Abstract
A study was conducted to evaluate the basic chemical composition, organic acids, volatile compound profiles, and overall acceptability of Surk cheese (acid cheese). The organic acids were determined by reverse phase high performance liqued chromatography method, and volatile compounds were analyzed by static headspace/gas chromatography/mass spectrometry technique. A total of 134 volatile compounds, including 42 esters, 40 terpenes, 15 alcohos, 11 free fatty acids, 6 ketones, 5 aldehydes, 4 alkenes, 4 phenyl propanoids, 3 phenolics, and 4 other compounds, were identified in the Surk cheeses. The main compounds were found to be carvacrol, γ-terpinene, p-cymene, hexanoic acid, octanoic acid, decanoic acid, butanoic acid, and eugenol. The mean total organic acid content of the Surk cheese was 1.71 g/100 g. The main organic acid in the Surk cheese was lactic acid (1067 mg/100 g), followed by acetic, propionic, oxalic, formic, citric, pyruvic, orotic, hippuric, and uric acids.
INTRODUCTION
The types of cheese can vary with the type of milk used, cheese-making procedure, ripening conditions and period, and the spices-herbs used,[Citation1] which affect flavour and texture characteristics of cheeses. In general, the unique flavour of a cheese is formed as a result of a complex balance between volatile and non-volatile compounds, which originate from fat, protein, and lactose. In Turkey, acid-type cheeses are usually produced by heating of acidified milk using indigenous microflora of milk or diluted yogurt. Surk cheese is traditionally made from diluted yogurt (ayran) through boiling. The ayran, the liquid remaining after the manufacture of butter from yogurt, is boiled for ˜30 min to precipitate milk proteins. The precipitate is pressed for 5–6 h to remove excess whey, and then added to various spices and herbs (peppermint, thyme, mint, cumin, black papper, cinnamon, and ginger) (at 0.1–0.3% each) and chili pepper (2–3%). After kneading with added salt (˜5%), the mixture is made into a conical strawberry or pear-like shape, weighing 150–200 g and 5–7 cm in diameter. Therefore, Surk is a cheese containing various spices and herbs produced by acid/heat combination in the southern part of Turkey, particularly in Antakya (Antioch). Surk cheese is usually consumed fresh without aging after air-drying in a shaded enviroment for 3–4 days or wrapped in parchment paper.[Citation2]
Nowadays, consumers demand a high quality of this product, which may benefit their health. Not many cheeses containing herbs and spices are produced in the world since these types of cheese are usually manufactured as a special tradition.[Citation2] However, the production of this type of specialty cheese continues to grow as a result of the increasing sophistication and multiculturalism of the consumer. Spices have long been used in foods due to their flavouring and antimicrobial effects on bacteri, fungi, and virus, and the antioxidant functional properties,[Citation3] whereby Surk cheese has a good marketing potential due to the special added herbs and spices. Spices and herbs used for Surk cheese-making can play an important role in promoting human health due to their anticancer, antioxidative, and anti-inflammatory properties.[Citation4] Treatments with herb extracts increased the stability of cheese against lipid oxidation and also resulted in the antibacterial and antioxidant activity.[Citation5]
A few studies have been reported on the microbial composition and gross chemical properties of Surk cheese,[Citation6 − Citation8] while no studies have been available on the unique taste and flavour of Surk cheese. Considering the specific procedure of Surk cheese manufacturing, Surk cheese may contain high levels of organic acids and volatile compounds, which may differ from those of the other cheeses due to the spices and herbs used. Therefore, the objectives of this study were: (1) to evaluate the basic chemical composition and overall acceptability of the cheeses, and (2) to determine the profiles of organic acids and volatile compounds in Surk cheese in regard to flavour.
MATERIALS AND METHODS
Preparation of Experimental Cheese Samples
Twelve Surk cheese samples (˜500 g) were collected from different villages, bazaars, and retail markerts of Antioch (Antakya). Two lots of each cheese were obtained from the same producer.
Gross-Chemical Analyses
Total solids content was determined by gravimetric method.[Citation9] Total nitrogen was measured by micro-Kjeldahl method,[Citation10] using the Gerhardt KB 40S digestion and Vapotest distillation systems (C. Gerhardt, Bonn, Germany). Fat, salt, titratable acidity, and pH were determined by Gerber, the potentiometric titration method,[Citation11,Citation12] titration using 0.1 N NaOH, and with a pH meter (Orion, Thermo, Beverly, MA, USA), respectively. Ash content was quantitated by dry ashing the samples in a muffle furnace at 550°C for 24 h.
Analysis of Organic Acids
The extraction and quantification of organic acids and lactose was performed according to the procedures described by Fernandez-Garcia and McGregor,[Citation13] with minor modifications. Organic acids and lactose were extracted with 5 mM H2SO4. For this purpose, 7 g of Surk cheese was dissolved in 30 mL of 5 mM H2SO4 and centrifuged at 7000× g for 7 min at 5°C. The upper layer was filtered through Whatman No. 1 filter paper, and then filtrate was filtered again through 0.45-μm syringe filters (Millex PVDF Millipore, Billerica, MA, USA). Seperations were carried out in an automated high performance liquid chromatography system (HPLC-20 AD Prominence, Shimadzu, Kyoto, Japan) using an ion exchange column (Aminex HPX-87 H, 300 × 7.8 mm, BIO-RAD, Hercules, CA, USA). Separation of organic acids was carried out at an isocratic flow rate of 0.6 mL min−1 at 55°C using 5 mM of sulfuric acid as a mobil phase. Organic acids and lactose were detected at 210 nm with a UV/VIS detector (SPD-20 AV, Shimadzu, Kyoto, Japan) and refractive index detector (RID-10A, Shimadzu, Kyoto, Japan), respectively. Organic acid and lactose standards were purchased from Sigma-Aldrich GmbH (Steinheim, Germany) and Supelco (Bellefonte, PA, USA), respectively. Organic acid and lactose solutions were prepared in distilled deionized water, filtered through a 0.45-μm syringe filter (PVDF, Millipore, Billerica, MA, USA) and injected into the HPLC system to provide standard lines based on the peak for each organic acid and lactose. Linear regression curve-based peak areas were calculated for the individual organic acid and lactose covering a broad range of concentrations (). The working solutions of standards were prepared at the five different concentrations. The triplicate injections were carried out on HPLC. The data points from calibration curves were subjected to a least square regression analysis. The coefficients of determination (R 2) obtained were 0.999. The R.S.D. values of f were in the range of 1.52–5% considered adequate to verify the linearity of the regression lines for analytical methods.
Table 1 Regression equations for the calibration curves and analysis of the linearity
Volatile Compound Analysis
The extraction and characterization of the volatile compounds were carried out by headspace (HS)/solid phase microextraction (SPME)/gas chromatography (GC)/mass spectrometry (MS) analysis, which were able to detect most of the volatile compounds. Samples of volatile analysis were prepared in triplicate from each cheese. For each sampling time, each cheese was cut into small pieces and placed in a chilled mortar and ground with a pestle. After several preliminary tests to optimize solid phase microextraction (SPME) system, 10 g of the homogenized cheese sample was immediately transferred in a 20-mL head space vial (Agilent, USA). The vials were sealed using crimp-top caps with TFE/silicone headspace septa (Agilent, USA) and immediately frozen at −20°C until use. Prior to analysis, frozen samples were thawed at 4°C overnight. At the time of solid phase microextraction analysis, the vials were placed in a water bath with temperature control and stirring. The sample vials were equilibrated for 30 min at 60°C in a water bath then a 50/30 μm DVB/CAR/PDMS (Supelco, Bellefonte PA, USA) fibre was exposed to the sample headspace for 40 min at 60°C. The fibre was conditioned for maximum performance at 260°C for 1 h before being placed in the subsequent sample. The extraction procedure was conducted by the modified method of Ziino et al.[Citation14] These sampling temperatures were also chosen after preliminary trials at different temperatures. After sampling, desorption of the volatile compounds from the fibre coating was carried out in the injection port of GC at 250°C during 3 min in splitless mode. The identification and quantification of volatile compounds were carried out on Agilent model 6890 GC and 5973 N mass spectrometry (MS) (Agilent, Palo Alto, CA, USA) equipped with a HP-INNOWAX capillary column (60 m × 0,25 mm id × 0.25 μm film thickness). Helium was used as the carrier gas at a flow rate of 1 mL min−1. The oven temperature program was initially held at 50°C for 1 min and then programmed from 50°C by a ramp of 5°C min−1 up to 100°C and then at 10°C min−1 to reach a final temperature of 230°C, which was held for 10 min. The mass selective (MS) detector was operating in the scan mode within a mass range of 33 to 330 m z−1 at 1 scan s−1, with electron energy of 70 eV. The interface line to MS was set at 250°C. The total analysis time was 30 min. The volatile compounds were preliminarily identified by a computer-matching of their mass spectral data supplemented with a Wiley7n.1 and Nist 02.L. GC-MS libraries, and then most of the identified were confirmed by GC retention time (RT) and MS ion spectra of authentic standards (Sigma-Aldrich, Milwaukee, WI, USA). The retention indices were also determined for all constituents by using homologous series of n-alkanes C5–C25. Results from the volatile analyses were expressed as the percentage of each compound's integrated area relative to the total integration of the compounds identified.
Consumer Preference
Sensory evaluation was performed by 10 experienced panelists who were accustomed to Surk cheese. Cheeses were removed from a refrigerator (4°C) 1 h prior to sensory evaluation, and kept at room temperature (22 ± 2°C). By using a 9-point hedonic sale (1 = dislike extremely, 5 = neither like nor dislike, 9 = like extremely), consumers rated overall acceptability. Cheeses were evaluated in duplicate by the panel members.
RESULTS AND DISCUSSION
Basic Chemical Composition
The basic chemical properties of Surk samples are shown in . The mean values ± SD for total solids, protein, fat, lactose, ash, salt, titratable acidity (TA), and pH were 45.45 ± 4.68 g/100 g, 15.64 ± 5.69 g/100 g, 6.60 ± 5.14 g/100 g, 2.21 ± 1.31 g/100 g, 6.33 ± 2.47 g/100 g, 4.95 ± 1.27 g/100 g, 1.67 ± 0.46 g/100 g, and 4.60 ± 0.36, respectively. The mean chemical properties of Surk cheeses were slightly different from the findings of Guler-Akın and Konar[Citation6] where mean values were: 44.3% for total solids; 9% for fat; 19% for protein; 8.4% for salt; 1.1% for titratable acidity; and 4.94 for pH. Surk cheeses had not been previously analyzed for lactose and ash contents. The mean nutritional composition of Surk cheeses confirmed that producers used for cheese-making about 5% salt and 10% spices, herb, and chilli pepper for cheese-making.These ratios were consistent with the values used for the traditional manufacturing method of Surk cheese.[Citation15]
Table 2 Nutritional composition (g/100 g cheese) of Surk cheeses
Organic acids
To our knowledge, this study is the first report on organic acids and volatile compounds of Surk cheese. A HPLC chromatogram sample for the organic acids of Surk cheese and standard organic acids used for calibration is shown in . Organic acids have two functional properties: preventing the development of spoilage and pathogenic microorganisms as natural preservatives, and improving the sensory characteristics of dairy products. Oxalic, orotic, citric, pyruvic, uric, lactic, formic, acetic, propionic, and hippuric acids were detected in Surk cheeses (). These organic acids appear in dairy products as a result of normal biochemical metabolism (citric, orotic, uric, or hippuric) and bacterial growth (lactic, acetic, pyruvic, propionic, and formic).[Citation16] The amount of organic acids showed a wide variation from cheese to cheese, with coeffient of variation ranging from 34.84 to 76.41%. This variability could be due to the differences in total protein (7.77–24.19%), fat (2–18%), and lactose (0.66–7.59%) contents of the cheeses, the cheese-making conditions, cheese ripening, or microbial load. Lactic acid ranged from 493.80 to 1791.72 with a mean of 1067.56 mg/100 g cheese. As expected, lactic acid was the principle organic acid in Surk cheeses since it is a major product of lactose catabolism by lactic acid bacteria in acid-type cheese.[Citation17] The maximum value of lactic acid was similar to the highest limit (17.4 mg/g) reported by Bevilacqua and Califano[Citation18] for various cheeses. Propionic and acetic acids were the second and the third most abundant organic acids with a mean of 241.76 mg/100 g and 143.79 mg/100 g cheese, respectively. So lactic, propionic, and acetic acids were the main organic acids in Surk cheeses. This result was similar to those reported by Fox et al.[Citation1] for acid-type cheeses, such as Cottage and Queso blanco. Too much pressing time to remove excess whey under the ambient temperatures (25–32°C) and air-drying a shaded enviroment for 3–4 days during Surk cheese manufacturing process could have caused an increase in heterofermantatif lactic acid bacteria, which might have been increased in acetic, propionic, and formic acids contents as well as lactic acid. Despite the unexpected, lactic, propionic, and acetic acids were followed by oxalic acid with a mean of 93.10 mg/100 g cheese. Oxalic acid can be produced from pyruvate by lactate dehydrogenase or from gylcolate by glycolate oxidase in plants.[Citation19] The synthesis of oxalic acid is increased in the mediums containing high lactose, such as whey.[Citation16] On the other hand, the high concentrations of oxalic acid detected in Surk cheeses may be related to spices or herbs used for cheese-making[Citation20] or the pasture on which the cows were feeding.[Citation21] Formic acid ranged from 25.49 to 160.89 with a mean of 71.12 mg/100 g cheese. Formic acid can be produced from pyruvic acid by a homofermantative bacteria, such as enterecocci, or from the deamination of serine by the certain Lactobacillus mesophilic.[Citation22] Among organic acids derived from mainly lactic or citric, pyruvic acid showed the lowest level with a mean of 16.37 mg/100 g cheese. Pyruvic acid acts as a substitute in various metabolic pathways for the formic and oxalic acids.[Citation23] As reported by Serra et al.,[Citation24] the low content of pyruvic acid may be reflected to an increase in microbial counts, which causes the synthesis of the other organic acids at the higher levels.
Table 3 Organic acids (mg/100 g cheese) identified in Surk cheeses accordingly
Figure 1 Chromatogram of: (A), (B) standard mixture; (C) Surk cheese sample. Peaks: (1) oxalic acid; (2) orotic acid; (3) citric acid; (4) pyruvic acid; (5) uric acid; (6) lactic acid; (7) formic acid; (8) acetic acid; (9) propionic acid; (10) hippuric acid.
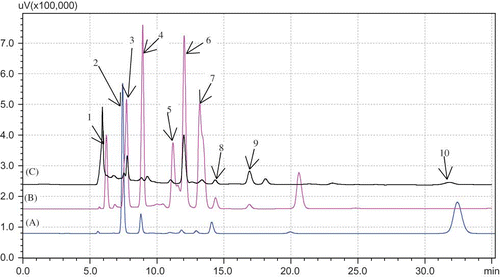
Citric acid is a product of body metabolism and an important organic acid. Its amount ranged from 14.89 to 106.09 mg/100 g. The similar results were obtained by Mullin and Emmons[Citation25] for various cheeses. In this study, acetic acid content was high in Surk cheeses with low citric acid. This finding confirmed that acetic acid could be produced from catabolism of citric acid. Orotic, uric, and hippuric acids are nonprotein nitrogenous compounds in milk. These acids were considerably lower in cheeses than the organic acids derived from mainly lactose or lactic acid. Orotic acid ranged from 1.42 to 18.55 with a mean of 7.93 mg/100 g. Orotic acid has been described as a growth factor for L. delbrueckiisubsp. bulgaricus and also as a precursor for the synthesis of nucleotides.[Citation13] Hippuric acid was not found in the four Surk samples. The maximum value of hippuric acid was 22.90 mg/100 g cheese. As Surk cheese is made from yogurt, the presence of hippuric acid is not a surprise since it was previously found in yogurt.[Citation26] Uric acid ranged from 1.14 to 3.15 with a mean of 2.31 mg/100 g cheese. The maximum value of uric acid found in Surk cheeses is close to the highest value of 3.3 mg/100 g reported in various cheeses by Bevilacqua and Califano.[Citation18] Uric acid was the lowest organic acid found in Surk cheeses.
Overall, low pyruvic, orotic, and hippuric acid levels are the results of increased consumption rates of orotic and hippuric acids. In addition, lactic, acetic, propionic, and formic acid levels could be due to the growth of both homofermantative and non starter lactic acid bacteria (NSLAB) in Surk cheeses, since this trend in organic acids is generally attributed to Lactobacillus spp. metabolism.[Citation27] Most spices and herbs could stimulate the growth of lactic acid bacteria present in Surk cheese. As expected, the Surk cheese with the highest total organic acid had the high titratable acidity and the low pH value.
Table 4 Volatile compounds identified in Surk cheeses according to chemical classes
Volatile Compounds
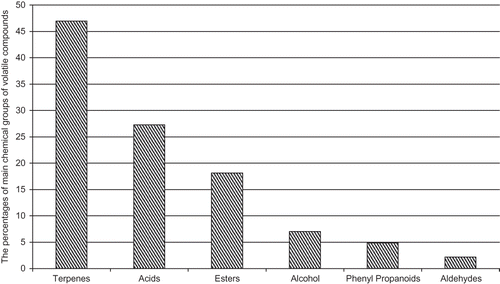
A GC-MS chromatogram sample for the volatile compound profiles of Surk cheese is shown in . A total of 134 volatile compounds were identified in Surk cheeses and were grouped according to chemical classes (). Terpenes were the most abundant compounds, accounting for 46.94% of total volatile compounds identified in all the Surk cheeses, followed by acids (27.25%), esters (18.2%), alcohols (7.23%), phenyl propanoids (4.85%), and aldehydes (2.17%) in decreasing order (). However, only 30 of 134 volatile compounds were found in all the Surk cheeses, which accounted for about 86.32% of total volatile identified (). Carvacrol (12.04%), γ-terpinene (11.02%), p-cymene (10.65%), hexanoic (7.29%), octanoic (6.57%), decanoic (5.54%), and butanoic (3.80%) acids were the most abundant volatile compounds identified in Surk cheeses (). Of volatile compounds identified in all of the Surk samples, ethyl hexanoate, methyl octanoate, ethyl octanoate, methyl benzoate, m-thymol, limonene, β-pinene, β-myrcene, γ-terpinene, p-cymene, butanoic, pentanoic, hexanoic, octanoic, and decanoic acids, 2-ethyl-1-hexanol, and benzenoethanol were previously determined in some cheese varieties, such as white cheese containing thyme, acid-type farm cheese.[Citation28,Citation29] As far as we know, 2-ethylhexyl (octyl) butanoate, methyl decanoate, ethyl decanoate, α-terpinene, β-cubebene, β-caryophyllene, carvacrol, p-cymen-7-ol, cuminic aldehyde, methyleugenol, and eugenol were identified for the first time in cheeses. These compounds could be related to milk or spices and herbs used for Surk cheese-making since carvacrol, γ-terpinene, m-tymol, p-cymene, cumin aldehyde, and β-pinene are important flavour constituents in thyme and caraway.[Citation30,Citation31] On the other hand, volatile compounds, such as linalool, eugenol/β-caryophyllene, cinnamic aldehyde/eugenol, carvacrol/thymol, eugenol, and β-caryophyllene, are the major flavour constituents in bacil, allspice, cinnamon, oregano, clove, and black pepper, respectively.[Citation32]
Table 5 The percentages of volatile compounds identified in all the Surk cheeses
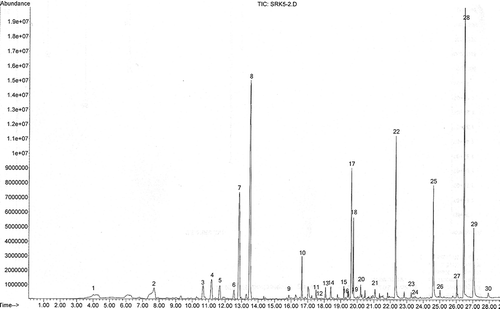
Figure 2 A typical chromatogram of one of the Surk cheese samples indicating volatile compounds identified in all of the Surk cheeses (see for Pik No.).
Free fatty acids were the second largest group of volatile compounds identified (). Butanoic, pentanoic, hexanoic, octanoic, and decanoic acids were identified in all of the samples, and accounted for about 25% of total volatile compounds. Hexanoic acid with a mean of 7.29% was the most abundant free fatty acid identified in Surk cheeses, which was followed by octanoic, decanoic, butanoic, and pentanoic acids in decreasing order. Similar results were reported by Delgado et al.[Citation33] for Torta del Casar cheese, produced by a plant coagulant without using starter. The high levels of short-chain free fatty acids are probably related to the high activity of esterase of Lactobacillus strains. This result is consistent to the high concentration of organic acids derived from Lactobacillus spp. metabolism.
The only 7 of 42 esters identified, including ethyl hexanoate, methyl octanoate, ethyl octanoate, 2-ethylhexyl butanoate, methyl decanoate, ethyl decanoate, and methyl benzoate, were found in all of the Surk samples (). Ethyl decanoate and ethyl octanoate were the most abundant esters identified in Surk samples. Esters formed by the esterification of free fatty acids and alcohols make a significant contribution to cheese flavour due to their low threshold values.[Citation34] However, esters, such as 2-ethylhexyl (octyl) butanoate and ethyl undecanoate, revealed the maximum level in cheese with a high score of overall acceptability, when compared with the other esters. To our knowledge, these esters have not been previously identified in cheeses, so they could provide unique and characteristic aromatic notes to Surk cheese. Ethly esters with fruity flavour notes may minimize the sharpness of rancidity derived from carboxylic acids.
Alcohols comprised the fourth group of volatile compounds, accounting for 7.03% of all the volatile compounds identified (). The only 3 of 15 alcohol compounds identified, including 2-ethyl hexen-1-ol (fatty alcohol), benzene-ethanol, and p-cymene-7-ol (cuminic alcohol), were found in all of the Surk samples (). These alcohols may play an important role in the characteristic flavour of Surk cheeses. Benzene-ethanol, a yeast metabolic product derived from phenyl alanine, is responsible for floral and rose flavour notes.[Citation34]
Considering phenyl propanoids, eugenol was the main propanoid identified in Surk cheeses. It revealed a wide variation among cheeses with a variation coefficient of 95%. As there is no standard method for the manufacturing of Surk cheeses, this situation could account for the use of pimento, cinnamon, and clove in different proportions by manufacturers since eugenol is the main volatile compound identified in those spices.[Citation32]
Cuminic aldehyde, the only one of five aldehyde compounds identified, was found in all of the Surk cheeses (). Cuminic aldehyde was the main aldehyde identified, whereas the other aldehydes were found in some samples at the trace levels. Ketones are common constituents of cheeses. However, in this study, they had the lowest relative proportion with a total value of 0.54%, compared to the other volatile compounds identified in Surk cheese. Ketone 2-nonanone only was identified in all the Surk cheeses at trace levels (). Ketones are mainly formed by decarboxylation of β-ketoacids occuring via β-oxidation of free fatty acids in cheeses.[Citation34] Spices and herbs used for Surk cheese-making could have inhibited oxidation of free fatty acids since volatile compounds derived from lipid oxidation, such as hexanal and propanal, were absent in the cheese samples.
In light of the other compounds, only diisopropyl ether was found in all of the Surk cheeses (). Dimethyl trisulfide, α-methyltoluene, and naphtalene were detected in some cheeses at the minimal levels. The sample with the low score of overall acceptability had the maximum level of diisopropyl ether (). Diisopropyl ether, α-methyltoluene, and naphtalene may be contamined to cheese by means of the external sources, such as pesticides, cleaning agents, pollution, and unclean herbs.
Consumer Preference
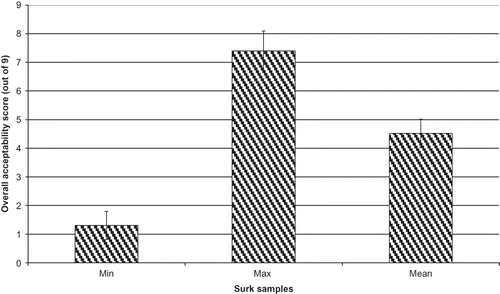
During the sensory analysis, none of the samples received the maximum score of 9 for overall acceptability. The maximum and minimum scores were 1.3 and 7.4 for overall acceptability, respectively (). In general, negative points in cheese samples with low overall acceptability scores were described as rancid, too pungent, too sour, and unidentified bitter taste by panelists. Surk cheese with the highest pentanoic, hexanoic, octanoic, and decanoic acids, and 2-ethyl hexyl butanoate (2.93%) and diisopropyl ether (2.58%), and with the lowest butanoic acid (1.28%), pyruvic (14.76 mg/100 g), and γ-terpinene (4.47%) levels received the lowest overall acceptability scores. Interestingly, the percentages of hexanoic, pentanoic, octanoic, and decanoic acid in the sample with the highest overall acceptability score was similar to each other and the ratio of each of those acids to butanoic acid was about 2.5. On the other hand, acetic acid content (80.75 mg/100 g) and the percentage of benezethanol (3.90%) in samples with a high score were similar to those of propionic acid and 2-ethyl-1-hexenol, respectively. In terms of the other volatile compounds, the sample with the highest overall acceptability score had the highest p-cymene (20%), limonene (3.36%), ethyl undecanoate (3.76%), eugenol (3.84%), and benzenethanol (3.93%). It is worth noting that the presence of volatile compounds and organic acids in the correct ratio are equally essential to produce a good flavour for Surk cheeses.
CONCLUSIONS
As compounds 2-ethylhexyl (octyl) butanoate, methyl decanoate, ethyl decanoate, α-terpinene, β-cubebene, β-caryophyllene, carvacrol, p-cymen-7-ol, cuminic aldehyde, methyleugenol, and eugenol were identified for the first time in cheeses, they could be characteristic for Surk cheese. The desirable flavour of Surk cheese appears to be closely related to the ratio of hexanoic, pentanoic, octanoic, and decanoic acids to butanoic acid, the balance between propionic and acetic acid, the high concentrations of p-cymene, limonene, ethyl undecanoate, eugenol, and benzenethanol. Furthermore, a long-term goal of our research is to determine the correlations between individual aroma compound and flavour attributes, and the free amino acid contents in Surk cheese produced under the same conditions.
ACKNOWLEDGMENTS
The author is thankful to Bahaaddin Yıldız, Hüseyin Noyan, Onur Coşkun, and Yunus Emre Şekerli for their assistance in conducting the experiments at the Food Engineering Department, Mustafa Kemal University, Hatay, Turkey. The author is also grateful to Professor Dr. Park. W. Young, Fort Walley State University, Fort Valley-George, for his proof reading.
Notes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ljfp.
REFERENCES
- Fox , P.F. , Guinee , T.P. , Cogan , T.M. and McSweeney , P.L.H. 2000 . Fundamentals of Cheese Science , 587 Gaithersburg , MD : Apsen Publishers Inc .
- Hayaloglu , A.A. and Farkye , N.Y. 2011 . “ Cheese/cheese with added herbs, spices condiments. In ” . In Encyclopedia of Dairy Sciences , Second Ed. , Edited by: Fuquay , J.W. , Fox , P.F. and McSweeney , P.L.H. Vol. 1; , 783 – 789 . San Diego : Academic Press . Vol.Eds.
- Ahmed , A.K. and Johnson , K.A. 2000 . Horticultural development of Australian native edible plants . Australian Journal of Botany , 48 : 417 – 426 .
- Ceylan , E. and Fung , D.Y.C. 2004 . Antimicrobial activity of spices . Journal of Rapid Methods and Automation in Microbiology , 12 : 1 – 55 .
- Shan , B. , Cai , Y.Z. , Brooks , J.D. and Corke , H. 2011 . Potential application of spice and herb extracts as natural preservatives in cheese . Journal Medical Food , 14 : 284 – 290 .
- Guler-Akin , M.B. and Konar , A. 2002 . Antakya piyasasinda satılan Surklerin bazı özellikleri . Harran Universitesi Ziraat Fakultesi Dergisi , 6 : 55 – 63 .
- Aygun , O. , Yaman , M. and Durmaz , H. 2007 . A survey on occurrence of Tyrophagus putrescentiae (Acari: Acaridae) in Surk, a traditional Turkish dairy product . Journal of Food Engineering , 78 : 878 – 881 .
- Aygun , O. , Essiz , D. , Durmaz , H. , Yarsan , E. and Aflatoxin , Altintas, L. 2009 . M1 levels in Surk samples, a traditional Turkish cheese from southern Turkey . Bulletin Environment Contaminant Toxicologly , 83 : 164 – 167 .
- AOAC . 2003 . Official Methods of Analysis. AOAC International: Washington , DC
- IDF . 1962 . Determination of the protein content of milk , Brussels , Belgium : IDF Standard 28 A. International Dairy Federation .
- TSI . 1995 . Standard of White Cheese. TS 591 , Ankara , Turkey : Türk Standartları Enstitüsü .
- TSI . 2001 . Cheese and processed cheese products—Determination of chloride content-potentiometric titration method. TS 4708 , Ankara , Turkey : Turkish Standard Institution .
- Fernandez-García , E. and McGregor , J.U. 1994 . Determination of organic acids during the fermentationand cold storage of yogurt . Journal of Dairy Science , 77 : 2934 – 2939 .
- Ziino , M. , Condurso , C. , Romeo , V. , Giuffrida , D. and Verzera , A. 2005 . Characterization of “Provola dei Nebrodi,” a typical Sicilian cheese, by volatiles analysis using SPME-GC/MS . International Dairy Journal , 15 : 585 – 593 .
- Hayaloglu , A.A. and Fox , P.F. 2008 . Cheeses of Turkey: 3 . Varieties containing herbs or spices. Dairy Science and Technology , 88 : 245 – 256 .
- Izco , J.M. , Tormo , M. and Jiménez-Flores , R. 2002 . Rapid simultaneous determination of organic acids, free amino acids, and lactose in cheese by capillary electrophoresis . Journal Dairy Science , 85 : 2122 – 2129 .
- Rodríguez-Alonso , P. , Centeno , J.A. and Garabal , J.I. 2011 . Biochemical study of industrially produced Arzúa-Ulloa semi-soft cows’ milk cheese: Effects of storage under vacuum and modified atmospheres with high-nitrogen contents . International Dairy Journal , 21 : 261 – 271 .
- Bevilacqua , A.E. and Califano , A.N. 1989 . Determination of organic acids in dairy products by high performance liquid chromatography . Journal of Food Science , 54 : 1076 – 1079 .
- Davies , D.D. and Asker , H. 1983 . Synthesis of oxalic acid by enzymes from lettıce leaves . Plant Physiology , 72 : 134 – 138 .
- Lianzhong , D. , Shiyue , D. , Yan , Z. , Yixu , L. and Songmei , Z. 1998 . A study on chemical composition of spices irradiated by electron beam . Radiatition Physical Chemistry , 52 : 49 – 52 .
- Franceschi , V.R. and Nakata , P.A. 2005 . Calcium oxalate in plants: Formation and function . Annual Review of Plant Biology , 56 : 41 – 71 .
- Skeie , S. , Lindberg , C. and Narvhus , J. 2001 . Development of amino acids and organic acids in Norvegia, influence of milk treatment and adjunct Lactobacillus . International Dairy Journal , 11 : 399 – 411 .
- McSweeney , P.L.H. and Sousa , M.J. 2000 . Biochemical pathways for the production of flavour compounds in cheese during ripening: A review . Lait , 80 : 293 – 324 .
- Serra , M. , Trujillo , A.J. , Guamis , B. and Ferragut , V. 2009 . Flavour profiles and survival of starter cultures of yoghurt produced from high-pressure homogenized milk . International Dairy Journal , 19 : 100 – 106 .
- Mullin , W. and Emmons , D.B. 1997 . Determination of organic acids and sugars in cheese, milk and whey by high performance liquid chromatography . Food Research International , 30 : 147 – 151 .
- Fernandez-García , E. and McGregor , J.U. 1997 . Fortification of sweetened plain yogurt with insoluble dieatary fiber . Zeitschrift für Lebensmittel-Untersuchung und Forschung , 204 : 433 – 437 .
- Menéndez , S. , Centeno , J.A. , Godínez , R. and Rodríquez-Otero , J.L. 2000 . Effects of Lactobacillus strains on the ripening and organoleptic characteristics of Arzúa-Ulloa cheese . International Journal of Food Microbiology , 59 : 37 – 46 .
- Foda , M.I. , Seleet , F.L. and El-Ghorab , A.H. 2008 . Sensory evaluation and related components of White herby cheese . International Journal of Dairy Science , 3 : 160 – 169 .
- Giuseppe , Z. , Manuela , G. , Marta , B. and Vincenzo , G. 2005 . Application of artificial neural network on mono- and sesquiterpenes compounds determined by headspace solid-phase microextraction–gas chromatography–mass spectrometry for the Piedmont ricotta cheese traceability . Journal of Chromatography A , 1071 : 247 – 253 .
- Kurkcuoglu , M. , Tumen , G. and Baser , K.H.C. 2001 . Essential oil constituents of Satureja boissieri from Turkey . Chemistry of Natural Compounds , 37 : 329 – 331 .
- Richter , J. and Schellenberg , I. 2007 . Comparison of different extraction methods for the determination of essential oils and related compounds from aromatic plants and optimization of solid-phase microextraction/gas chromatography . Analytical Bioanalytical Chemistry , 387 : 2207 – 2217 .
- Tajkarimi , M.M. , Ibrahim , S.A. and Cliver , D.O. 2010 . Antimicrobial herb and spice compounds in food . Food Control , 21 : 1199 – 1288 .
- Delgado , F.J. , González-Crespo , J. , Cava , R. , García-Parra , R. and Ramírez , R. 2010 . Characterisation by SPME-GC-MS of the volatile profile of a spanish soft cheese PDO torta del Casar during ripening . Food Chemistry , 118 : 182 – 189 .
- Molimard , P. and Spinnler , H.E. 1996 . Compound involved in the flavor of surface mold-ripened cheeses: Origins and properties . Journal of Dairy Science , 79 : 169 – 184 .