Abstract
In this study, phenolic compounds of Rhododendron honey (also known as mad honey) samples collected from the Black Sea Region were identified using high performance liquid chromatography-diode array detector system. The major phenolic substances in Rhododendron honeys were found to be chlorogenic and coumaric acids with the amounts of 0.11–191.54 mg/kg and 0–82.83 mg/kg, respectively. Gallic and ferulic acids were detected in the most honey samples. Additionally, significant correlations were determined between the phenolic substances. The present study showed that Rhododendron honeys contained higher quantities of phenolic acids than flavonoids. Chlorogenic and coumaric acids were the dominant phenolic substances detected in honey samples.
INTRODUCTION
Honey is a natural food product produced by honey bees (Apis mellifera), using nectar collected from many plants. The composition of honey varies with differences in floral sources, climate, and environmental conditions, as well as contribution of a beekeeper.[Citation1,Citation2] Honey is a highly concentrated solution of a complex mixture of sugars; namely, fructose (38%) and glucose (31%); honey also contains other constituents such as minerals, proteins, vitamins, organic acids, flavonoids, phenolic acids, enzymes, and other phytochemicals in small quantities.[Citation3] White[Citation4] reported that honey is a remarkably complex natural liquid which contains at least 181 substances. Many authors reported that honey is rich in natural antioxidant compounds which are effective in reduction of the heart disease risk, cancer, immune-system decline, cataracts, and different inflammatory problems.[Citation3,Citation5] The main sources of antioxidant compounds are medicinal and aromatic herbs, as well as fruits and leaves of some plants which biosynthesize phytochemicals possessing antioxidant activity.[Citation6] Bees collect nectar from many plants which are rich in antioxidative compounds and consequently transfer bioactive components from original plant to the honey.[Citation7] For that reason, the composition and antioxidant activity of honey depend on the floral source, and also seasonal and climatic conditions.[Citation8,Citation9] The major compounds which are responsible for the antioxidant activity of the honey are mainly flavonoids, phenolic acids, carotenoids, and some enzymes.[Citation10,Citation11] It was also reported that honey is similar in terms of antioxidant capacity to many fruits and vegetables on a fresh weight basis.[Citation12] Polyphenols or phenolic compounds are the products of secondary metabolism of plants. They can be categorized into two important classes as flavonoids and phenolic acids. Evaluation of phenolic profile of different kind of honeys has been a very important item in recent years because the antioxidant power of honey is related to its phenolic profile. Additionally, phenolic compounds are suitable markers for detection of botanical origin of the honeys.[Citation13] For instance, hesperidin and homogentisic acid were proposed as a marker for Citrus and Arbutus honeys, respectively.[Citation14,Citation15] It was also reported that flavones, flavanones, isoflavones, and other polyphenols were used for the classification of honeys.[Citation14] A considerable number of studies have recently been conducted on determination of the flavonoid and phenolic profiles, dealing with identification of the specific compounds having a potential to be utilized as a floral marker for honeys.[Citation16,Citation17]
Rhododendron (Rhododendron spp.) honey, which is locally called “mad honey” or “toxic honey”, has been known since ancient times and widely produced in Black Sea Region in Turkey. The honey is used as a tool of alternative medicine for the treatment of several diseases and it is marketed with relatively high cost in Black Sea Region. Küçük et al.[Citation1] reported that the symptoms of poisoning due to the consumption of Rhododendron honey in large amounts are severe vertigo, arterial hypotension, and bradycardia. The honey contains andromedotoxin, being responsible for the poisoning cases in humans.[Citation18]
To the best knowledge of the authors, in literature no study has appeared so far on determination of phenolic profile of the Rhododendron honeys. Even if the study of Silici et al.[Citation19] has appeared to be the only investigation where the total phenolic content, antiradical, antioxidant, and antimicrobial activities of the Rhododendron honeys were determined; the phenolic profile of Rhododendron honeys collected from different regions in Turkey was not identified in this study. Therefore, in the present study, 12 Rhododendron honeys were collected from different cities in Black Sea Region and their phenolic profiles were identified using high pressure liquid chromatography-diode array detector (HPLC-DAD) system.
MATERIALS AND METHODS
Materials
Honey samples were collected from different locations (Artvin, Trabzon, Ordu, Hopa, and Zonguldak) in the Black Sea Region of Turkey. Gallic acid, chlorogenic acid, caffeic acid, coumaric acid, catechin, epicatechin, ferulic acid, quercetin, pinocembrin, myricerin, luteolin, kaempferol, chrysin, vanilic acid, benzoic acid, cinnamic acid, isohamneti, pinostrobin, ellagic acid, and syringic acid were purchased from Sigma (St. Louis, MO, USA).
Methods
Pollen analysis
Twelve honey samples were identified according to their pollen frequencies using the methods of Louveaux et al.[Citation20] and Von der Ohe et al.[Citation21] Pollen grains were observed and counted using a microscope and compared with those in the reference slides for classification. Botanical classification was conducted for the honeys whose dominant pollen spectrum were higher than 45%.
HPLC-DAD analysis for the profile of the phenolic compounds in Rhododendron honeys
Rhododendron honey samples were prepared and extracted with ethyl acetate according to the procedure reported by Aljadi and Yusoff.[Citation22] Solid phase extraction technique was used for the recovery of phenolics from honey samples with some modification of the proposed method by Dimitrova et al.[Citation23] Dry honey extracts were dissolved in acidified deionised water (pH 3.5), and after that, the dissolved phenolics were adsorbed onto preconditioned isolute C18 column. 5 ml each of methanol and acidified water (pH 3.5) were passed at a drop wise flow rate to precondition the cartridge and then, phenolic extracts were passed through the cartridge to obtain effective adsorption of the phenolic compounds. At the end of the process, the adsorbed phenolic compounds were eluted from the cartridges by passing 3–5 ml of methanol-water solution (50%, v/v) at a drop wise flow rate. HPLC-DAD system (HPLC-DAD, Agilent 1100 Series, Manual injection quaternary pump, thermostatted column compartment, Diode Array and Multiple Wavelength Detectors, USA) equipped with a C18 column (4.6 × 250 mm, 5 μm particle size) thermostated at 35°C was used for the identification of phenolics. For the separation of extracted phenolic compounds, a gradient elution technique proposed by Weston et al.[Citation24] was used with some modifications. Solvent (A) was methanol and solvent (B) was 5% formic acid prepared with distilled water. Solvent flow rate was set to be 1.0 mL/min. The gradient profile of the system was 15% solvent A and 85% solvent B at the initial stage; 20% solvent A and 80% solvent B at 10 min; 40% solvent A and 60% solvent B at 30 min; 70% solvent A and 30% solvent B at 50 min; and 15% solvent A and 85% solvent B at 60 min. The chromatograms were obtained at 280 nm because most of the phenolic compounds show reasonably high absorbance at this wavelength value.[Citation25] The phenolic substances were identified by comparing the chromatographic retention times with those of standard phenolics.
Table 1 Geographical and botanical origin of Rhododendron honeys.[Citation19]
RESULTS AND DISCUSSION
shows the geographical and botanical origin of Rhododendron honeys collected from different areas of the Black Sea region in Turkey. The pollen content of honeys was found in the range of 17.64–65.62%. Silici et al.[Citation19] reported that a honey is considered mainly from a plant (monofloral) if the pollen frequency of this plant is higher than 45% in the honey. The low pollen frequency is due to the short flowering period and large pollen grains of Rhododendron plants. In the present study, the pollen frequency of two samples obtained from different areas was found to be lower than 45%. Persano Oddo et al.[Citation26] reported that Rhododendron honeys are characterized with slow granulation and variable size crystals for physical states. Generally, the color of Rhododendron honeys is water white when it is liquid and white to light when it is crystallized and they have a delicate floral odor and aroma. Color of honeys is important because a strong correlation was found between antioxidant activity and color of honey. Honeys with dark color have a higher total phenolic content compared to those which have lighter color.[Citation27] The smell and flavor of the Rhododendron honeys are very weak, herbaceous, and fruity.
Twenty phenolic markers were used for chromatographic comparison of data, but only 9 of the 20 phenolics were detected in Rhododendron honeys. Five of these phenolics were characterized to be phenolic acids and the rest of the compounds were flavonoids. The phenolic profiles detected in the honey samples collected from different regions were quite different. HPLC chromatograms for standards and samples are illustrated in . shows the standard solution results and and 1c present the phenolic profile of Artvin1 and Trabzon1 honey samples which are rich in phenolic compounds.
Figure 1 HPLC-DAD chromatogram of standards (a) and Rhododendron honeys collected from Artvin1 (b) and Trabzon3 (c) cities in the Black Sea Region in Turkey. The peaks correspond to the following: (1) gallic acid; (2) catechin; (3) chlorogenic acid; (4) caffeic acid; (5) epicatechin; (6) coumaric acid; (7) ferulic acid.
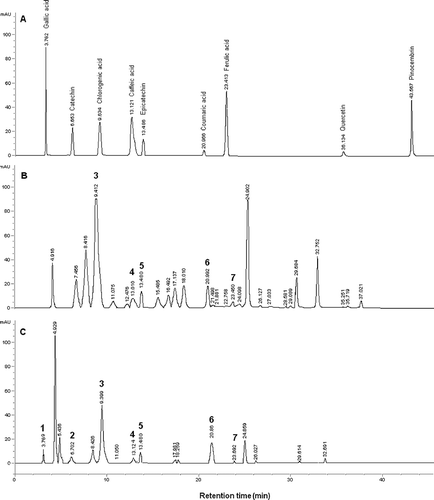
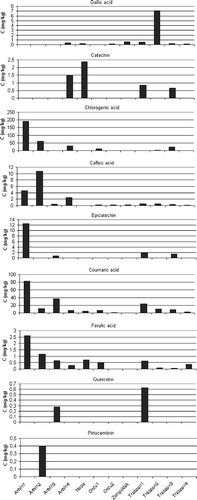
The results showed that the most abundant phenolic compound was the chlorogenic acid in the Rhododendron honeys collected from Artvin and it was found to be 71.31 mg/kg as mean of all samples from Artvin region. Significant differences in phenolic composition of Rhododendron honeys collected from different areas were also observed. The second major phenolic compound was coumaric acid with the amount of 34.42 mg/kg in honeys collected from Artvin. On the contrary, coumaric acid was the most abundant (11.30 mg/kg) in honeys collected from Trabzon while the chlorogenic acid was the second (6.41 mg/kg). Coumaric acid was determined in honey samples collected from all regions except Zonguldak and the maximum quantity was found to be 82.82 mg/kg in honey samples obtained from Artvin1. Socha et al.[Citation28] reported that p-coumaric acid was identified in the highest quantity for honeys and the amount of coumaric acid influenced the antioxidant activity and total phenolic content of honeys. A linear correlation (r = 0.990) was determined between coumaric acid and total phenolic levels of the honey samples. Chlorogenic acid was detected in only three honey samples. And also, it was reported that chlorogenic acid was identified in Australian and New Zealand honeys.[Citation29] Rhododendron honeys collected from Zonguldak were poor with regards to phenolic profile and only gallic acid, chlorogenic acid, and caffeic acid were detected in these samples. Chlorogenic acid and caffeic acid were detected in all 12 samples and pinocembrin was detected only in one sample (Artvin2). Tomás-Barberán et al.[Citation16] reported that the levels of caffeic acid were found in European honeys with the range of 20–160 μg/100 g, which was the highest amount being found for sunflower honeys. Generally, gallic acid was not detected or detected in little quantity in honey samples collected from Artvin regions but the highest quantity of gallic acid was determined to be 7.13 mg/kg in Trabzon2 sample (). Gallic acid was determined in natural honeys and it was reported that the level of gallic acid was found to be 82–330 μg/100 g in Malaysian honeys.[Citation22] Gallic acid was determined to be dominant phenolic acid in some Australian and New Zealand honeys. It was established that gallic acid was the main constituent of the phenolic profile and a potential marker of the origin of a honey.[Citation29] Socha et al.[Citation28] found a significant linear correlation between gallic acid and total phenolic contents. The other commonly detected phenolic acid was ferulic acid and identified in all the honey samples except for those coded as Ordu2 and Zonguldak. Socha et al.[Citation28] reported that the ferulic acid was determined in the range of 13.2–178.6 μg/100 g in honeys. Quercetin was found in small quantities only in two Rhododendron honey samples collected from Artvin and Trabzon (0.28 and 0.63 mg/kg, respectively). Petrus et al.[Citation30] reported that the major phenolics of Rhododendron honeys collected from Italy were galangin and kaempferol. Quercetin was found to be lower than 0.3 mg/kg, which was similar to the results in this study.
Figure 2 Phenolic compound profile of Rhododendron honeys collected from different cities in the Black Sea Region in Turkey (C: concentration).
shows the mean share (%) of individual phenolic compounds in total phenolics of Rhododendron honeys. As stated before, chlorogenic and coumaric acid were the most abundant phenolic compounds in the Rhododendron honeys collected from Artvin and Trabzon and their ratio was determined to be 61.7 and 52.0% as mean share of detected total phenolics, respectively. Coumaric acid was found to be 29.8% as mean share of detected total phenolics in Artvin honeys, and chlorogenic acid was found to be 29.5% as mean share of detected total phenolics in Trabzon honeys. Quercetin and pinocembrin were detected to be lower than 1% in Rhododendron honeys collected from Artvin and Trabzon. It can be said that the studied Rhododendron honey samples were rich in terms of phenolic acids, but the quantity of the flavonoids was quite low. Kenjeríc et al.[Citation31] investigated the flavonoid profile of Robinia honeys produced in Croatia and reported that quercetin was one of the flavonoid substances, which was found in relatively low quantity. Yao et al.[Citation32] found that the characteristic phenolic compounds of Australian Eucalyptus honey were gallic, chlorogenic, ellagic, and coumaric acid. In another study, Yao et al.[Citation29] investigated the phenolic acids in Leptospermum honeys and reported that gallic and coumaric acids were the main phenolic acids of Australian jelly bush honey. These results were comparable with those reported in this study in terms of the phenolic profiles of Turkish Rhododendron honey. Olthof et al.[Citation33] stated that chlorogenic and caffeic acids had in vitro antioxidant activity, and inhibitors of the N-nitrosation reaction and could inhibit DNA damage.
Figure 3 Mean share (%) of individual phenolic compounds in total phenolics of Rhododendron honeys collected from Artvin and Trabzon cities in the Black Sea Region.
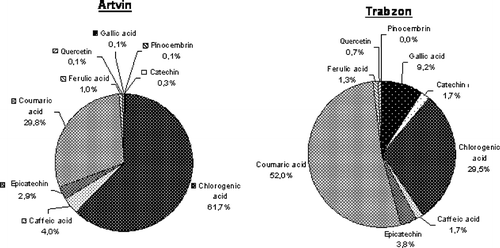
Truchado et al.[Citation34] investigated that the flavonoid glycosides of 27 different honey samples and reported that the pinobanksin was the most abundant flavonoid in one of the Rhododendron honey samples obtained from Italy. But in this study, pinobanksin was not detected. Silici et al.[Citation19] reported that the phenolic content of honey was strongly affected by the botanical and geographical origin as well as climatic characteristic of the region. Pinocembrin was detected in only one honey sample coded as Artvin 2. Pinocembrin is generally a well known main flavonoid present in propolis, having antioxidant activity.[Citation12]
In order to determine the relationships between the phenolic compounds in the honey samples, the Pearson's correlation test analysis was performed. shows the correlation coefficients of the phenolic substances. Positive correlations were determined between the contents of phenolic acid and flavonoid compounds. For example, a significant positive correlation was observed between epicatechin and chlorogenic acid (r = 0.918) and between coumaric (r = 0.928) and ferulic acids (r = 0.865). A positive correlation between pinocembrin and caffeic acid was also determined (r = 0.906). As can be seen in , all significant correlations (p < 0.05) were found to be positive. Similarly, Kim et al.[Citation35] indicated that there were significant correlations between the quantities of hulled rice flavonoids. Also, Javanmardi et al.[Citation6] reported that there were significant correlations between the quantities of certain phenolic acids in leaves and flowers of Basil (Ocimum basilicum). Especially, positive correlations were observed between rosmarinic and p-coumaric acids and between vanillic and ferulic acids.
Table 2 Correlation coefficients between the phenolic compounds of Rhododendron honeys
CONCLUSION
The present study revealed that Rhododendron honeys collected from the Black Sea Region of Turkey showed a great variation in phenolic acid composition as compared to that in flavonoid composition. Pollen analysis could be used effectively to identify the origin of the honey samples collected. Chlorogenic and coumaric acids were found to be the dominant phenolic substances in the honey samples. Furthermore, Pearson's correlation analysis indicated that there was a significant positive correlation between the quantities of phenolic acids. However, these correlations should be investigated in more detail for other honey types that were not considered in this study.
REFERENCES
- Küçük , M. , Kolaylı , S. , Karaoğlu , Ş. , Ulusoy , E. , Baltacı , C. and Candan , F. 2007 . Biological activities and chemical composition of three honeys of different types from Anatolia . Food Chemistry , 100 : 526 – 534 .
- Anklam , E. 1998 . A review of the analytical methods to determine the geographical and botanical origin of honey . Food Chemistry , 63 : 549 – 562 .
- Bertoncelj , J. , Dobersék , U. , Jamnik , M. and Golob , T. 2007 . Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey . Food Chemistry , 105 : 822 – 828 .
- White , J.W. 1975 . “ Composition of honey ” . In Honey, A Comprehensive Survey , Edited by: Crane , E. 157 – 206 . New York : Crane, Russak and Company .
- Viuda-Martos , M. , Ruiz-Navajas , Y. , Fernández-López , J. and Pérez-Álvarez , J.A. 2008 . Functional properties of honey, propolis and royal jelly . Journal of Food Science , 73 : 117 – 124 .
- Javanmardi , J. , Khalighi , A. , Kashi , A. , Bais , H.P. and Vivanco , J.M. 2002 . Chemical characterization of basil ( Ocimum basillicum L.) found in local accessions and used in traditional medicines in Iran . Journal of Agriculture and Food Chemistry , 50 : 5878 – 5883 .
- Baltrusaityte , V. , Venskutonis , P.R. and Ceksteryte , V. 2007 . Radical scaenging activity of different floral origin honey and beebread phenolic extracts . Food Chemistry , 101 : 502 – 514 .
- Al-Mamary , M. , Al-Meeri , A. and Al-Habori , M. 2002 . Antioxidant activities and total phenolics of different types of honey . Nutrition Research , 22 : 1041 – 1047 .
- Gheldof , N. , Wang , X.H. and Engeseth , N.H. 2002 . Identification and quantification of antioxidant components of honeys from various floral sources . Journal of Agriculture and Food Chemistry , 50 : 5870 – 5877 .
- Idris , Y.M.A. , Mariod , A.A. and Hamad , S.I. 2011 . Physicochemical properties, phenolic contents and antioxidant activity of Sudanese honey . International Journal of Food Properties , 14 : 450 – 458 .
- Herken , E.N. , Erel , O. , Güzel , S. , Çelik , H. and Ibanoglu , S. 2009 . Total antioxidant, phenolic compounds, and total oxidant status of certified and uncertified Turkey's honeys . International Journal of Food Properties , 12 : 461 – 468 .
- Gheldof , N. and Engeseth , N.J. 2002 . Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples . Journal of Agriculture and Food Chemistry , 8 : 3050 – 3055 .
- Pulcini , P. , Allegrini , F. and Fast , Festuccia, N. 2006 . SPE extraction and LC-ESI-MS-MS analysis of flavonoids and phenolic acids in honey . Apiacta , 41 : 21 – 27 .
- Ferreres , F. , Garcia-Viguera , C. , Tomas-Lorente , F. and Tomas-Barberan , F.A. 1993 . Hesperetin, a marker of the floral origin of citrus honey . Journal of Science Food and Agriculture , 61 : 121 – 123 .
- Cabras , P. , Angioni , A. , Tuberoso , C. , Floris , I. , Reniero , F. , Guillou , C. and Ghelli , S. 1999 . Homogentisic acid : A phenolic acid as marker of strawberry-tree (Arbutus unedo) honey . Journal of Agriculture and Food Chemistry , 47 : 4064 – 4070 .
- Tomás-Barberán , F.A. , Martos , I. , Ferreres , F. , Radovic , B.S. and Anklam , E. 2001 . HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys . Journal of the Science of Food and Agriculture , 81 : 485 – 496 .
- Martos , I. , Ferreres , F. and Tomás-Barberán , F.A. 2000 . Identification of flavonoid markers for the botanical origin of eucalyptus honey . Journal of Agriculture and Food Chemistry , 48 : 1498 – 1502 .
- Sutlupinar , N. , Mat , A. and Satganoglu , Y. 1993 . Poisoning by toxic honey in Turkey . Archives of Toxicology , 67 : 148 – 150 .
- Silici , S. , Sagdic , O. and Ekici , L. 2010 . Total phenolic content, antiradical, antioxidant, and antimicrobial activities of Rhododendron honeys . Food Chemistry , 121 : 238 – 243 .
- Louveaux , J. , Maurizio , A. and Vorwohl , G. 1978 . International commission for bee botany of IUBS . Methods of melissopalynology. Bee World , 59 : 139 – 157 .
- Von der Ohe , W. , Persano-Oddo , L. , Piana , M.L. , Morlot , M. and Martin , P. 2004 . Harmonised methods of melissopalynology . Apidologie , 35 : 18 – 25 .
- Aljadi , A.M. and Yusoff , K.M. 2003 . Isolation and identification of phenolic acids in Malaysian honey with antibacterial properties . Turkish Journal of Medicinal Sciences , 33 : 229 – 236 .
- Dimitrova , B. , Gevrenova , R. and Anklam , E. 2007 . Analysis of phenolic acids in honeys of different floral origin by solid-phase extraction and high performance liquid chromatography . Phytochemical Analysis , 18 : 24 – 32 .
- Weston , R.J. , Brocklebank , L.K. and Lu , Y. 2000 . Identification and quantitative levels of antibacterial components of some New Zealand honeys . Food Chemistry , 70 : 427 – 435 .
- Seo , A. and Morr , V. 1984 . Improved high performance liquid chromatographic analysis of phenolic acids and isoflavonoids from soybean protein products . Journal of Agriculture and Food Chemistry , 32 : 530 – 533 .
- Persano Oddo , L. , Piazzai , M.G. , Sabatini , A.G. and Accorti , M. 1995 . Characterization of unifloral honeys . Apidologie , 26 : 453 – 465 .
- Beretta , G. , Granata , P. , Ferrero , M. , Orioli , M. and Facino , R.M. 2005 . Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics . Analytica Chimica Acta , 533 : 185 – 190 .
- Socha , R. , Juszczak , L. , Pietrzyk , S. and Fortuna , T. 2009 . Antioxidant activity and phenolic composition of herb honeys . Food Chemistry , 113 : 568 – 574 .
- Yao , L. , Datta , N. , Tomás-Barberán , F.A. , Ferreres , F. , Martos , I. and Flavonoids , Singanusong, R. 2003 . phenolic acids, and abscisic acid in Australian and New Zealand Leptospermum honeys . Food Chemistry , 81 : 159 – 168 .
- Petrus , K. , Schwartz , H. and Sontag , G. 2011 . Analysis of flavonoids in honey by HPLC coupled with colorometric electrode array detection and electrospray ionization mass spectrometry . Analytical and Bioanalytical Chemistry , 400 : 2555 – 2563 .
- Kenjeríc , D. , Mandíc , M.L. , Primorac , L. , Bubalo , D. and Perl , A. 2007 . Flavonoid profile of Robinia honeys produced in Croatia . Food Chemistry , 102 : 683 – 690 .
- Yao , L. , Jiang , Y. , Singanusong , R. , Datta , N. and Raymont , K. 2004 . Phenolic acids and abscisic acid in Australian Eucalyptus honeys and their potential for floral authentication . Food Chemistry , 86 : 169 – 177 .
- Olthof , M.R. , Hollman , P.C.H. and Katan , M.B. 2001 . Chlorogenic acid and caffeic acid are absorbed in humans . Journal of Nutrition , 131 : 66 – 71 .
- Truchado , P. , Ferreres , F. and Tomas , F.A. 2009 . Liquid chromatography–tandem mass spectrometry reveals the widespread occurrence of flavonoid glycosides in honey, and their potential as floral origin markers . Journal of Chromatography A , 1216 : 7241 – 7248 .
- Kim , J.K. , Lee , S.Y. , Chu , S.M. , Lim , S.H. , Suh , S.C. , Lee , Y.T. , Cho , H.S. and Ha . 2010 . S.H. Variation and correlation analysis of flavonoids and caretenoids in Korean pigmented rice (Oryza sativa L.) cultivars . Journal of Agriculture and Food Chemistry , 58 : 12804 – 12809 .
