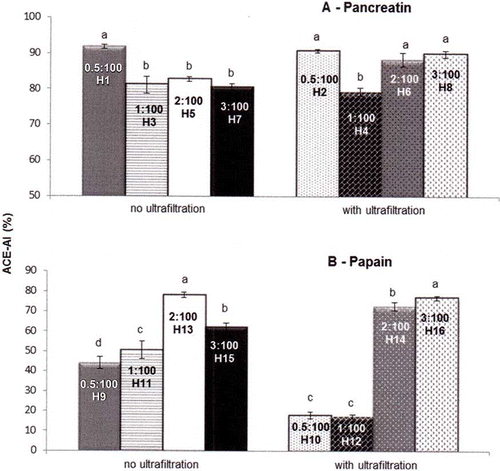
The ACE-IA decreased sharply for samples hydrolyzed with pancreatin in the absence of UF, being reduced from an E:S of 0.5:100 (91.88%) to 1:100 (81.14%), and it remained constant thereafter until an E:S of 3:100 (89.96%). In the presence of UF, the inhibitory activity also showed a sharp drop from an E:S of 0.5:100 (90.73%) to 1:100 (79.28%), but it returned to the initial values at an E:S of 2:100 (88.35%) and 3:100 (89.96%).
The inhibitory activity of samples hydrolyzed with papain in the absence of UF increased when the E:S moved from 0.5:100 (44.15%) to 2:100 (78.17%), where it reached its maximum, and decreased after this point (E:S of 3:100 = 62.33%). Using UF, no change was observed for ACE-IA when the E:S changed from 0.5:100 (17.92%) to 1:100 (17.29%). However, a sharp increase of ACE-IA was observed when the E:S went from 1:100 (17.29%) to 2:100 (72.76%), and a smaller increase was observed from this point until an E:S of 3:100 (76.86%), where it reached its maximum.
In certain cases, the use of a lower E:S ratio may produce hydrolysates with higher ACE-inhibitory activity; this finding could be related to the existence of optimal conditions for hydrolysis. Outside of these conditions, ACE-inhibitory peptides may degrade rather than form, which would decrease inhibitory activity.[Citation6] In the only study of the effect of E:S ratio on the ACE-inhibitory activity of protein hydrolysates, Guo et al.[Citation24] showed that the action of a protease from Lactobacillus helveticus on WPC led to an increase of ACE-IA when the E:S moved from 0.2:100 to 0.8:100, where it reached its maximum value (63%) and subsequently remained unchanged until an E:S of 1.2:100.
Effect of UF
The effect of UF on the ACE-inhibitory activity of protein hydrolysates is shown in . To keep the other parameters constant, analysis included eight groups, four E:S ratios (0.5:100, 1:100, 2:100, and 3:100) for each enzyme (pancreatin and papain). These four groups are shown in each part (a and b) of . The absence of UF was not beneficial when pancreatin was used, because this resulted in protein hydrolysates with either lower ACE-IA values (82.86% for E:S of 2:100 and 80.74% for 3:100) than those obtained in the presence of UF (88.35% for E:S of 2:100 and 89.96% for 3:100) or with no significant differences between ACE-IA values for both types of treatment (91.88% for E:S 0.5:100 and 81.14% for 1:100, without UF; 90.73% for E:S 0.5:100 and 79.28% for 1:100, with UF). However, when using papain in absence of UF, it was possible to obtain hydrolysates with higher ACE-IA than in presence of UF in three cases (17.92% for E:S 0.5:100, 17.29% for 1:100 and 72.76% for 2:100, with UF; 44.15% for E:S 0.5:100, 50.46% for 1:100, and 78.17% for 2:100, without UF). Only in one case (E:S 3:100) was this situation reversed. These findings indicate that the absence of UF can be advantageous depending on the hydrolytic conditions used.
Figure 3 Effect of ultrafiltration on the ACE-inhibitory activity of enzymatic hydrolysates from whey protein concentrate. UF = ultrafiltration; ACE = angiotensin-converting enzyme; ACE-IA = ACE-inhibitory activity; E:S = enzyme:substrate ratio. These results represent the averages of triplicates. Values are means with their standard errors depicted by vertical bars. Different letters are significantly different (p < 0.05) for the same group of different hydrolysates.
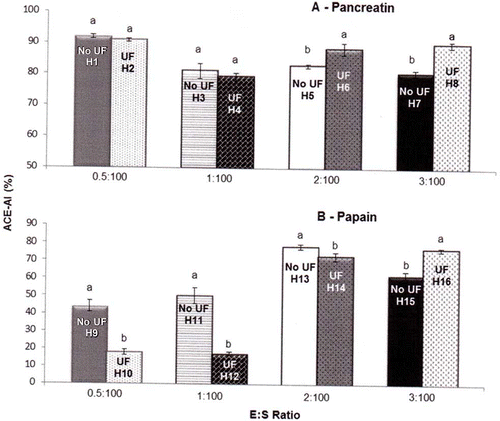
The higher ACE-IA values obtained in the absence of UF could be explained by two factors associated to the reduction of the inhibitory activity when using UF. First, according to Raghavan and Kristinsson,[Citation6] in whole hydrolysates (not subjected to any process of separation of peptides) there is a synergistic action between all peptides found in the sample. Second, depending on the conditions employed, the UF process could cause the retention of peptides with structures (aromatic amino acids or proline at the C-terminal position) that favor ACE-inhibitory activity.
Only two studies examined the influence of UF on the ACE-inhibitory activity of enzymatic hydrolysates from milk proteins. Mullally et al.[Citation23] used two protein sources, specifically, WPC and β-lactoglobulin, which were hydrolyzed by trypsin and subsequently subjected to UF. These authors showed that no change and a reduction of ACE-IA were observed for WPC and β-lactoglobulin hydrolysates, respectively, when a 10 KDa cut-off membrane was used. Therefore, it can be inferred that the absence of UF was beneficial only for this second protein, as was observed in the present work when employing papain to hydrolyze WPC.
In the other study, carried out by Jiang et al.,[Citation4] the yak milk casein was hydrolyzed by a neutrase, and a 10 KDa cut-off UF membrane was used. The result showed no influence of this process on ACE-IA, which was similar to the result obtained for the hydrolysates not subjected to UF in the present work. Taken as a whole, the results obtained both here and in other studies indicate that although UF is considered by some authors to be a method for enriching protein hydrolysates with peptides exhibiting ACE-inhibitory activity,[Citation4] this finding is not always confirmed and depending on the conditions employed, especially in the preparation of protein hydrolysates, the absence of this process can favor ACE-IA, which may lower the costs of scaling-up the process.
CONCLUSIONS
In general, WPC hydrolysates showed significant ability to inhibit ACE. The type of enzyme, E:S ratio and the use of UF affected this property, and the best results for ACE-inhibitory activity were obtained when pancreatin was used in an E:S ratio of 0.5:100 (90.73% in the presence or 91.88% in the absence of UF) or 3:100 (89.96% in the presence of UF).
ACKNOWLEDGMENTS
The authors thank the FINEP and the CNPq for financial support.
REFERENCES
- Sociedade Brasileira de Cardiologia; Sociedade Brasileira de Hipertensão; Sociedade Brasileira de Nefrologia . 2006 . V Diretrizes Brasileiras de Hipertensão Arterial; São Paulo 48 pp
- Guyton , A.C. and Hall , J.E. 2006 . Tratado de Fisiologia Médica , 11th , 1148 Elsevier : Rio de Janeiro . Ed
- Costa , E.L , Gontijo , J.A.R. and Netto , F.M. 2007 . Effect of heat and enzymatic treatment on the antihypertensive activity of whey protein hydrolysates . International Dairy Journal , 17 ( 6 ) : 632 – 640 .
- Jiang , J. , Chen , S. , Ren , F. , Luo , Z. and Zeng , S.S. 2007 . Yak milk casein as a functional ingredient: Preparation and identification of angiotensin-I-converting enzyme inhibitory peptides . Journal Dairy Research , 74 ( 1 ) : 18 – 25 .
- Otte , J. , Shalaby , S.M.A. , Zakora , M. and Nielsen , M.S. 2007 . Fractionation and identification of ACE-inhibitory peptides from α-lactalbumin and β-casein produced by thermolysin-catalysed hydrolysis . International Dairy Journal , 17 ( 12 ) : 1460 – 1472 .
- Raghavan , S. and Kristinsson , H.G. 2009 . ACE-inhibitory activity of tilapia protein hydrolysates . Food Chemistry , 117 ( 4 ) : 582 – 588 .
- Nagaia , T. , Nagashima , T. , Abeb , A. and Suzuki , N. 2006 . Antioxidative activities and angiotensin i-converting enzyme inhibition of extracts prepared from chum salmon (oncorhynchus keta) cartilage and skin . International Journal of Food Properties , 9 ( 4 ) : 813 – 822 .
- Lee , D.H. , Kima , J.H. , Park , J.S. , Choi , Y.J. and Lee , J.S. 2004 . Isolation and characterization of a novel angiotensina-I-converting enzyme inhibitory peptide derived from the edible mushroom Tricholoma giganteum . Peptides , 25 ( 4 ) : 621 – 627 .
- Wu , J. , Aluko , R.E. and Muir , A.D. 2009 . Production of angiotensin-I-converting enzyme inhibitory peptides from defatted canola meal . Bioresource Technology , 100 ( 21 ) : 5283 – 5287 .
- Pedroche , J. , Yust , M.M. , Lqari , H. , Megias , C. , Girón-Calle , J. , Alaiz , M. , Vioque , J. and Millán , F. 2007 . Obtaining of Brassica carinata protein hydrolysates enriched in bioactive peptides using immobilized digestive proteases . Food Research International , 40 ( 7 ) : 931 – 938 .
- Gao , B. and Zhao , X.H. 2011 . “ Modification of soybean protein hydrolysates by alcalase-catalyzed plastein reaction and the ACE-inhibitory activity of the modified product in vitro ” . In International Journal of Food Properties DOI:10.1080/10942912.2010.511755
- Kuba , M. , Tana , C. , Tawata , S. and Yasuda , M. 2005 . Production of angiotensin-I-converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase . Process Biochemistry , 40 ( 6 ) : 2191 – 2196 .
- Li , G.H. , Le , G.W. , Liu , H. and Shi , Y.H. 2005 . Mung-bean protein hydrolysates obtained with alcalase exhibit angiotensin-I-converting enzyme inhibitory activity . Food Science and Technology International , 11 ( 4 ) : 281 – 287 .
- Lee , J.S. , Yoo , M.A. , Koo , S.H. , Baek , H.H. and Lee , H.G. 2008 . Antioxidant and ACE inhibitory activities of soybean hydrolysates: effect of enzyme and degree of hydrolysis . Food Science and Biotechnology , 17 ( 4 ) : 873 – 877 .
- Torruco-Uco , J. , Chel-Guerrero , L. , Martínez-Ayala , A. , Dávila-Ortíz , G. and Betancur-Ancona , D. 2009 . Angiotensin-I-converting enzyme inhibitory and antioxidant activities of protein hydrolysates from Phaseolus lunatus and Phaseolus vulgaris seeds . Food Science and Technology , 42 ( 10 ) : 1597 – 1604 .
- Kim , J.M. , Whang , J.H. and Suh , H.J. 2004 . Enhancement of angiotensin-I-converting enzyme inhibitory activity and improvement of the emulsifying and foaming properties of corn gluten hydrolysate using ultrafiltration membranes . European Food Research and Technology , 218 ( 2 ) : 133 – 138 .
- Ma , M.S. , Bae , I.Y. , Lee , H.G. and Yang , C.B. 2006 . Purification and identification of angiotensin-I-converting enzyme inhibitory peptide from buckwheat (Fagopyrum esculentum Moench) . Food Chemistry , 96 ( 1 ) : 36 – 42 .
- Hartmann , R. and Meisel , H. 2007 . Food-derived peptides with biological activity: from research to food applications . Current Opinion in Biotechnology , 18 ( 2 ) : 163 – 169 .
- Sgarbieri , V.C. 2004 . Propriedades fisiológicas-funcionais das proteínas do soro de leite . Revista de Nutrição , 17 ( 4 ) : 397 – 409. .
- Brans , G. , Schroën , C.G.P.H. , Van Der Sman , R.G.M. and Boom , R.M. 2004 . Membrane fractionation of milk: state of the art and challenges . Journal of Membrane Science , 243 ( 1–2 ) : 263 – 272 .
- Wu , J. , Aluko , R.E. and Muir , A.D. 2002 . Improved method for direct high-performance liquid chromatography assay of angiotensin-I-converting enzyme-catalyzed reactions . Journal of Chromatography A , 950 ( 1–2 ) : 125 – 130 .
- Pimentel-Gomes , F. 2000 . Curso de Estatística Experimental , 14th , 468 Nobel : Piracicaba . Ed
- Mullally , M.M. , Meisel , H. and Fitzgerald , R.J. 1997 . Angiotensin-I-converting enzyme inhibitory activities of gastric and pancreatic proteinase digests of whey proteins . International Dairy Journal , 7 ( 5 ) : 299 – 303 .
- Guo , Y. , Pan , D. and Tanokura , M. 2009 . Optimisation of hydrolysis conditions for the production of the angiotensin-I converting enzyme (ACE) inhibitory peptides from whey protein using response surface methodology . Food Chemistry , 114 ( 1 ) : 328 – 333 .
- Frenhani , P.B. and Burini , R.B. 1999 . Mecanismos de absorção de aminoácidos e oligpeptídeos . Arquivos de Gastroenterologia , 36 ( 4 ) : 227 – 237 .
- Hernández-Ledesma , B. , Recio , I. , Ramos , M. and Amigo , L. 2002 . Preparation of ovine and caprine β-lactoglobulin hydrolysates with ACE-inhibitory activity. Identification of active peptides from caprine β-lactoglobulin hydrolyzed with thermolysin . International Dairy Journal , 12 ( 10 ) : 805 – 812 .
- Beynon , R. and Bond , J.S. 2001 . Proteolytic Enzymes , 2nd , 340 Oxford : Oxford University Press . Ed
