Abstract
A review of the literature identified that there is significant scatter in the data for the thermal conductivity of cakes (including muffins). Most data have been obtained using the line source or thermal conductivity probe method. In this work the thermal conductivity of two types of cakes (sponge cake and yellow cake) were measured over a range of porosities, using a transient comparative method. The thermal conductivity data measured in this work were generally lower than the data measured by the probe or line source method; however, they appeared to lie on a similar curve to the data collated by previous workers who measured thermal conductivity at porosities outside the range considered in this work. The thermal conductivity of the cakes was modelled assuming the cakes were binary materials made up of a condensed phase and air, using equations that did not contain any empirical parameters. Of the eight thermal conductivity models considered, the effective medium theory model provided the most accurate predictions for thermal conductivity as a function of porosity.
INTRODUCTION
Thermal conductivity is an important parameter for modelling thermal processes including cooking, drying, chilling, and freezing.[Citation1,Citation2] Such models allow for process optimisation to improve quality and efficiency.[Citation1 − Citation5] Rask[Citation3] and Baik et al.[Citation6] have reviewed published data for the thermal conductivity of bakery products. By comparison with breads, relatively few data for cake(s) currently exist. Sweat[Citation7] measured the thermal conductivity both at the centre and near the edge of yellow cakes using the line-source method[Citation8] at 22°C. Tou and Tadano[Citation9] calculated the thermal conductivity of muffins (essentially a small cake) from thermal diffusivity, density, and specific heat capacity data. Baik et al.[Citation5] measured the thermal conductivity of a cup-cake at various stages of the baking process using the line source method at 20°C. Reyes et al.[Citation10] measured the thermal conductivity of different cakes and muffins using the thermal conductivity probe (temperature unspecified).[Citation8] Other than these, there are no thermal conductivity data in widely available media (e.g., international peer-reviewed journals, or monographs published by the major scientific publishing houses).
shows the thermal conductivity data for cakes from the sources referred to above[Citation5,Citation7,Citation9,Citation10] plotted as a function of porosity, at (or near) 20°C. Note that some of the thermal conductivity data was cited with corresponding bulk densities rather than porosities, and so the porosities were estimated from the bulk density, using the following relationship:
Figure 1 Thermal conductivity data for cakes (including muffins) from the literature,[Citation5,Citation7,Citation9,Citation10] plotted as a function of porosity.
![Figure 1 Thermal conductivity data for cakes (including muffins) from the literature,[Citation5,Citation7,Citation9,Citation10] plotted as a function of porosity.](/cms/asset/8e0e86ee-3ade-46f6-809c-846444c33239/ljfp_a_692749_o_f0001g.jpg)
If the density of the condensed phase (i.e. the non-air components of the cake) was not specified it was estimated from EquationEq. (2):
If the composition of the cake was not specified then ρa was assumed to be 1400 kg m−3, which is a representative value for cakes (and was the average value obtained from experiments described in this article). shows that the data-point from Tou and Tadano[Citation9] is a clear outlier. A thermal conductivity value of 0.7 W m−1 K−1, which is higher than for pure water, seems too high for a porous food product. This may perhaps be attributed to the fact that it was determined from other physical property data, and hence would include the uncertainty of three different measurements. Alternatively, it may simply have been a data-entry or typographical error. shows the data without the point from Tou and Tadano,[Citation9] and a clear dependence on porosity is observed, with thermal conductivity decreasing as thermal porosity increases, as would be expected since the thermal conductivity of air is an order of magnitude lower than the thermal conductivities of the major food components.[Citation8] However, the scatter in the data seems quite high, particularly when the three data-points from Reyes et al.[Citation10] near ϵ = 0.7 are considered. Reyes et al.[Citation10] did not state the composition of the cakes they tested (other than its porosity), which makes it difficult to assess whether moisture content is partly attributable to this scatter, although it seems unlikely it could be solely responsible. The lack of composition data also makes it difficult to apply predictive models based on composition to the data.
Figure 2 Thermal conductivity data for cakes (including muffins) from the literature,[Citation5,Citation7,Citation10] plotted as a function of porosity (outlying data removed).
![Figure 2 Thermal conductivity data for cakes (including muffins) from the literature,[Citation5,Citation7,Citation10] plotted as a function of porosity (outlying data removed).](/cms/asset/615024f5-3ddf-4b77-80f5-0dcf537dde0e/ljfp_a_692749_o_f0002g.jpg)
Sweat's work[Citation7] also contains a lot of scatter (see data points around ϵ = 0.8), and his data are higher on average than that of Baik et al.[Citation5] and Reyes et al.[Citation10] (note that Sweat's ‘center’ data, rather than the ‘edge’ data were used in plotting and ). Baik et al.[Citation5] provided composition data, however they did not perform many measurements in the region between 0.4 < ϵ < 0.6 (they measured thermal conductivity and density as a function of baking time, rather than thermal conductivity as a function of porosity). Other than the data of Tou and Tadano[Citation9] (who did not actually measure thermal conductivity directly), all the experimental data for the thermal conductivity of cakes were measured using the line source method or conductivity probe (which apply essentially the same measurement principle[Citation8]).
This article describes thermal conductivity measurements of cakes made from two different recipes (‘sponge cake’ and ‘yellow cake’) over a range of porosities, using a transient comparative method described previously. The line source/probe method performs localised thermal conductivity measurements, which means that for heterogeneous media such as porous foods, local variations on composition (including porosity) could introduce measurement bias. By contrast, the transient comparative method used in this work measures thermal conductivity based on heat transfer through the entire sample, and hence is a volume-averaged measurement rather than a localised measurement.
MATERIALS AND METHODS
Thermal Conductivity Measurement
The thermal conductivity measurement was achieved using a transient-comparative method that has been described in detail previously.[Citation8,Citation11] Briefly, a spherical sample of the material is cooled (or heated) in a bath alongside a reference material whose thermal properties are known. The thermal conductivity of the sample can be determined from EquationEq. (3):[Citation11]
The experimental apparatus used in these measurements is illustrated schematically in . The sample containers were flanged, 2 mm thick aluminium hemispheres with internal radii of approximately 75 mm. The test and reference samples were allowed to reach thermal equilibrium overnight before being placed in the stirred ice/water bath (maintained at 0°C) and cooled from approximately 20°C to approximately 1°C. The ice/water bath was sufficiently agitated that the Biot numbers were high (>100), and hence minor variations in the surface heat transfer coefficient would not have had significant effects on the cooling time (refer to Carson et al.[Citation11] for elaboration on this point). Also, since both the test sample and reference were subjected to the same cooling conditions, the effects of transient variations in heat transfer coefficients would have been cancelled by the time thermal conductivity was calculated from EquationEq. (3). Temperatures were measured by calibrated T-type thermocouples and were recorded at regular time intervals (typically every 60 s). The experiments lasted approximately three hours depending on the porosities of the samples.
Figure 3 Experimental apparatus for the transient comparative method used to measure thermal conductivity in this work (for a full description refer to Carson et al.[Citation11]).
![Figure 3 Experimental apparatus for the transient comparative method used to measure thermal conductivity in this work (for a full description refer to Carson et al.[Citation11]).](/cms/asset/aad515e3-9d1a-40f5-9d57-ba08c6fb213c/ljfp_a_692749_o_f0003g.gif)
Cake Preparation
lists the relative amounts of the raw ingredients used for the sponge and yellow cakes. All ingredients were obtained from a local food retailer. The batter for the sponge cakes was mixed using the ‘creaming’ method in which the oil and sugar are mixed first, followed by the eggs, and finally the milk and the flour (sieved) in alternate small portions.[Citation12] The baking powder was added at the same time as the flour and the amount was varied in order to vary the final porosity of the cake. The cake batter was then poured into the 75 mm radius hemispherical aluminium sample containers.
Table 1 Mass basis percentage composition of sponge and yellow cakes in terms of raw ingredients
Table 2 Mass basis percentage composition of sponge and yellow cakes, and sponge and yellow cake reference samples in terms of basic food components
The cakes were baked in the sample containers at 170°C for as long as was required for the centre of the cake to cook (typically between 1 and 1.5 h). At the completion of the baking process the cakes were allowed to cool overnight. The next day the excess cake was cut from the tops of the containers in order that the two hemispheres could be attached together after the thermocouple had been placed at the centre of the sphere. The moisture content of a sample taken from the off-cut was determined by drying at 104°C until no further weight loss was observed. shows the mass basis chemical composition of sponge cake and yellow cake samples (at the completion of the baking process). The basic chemical compositions of the cake ingredients were determined from the ASHRAE Handbook of Refrigeration.[Citation13]
Preparation of Reference Samples
Since the thermal conductivity measurement procedure was a comparative method, reference samples were required. The reference samples were prepared such that they had similar composition to cake samples, but with no air. For this reason baking powder and eggs were not used in the reference samples in order to prevent entrainment of air during the mixing process, or gas evolution over time. Extra milk was added in order to compensate for the removal of the protein and water due to the removal of egg from the ingredients. Other ingredient amounts were also altered slightly to produce compositions of the references as close as possible to the condensed phase (non-air components) of the cakes. The mass basis compositions of the reference samples are shown in . In order that the thermal conductivities of the cakes might be stated in absolute units rather than in relative terms, the thermal conductivities of the reference samples were measured using a Hukseflux™ TP08 thermal conductivity probe,[Citation8,Citation14] and the specific heat capacity was calculated as the weighted arithmetic mean of the specific heat capacity of components.[Citation11,Citation13]
RESULTS AND DISCUSSION
Two replicate thermal conductivity measurements were performed for each porosity value. The agreement between replicate measurements was good, being typically less than 2%. shows the thermal conductivities of the reference samples and the thermal conductivities of the cakes as a function of porosity. The estimated uncertainty in the thermal conductivity data was ± 10%. Since a transient method was employed, the measurements were performed over a range of temperatures, so a mid-range value of 10°C is probably the best temperature to cite as the measurement temperature.
Table 3 Thermal conductivities of the reference samples and the thermal conductivities of the cakes as a function of porosity measured using the transient comparative method
Comparison with Other Data
shows the thermal conductivity data measured for sponge cakes and yellow cakes along with the data from Baik et al.,[Citation5] Sweat,[Citation7] and Reyes et al.[Citation10] The scatter in the data from this work is significantly less than the scatter in the data of Sweat,[Citation7] and Reyes et al.[Citation10] There is little difference between the sponge cake data and yellow cake data measured in this work, suggesting that the minor differences in composition due to different recipes, should not result in significant differences in thermal conductivity. Since the moisture contents of the cakes in this work and the data from Baik et al.[Citation5] and Sweat[Citation7] were all in a similar range, it would be expected that cakes with similar porosities should have similar thermal conductivities; however, shows that the data of Sweat are consistently approximately twice the values of the data in this work and the data of Baik et al.,[Citation5] for similar porosities. By contrast, the data measured in this work appear to lie on a similar curve to the data of Baik et al.,[Citation5] and hence may be considered to be in relative agreement with them.
Figure 4 Thermal conductivity data measured in this work plotted with the data from Baik et al.,[Citation5] Sweat,[Citation7] and Reyes et al.[Citation10]
![Figure 4 Thermal conductivity data measured in this work plotted with the data from Baik et al.,[Citation5] Sweat,[Citation7] and Reyes et al.[Citation10]](/cms/asset/21bd50e3-c4ea-47f4-aded-a53ad7ab9816/ljfp_a_692749_o_f0004g.jpg)
Modelling the Effect of Porosity on Thermal Conductivity
Ideally the thermal conductivity of foods could be predicted based on composition data alone, with little or no information required about its structure. A number of models that have been used to predict the thermal conductivity of foods (including the widely used Krischer model[Citation5,Citation8,Citation15]) contain parameters whose parameter must, in practice, be determined empirically. However, if a measurement needs to be made in order to use a model, it can hardly be described as ‘predictive’. In assessing the suitability of models for predicting the thermal conductivities of cakes, only those which do not contain any empirical parameters were considered.
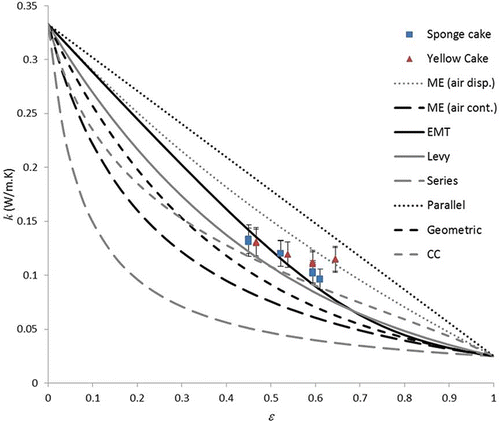
shows plots of the predictions of the following thermal conductivity models, along with the experimental data:
Figure 5 Thermal conductivity data measured in this work plotted against predictions of eight effective thermal conductivity models.
Effective Medium Theory (EMT):[Citation16] (7)
Maxwell-Eucken (air dispersed):[Citation17,Citation18] (8)
Maxwell-Eucken (air continuous):[Citation17,Citation18] (9)
Co-continuous (CC) model:[Citation20] (13)
Since the Maxwell-Eucken and Levy models were derived for binary mixtures, the cakes were assumed to be made up of air and a condensed phase (containing water and food solids). In this study, the thermal conductivity of the condensed phase was that of the reference samples, which were measured; however, if the thermal conductivity of the condensed phase was unknown, it could be estimated using the Parallel model (when the Parallel model was used to estimate the thermal conductivities of the condensed phase in this work, the predicted values were within ± 20% of the measured values).
shows that the best predictions were obtained by the EMT model, which passed through the error bars for all the sponge cake data, but under-predicted the data for the yellow cakes at higher porosities. The Levy and CC models followed the basic trend of the data, but consistently under-predicted the thermal conductivity. The Maxwell-Eucken model with air as the dispersed phase generally over-predicted the thermal conductivity, but passed through the error bars of the yellow cake data at the highest porosity. The experimental data is essentially enclosed in a region bounded above by the Maxwell-Eucken model with air as the dispersed phase, and below by the EMT model, as has been predicted for foam-like materials.[Citation21] While a model which incorporates an empirical parameter might achieve a better fit to the data, it appears that of the models considered, the EMT model is the best for accounting for the effect of porosity on thermal conductivity in cakes.
CONCLUSION
The thermal conductivity data measured using the transient comparative method were generally lower than the data measured by the probe or line source method. However, they appeared to lie on a similar curve to the data collated by Baik et al.,[Citation5] who measured thermal conductivity at porosities outside the range considered in this work. The thermal conductivity of the cakes was modelled as a binary material made up of a condensed phase and air, using equations that did not contain any empirical parameters. Of the eight thermal conductivity models considered, the EMT model provided the most accurate predictions for thermal conductivity as a function of porosity.
NOMENCLATURE
| c | = |
Specific heat capacity (J kg−1 K−1) |
| F | = |
Intermediate variable defined by EquationEq. (11) |
| G | = |
Intermediate variable defined by EquationEq. (12) |
| k | = |
Thermal conductivity (W m−1 K−1) |
| R | = |
Sample container radius (m) |
| t | = |
Time (s) |
| T | = |
Temperature |
| v | = |
Volume fraction |
| ɛ | = |
Porosity (i.e., volume fraction of air) |
| λ | = |
Function of the Biot number (defined in Tou and Tadano,[9] but approximately equal to π) |
| ρ | = |
Density (kg m−3) |
| σ | = |
Slope of cooling curve (s−1) |
Subscripts
| a | = |
Property of air |
| c | = |
Property of condensed phase |
| e | = |
Effective property |
| i | = |
Component index |
| ref | = |
Property of reference sample |
| ∞ | = |
Thermal reservoir (temperature) |
Notes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ljfp.
REFERENCES
- Krokida , M.K. , Pangiotou , N.M. , Maroulis , Z.B. and Saravacos , G.D. 2001 . Thermal conductivity: Literature data compilation for foodstuffs . International Journal of Food Properties , 4 : 111 – 137 .
- Krokida , M.K. , Michailidis , P.A. , Maroulis , Z.B. and Saravacos , G.D. 2002 . Literature data of thermal conductivity of foodstuffs . International Journal of Food Properties , 5 : 63 – 111 .
- Rask , C. 1989 . Thermal properties of dough and bakery products: a review of published data . Journal of Food Engineering , 9 : 167 – 193 .
- Sablani , S.S. , Marcotte , M. , Baik , O.D. and Castaigne , F. 1998 . Modeling of simultaneous heat and water transport in the baking process: A review . Lebensmittel Wissenshaft. Technologie , 31 : 201 – 209 .
- Baik , O.D. , Sablani , S.S. , Marcotte , M. and Castaigne , F. 1999 . Modeling the thermal properties of a cup cake during baking . Journal of Food Science , 64 : 295 – 299 .
- Baik , O.D. , Sablani , S.S. , Marcotte , M. and Castaigne , F. 2001 . Thermal and physical properties of bakery products . Critical Reviews in Food Science and Nutrition , 41 : 321 – 352 .
- Sweat , V.E . Experimental measurement of the thermal conductivity of a yellow cake. Proc. 13th Int. Conf. on Thermal Conductivity 213 – 216 . Lake Ozark , MO Nov. 5–7, 1973 (cited in Baik et al.[4]).
- Rahman , M.S. and Properties , Food . 2009 . Handbook , 2nd Ed. , Boca Raton : CRC Press .
- Tou , K. and Tadano , T. 1991 . Study on effective thermal diffusivity of muffin during baking. Bull. Coll. Agric. Vet. Med., Nihon Univ. Vol. No. 48 , 199 – 204 . cited in Baik et al.[4]
- Reyes , C. , Barringer , S.A. , Uchummal-Chemminian , R. and Kaletunc , G. 2006 . Thermal conductivity models for porous baked foods . Journal of Food Processing and Preservation , 30 : 381 – 392 .
- Carson , J.K. , Lovatt , S.J. and Tanner , D.J. 2004 . Cleland, A.C. Experimental measurements of the effective thermal conductivity of a pseudo-porous food analogue over a range of porosities and mean pore sizes . Journal of Food Engineering , 63 : 87 – 95 .
- Pyler , E.J. 1973 . Baking Science and Technology , Chicago : Siebel Publishing Co. .
- ASHRAE Handbook of Refrigeration . 1998 . American Society of Heating, Refrigeration and Air-conditioning Engineers Atlanta GA
- User manual – TP08 Small Size Non-Steady-State Probe for Thermal Conductivity Measurement, Art no 5000-003 Version 0607, Hukseflux www.hukseflux.com (http://www.hukseflux.com)
- Krischer , O. 1963 . Die wissenschaftlichen Grundlagen der Trocknungstechnik (The Scientific Fundamentals of Drying Technology) , Springer-Verlag, Berlin .
- The Electrical , Landauer, R. 1952 . Resistance of Binary Metallic Mixtures . Journal of Applied Physics , 23 : 779 – 784 .
- Maxwell , J.C. 1954 . A Treatise on Electricity and Magnetism , 3rd Ed. , New York : Dover Publications Inc. .
- Eucken , A. 1940 . Allgemeine Gesetzmäßigkeiten für das Wärmeleitvermögen verschiedener Stoffarten und Aggregatzustände . Forschung Gabiete Ingenieur , 11 : 6 – 20 .
- Levy , F.L. 1981 . A modified Maxwell-Eucken equation for calculating the thermal conductivity of two-component solutions or mixtures . International Journal of Refrigeration , 4 : 223 – 225 .
- Wang , J.F. , Carson , J.K. , North , M.F. and Cleland , D.J. 2008 . A new structural model of effective thermal conductivity for heterogeneous materials with co-continuous phases . International Journal of Heat and Mass Transfer , 51 : 2389 – 2397 .
- Carson , J.K. , Lovatt , S.J. , Tanner , D.J. and Cleland , A.C. 2005 . Thermal conductivity bounds for isotropic, porous materials . International Journal of Heat and Mass Transfer , 48 : 2150 – 2158 .
