Abstract
The objective of this research was to conduct an experimental and theoretical investigation into the antioxidant properties of thymol and 2,5,6-trifluorothymol. Calculations based on the density functional theory were performed using the B3LYP exchange-correlation functional and the 6-311G(d,p) standard basis set to determine the carbon-fluorine bond dissociation enthalpy of 2,5,6-trifluorothymol and thymol in the gas phase and in solution in water and in methanol. Computed delta binding dissociation energies and delta ionization potential values of theoretical 2,5,6-trifluorothymol, test of total phenolics, and 1,1-diphenyl-2-picrylhydrazyl assay allowed the authors to conclude that both thymol and 2,5,6-trifluorothymol have a protective antioxidant action.
INTRODUCTION
Thymol (2-isopropyl-5-methylphenol), a monoterpene phenol naturally occurring in the essential oil of the popular culinary herb thyme, is among the phenolic compounds identified as effective agents for inhibition of oxidation of materials.[Citation1,Citation2] The antioxidant behavior of phenolic compounds is due to existing hydroxyl groups in their structures that act to remove free radicals from the considered environment.[Citation3] The phenolic antioxidant, which has at least one hydroxyl group, is designated by the ArOH symbol, highlighting the importance of the hydroxyl group. Formation of ArO· free radicals in ArOH-related antioxidant compounds could prevent the chain reactions of the products of the oxidation processes in biological systems.[Citation4] Due to the importance of this process, several works have focused on determining the procedures for inhibition of free radical distribution by ArOH-related antioxidants.[Citation5,Citation6] This study investigated the properties of the ArOH-related compound thymol, which can be found naturally in thyme oil and horsemint, as an antioxidant agent. Additionally, we investigated the properties of 2,5,6-trifluorothymol as a derivative of thymol to detect the effects of fluorination on the antioxidation process. The molecular structure of thymol is similar to 2,5,6-trifluorothymol, but lacks the three F groups in the ortho and para positions ().
The relationship between free radicals and disease has been the subject of much investigation. A number of biochemical reactions in the human body produce reactive oxygen species, which, unless they are scavenged by cellular components, can lead to disease.[Citation7] Recent research has verified that a diet of foods rich in antioxidants can help prevent cancer and cardiovascular disease, neurodegenerative disease (e.g., Parkinson's and Alzheimer's diseases), inflammation, and problems caused by aging of the cells and skin.[Citation8– Citation10]
In one previous study, we examined the microbial transformation of onopordopicrin by Aspergillus niger, and four compounds were obtained. Their structures were identified on the basis of chemical and spectroscopic data. All four compounds were novel.[Citation11] In other studies, microbial transformation of citral, geraniol, nerol, menthol, and myrcene by various fungi, including A. niger, Pseudomonas sp., and Penicillium sp., produced a number of compounds, such as 1,8-cineole, 2,6-dimethyloctane, α-pinene, α-terpineol, cis-p-menthan-7-ol, dihydrolinalool, γ-terpinene, linalool, limonene, p-cymene, p-menth-1-ene, sabinene, and trans-p-menthan-1-ol.[Citation12– Citation17] In the current work, we investigated the mechanism of formation of the ArO· radical for thymol and 2,5,6-trifluorothymol by performing density functional theory (DFT) calculations.[Citation18] Calculations were performed in a vacuum and in solvent phases, employing water and methanol as solvents.[Citation1,Citation19] It is noted that the bond dissociation enthalpy (BDE) of the O–H bond is an important factor in investigating the formation of ArO· radicals. Furthermore, the stability of the produced ArO· structure determines the antioxidant efficiency of ArOH.[Citation18]
RESULTS AND DISCUSSION
Adequacy of the Theoretical Methods Employed
Thymol and related compounds are important for their potential use as antioxidant agents. Knowing the mechanism of formation of radical structures for thymol and its related compounds is important for better understanding the concepts of antioxidation of thymol structures. As structural modifications of thymol could influence the properties of thymol, we also investigated the properties of fluorinated thymol, 2,5,6-trifluorothymol. The fluorine atom is an electronegative atom, which could change the properties of the thymol structure. Hence, investigating such modified structures could better demonstrate the antioxidation properties of thymol and its related structures.[Citation20]
Table 1 Total electronic energies (Ee), zero-point vibrational energies (ZPE), thermal corrections to enthalpies (TCH), energies at 0 K (E0), and enthalpies at 298 K (H298) for thymol in vacuum, water, and methanol
Table 2 Total electronic energies (Ee), zero-point vibrational energies (ZPE), thermal corrections to enthalpies (TCH), energies at 0 K (E0), and enthalpies at 298 K (H298) for 2,5,6-trifluorothymol in vacuum, water, and methanol
The results shown in indicate that the properties of thymol with respect to the values of energies vary from one phase to another. It is important to note that being in a vacuum reveals some distinct properties for matter, but when the solvent effects are included in the calculations, the results are changed. Interestingly, our results indicated that the radical structure of thymol is more stable in water than in other phases. Comparing the values of enthalpies, the most stable neutral structure of thymol was observed for the water phase, followed by the methanol phase, with the vacuum phase having the least stability. Comparing the values of antioxidant activity under experimental and theoretical conditions shows the polar solvent to have a high effect.[Citation20,Citation21] The framework of the self-consistent isodensity polarizable continuum model was employed to compute water and methanol solvent effects. The geometries optimized in the gas phase were used to obtain single-point energies.[Citation22]
shows the evaluated parameters for the 2,5,6-trifluorothymol structure in the three phases of vacuum, water, and methanol. Different parameters were obtained compared to those obtained with thymol. This trend could mean that the properties of thymol structure were affected by fluorine modification. For the radical structure of 2,5,6-trifluorothymol in different phases, similar results were observed. The values of enthalpies obtained indicated that, as with thymol, the most stable neutral structure of 2,5,6-trifluorothymol was realized in the water phase, followed by the methanol phase, with the vacuum phase again showing the least stability. Thus, it can be concluded that modification of thymol to 2,5,6-trifluorothymol did not change its stability.
presents the values of ΔBDE for thymol and 2,5,6-trifluorothymol in the different phases of vacuum, water, and methanol. The reference value used for obtaining these parameters was phenol, for which the differences between the BDE of phenol and the investigated materials were obtained. The results indicated that the structures of fluorinated thymol are better than thymol in vacuum, water, and methanol. There were no notable variations among the values of delta binding dissociation energies (ΔBDE) for the different phases of thymol, but the values differed for 2,5,6-trifluorothymol in a vacuum as opposed to in solution. Moreover, the structures of 2,5,6-trifluorothymol show better properties in comparison to thymol ().
Table 3 ΔBDE for thymol and 2,5,6-trifluorothymol in vacuum, water, and methanol (kcal/mol)
Figure 2 (a) Structure of isothymol, (b) resonance forms of the 2,5,6-trifluorothymol radical, and (c) bond lengths in Å 2,5,6-trifluorothymol.
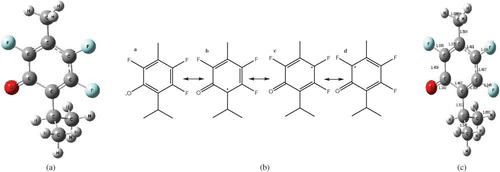
Wright et al.[Citation4] reported a hydrogen atom transfer (HAT) mechanism in aqueous solutions with ΔBDE of 10 kcal/mol and delta ionization potential (ΔIP) up to about 36 kcal/mol, and antioxidant activity predominantly set for compounds with ΔIP above about 45 kcal/mol. shows that neither 2,5,6-trifluorothymol nor thymol fulfills these requirements for the mechanism via SET, and as such the antioxidant effect of both compounds in the presence of water and methanol will follow a mechanism via HAT. The stability of the radical can be explained from the point of view of its resonance forms. Optimized geometry showed the structure resonance to have a higher stability in water.
Antioxidant Activity of Methanol Extract
Amount of 1,1-diphenyl-2-picrylhydrazyl (DPPH)
To create the methanol extract, a stock solution (10 mg/ml) of the oil was dissolved in methanol. The stable radical DPPH was reduced by the methanol extract to the yellow-colored diphenylpicrylhydrazine with an IC50 value of 0.5 38 ± 0.02 μg/ml. The methanol extract had the strongest free radical scavenging activity.
Amount of total phenolics
Folin-Ciocalteu reagent's effect on absorbance in the methanol extract solutions compared with the standard solution equivalents. The amount of total phenolics was highest in the methanol extract (0.36 ± 0.06 mg gallic acid/g). Comparison of the results revealed that the phenolic content was high in polar extracts. The presence of polar phenolics thus appears to be fundamental in the evaluation of free radical scavenging. The highest activity, seen for the polar sub-fraction of the methanol extract, reflects the radical scavenging characteristics of these phenolics. The key role phenolic compounds play as free radical scavengers has been reported elsewhere.[Citation23] It should be kept in mind that radical scavenging activity is only one of numerous mechanisms contributing to overall activity and creating synergistic effects. The role of the volatile components present in these extracts in the total antioxidant activities of the non-polar methanol extract should not be disregarded.[Citation20]
MATERIALS AND METHODS
Computational Details
DFT calculations were used to investigate the structure and properties of thymol and 2,5,6-trifluorothymol. DFT calculations were performed using the B3LYP exchange-correlation functional, the 6-311G(d,p) standard basis set, and the Gaussian 98 electronic structure calculation package. To detect the properties, we first optimized the original models to obtain the minimum-energy structures. We then performed frequency calculations to obtain the required values for total energies,[Citation24– Citation26] zero-point energies, thermal correction to enthalpies, energies at zero Kelvin, and enthalpies. Calculations were performed on thymol and 2,5,6-trifluorothymol in vacuum, water, and methanol. The symbolic form of thymol ArOH can be converted to the ArO· radical by releasing one hydrogen atom. The authors calculations were performed for both ArOH and the ArO· counterpart to evaluate their properties in vacuum, water, and methanol. For the investigated structures, values of ΔBDE were obtained by measuring differences between the investigated structures and the reference phenol.
Antioxidant Activity
DPPH assay
The DPPH assay is generally considered to involve a HAT reaction, but based on kinetic data, an electron transfer mechanism should also be considered. The radical scavenging activity (RSA) of the methanol extract of thymol was determined using a published DPPH and RSA assay method with minor modifications. Stock solutions (10 mg/ml each) of the oils and the synthetic standard antioxidant butylated hydroxytoluene (BHT) were dissolved in methanol. The solutions were diluted to concentrations ranging from 1 to 5 × 10−10 mg/ml. For each concentration, 2 ml were mixed with an equal amount of 80 μg/ml DPPH methanol solution and left for 30 min in the dark at room temperature to allow any reaction to occur. Ultraviolet absorbance of these solutions was recorded on a spectrometer at 517 nm using a blank containing the same concentration of oil or BHT without DPPH. Inhibition of free radical DPPH in percent (I%) was calculated using the following formula:
Determination of total phenolic compounds
Studies of other food products have determined phenolic compound levels in order to establish antioxidant activity.[Citation27,Citation28] The Folin-Ciocalteu method was employed to determine total phenolic compound contents.[Citation22] Each extract sample (0.5 ml) was mixed with 2.5 ml of 0.2 N Folin-Ciocalteu reagent for 5 min, and after that 2.0 ml of 75 g/l sodium carbonate was added, and the resulting mixture allowed to incubate at room temperature for 2 h. The absorbance of reaction was then measured at 517 nm. Results were expressed as gallic acid equivalents. The colorimetric method was used to determine the flavonoid content of each extract:[Citation29] 0.5 mL solution of each methanol extract was mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water, and allowed to stand for 30 min at room temperature. The absorbance of the reaction mixture was measured at 415 nm with a double beam Perkin Elmer UV/Visible spectrophotometer (UV-Visible EZ201, Perkin Elmer, USA). Total flavonoid contents were calculated as quercetin from a calibration curve.
CONCLUSIONS
In this article, it is shown that thymol is a potential antioxidant because of the high stability of its radicals. The study was concerned with the determination of the BDE and the IP values according to the mechanism proposed in the literature for free radical scavenging activity. Both experimental and theoretical results allowed us to determine that thymol has a protective antioxidant action.
Notes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ljfp.
REFERENCES
- Aeschbach , R. , Loliger , J. , Scott , B.C. , Murcia , A. , Butler , J. , Halliwell , B. and Aruoma , O.I. 1994 . Evaluation of the antioxidant actions of gallic acid and its derivatives . Food and Chemical Toxicology , 32 : 31 – 36 .
- Halliwell , B. and Gutteridge , J.M.C. 1989 . Free Radicals in Biology and Medicine , 2nd , Oxford : Clarendon Press .
- Huang , D. , Ou , B. and Prior , R.L. 2005 . The chemistry behind antioxidant capacity assays . Journal of Agricultural and Food Chemistry , 53 : 1841 – 1856 .
- Wright , J.S. , Johnson , E.R. and DiLabio , G.A. 2001 . Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects and application to major families of antioxidants . Journal of the American Chemical Society , 123 : 1173 – 1183 .
- Hernández , C.A. , Martínez Manchado , C. , Silva , J. and Herrera , J.C. 1999 . A novel approach to quantitative structure-activity relationships in antioxidants . Ciencia , 7 ( 1 ) : 78 – 87 .
- Rosas-Romero , A.J. and Saavedra , G. 2005 . Screening Bolivian plants for antioxidant activity . Pharmaceutical Biology , 43 ( 1 ) : 79 – 86 .
- Halliwell , B. , Gutteridge , J.M.C. and Cross , C.E. 1992 . Free radicals, antioxidants and human disease: where are we now? . Journal of Laboratory and Clinical Medicine , 119 : 598 – 620 .
- Ames , S.N. , Shigrenaga , M.K. and Hagen , T.M. 1993 . Oxidants, antioxidants and degenerative diseases of aging . Proceedings of the National Academy of Sciences of the United States of America , 90 : 7915 – 7922 .
- Gerber , M. , Boutron-Ruault , M.C. , Hercberg , S. , Riboli , E. , Scalbert , A. and Siess , M.H. 2002 . Food and cancer: State of the art about the protective effect of fruits and vegetables . Bulletin du Cancer , 89 : 293 – 312 .
- Kris-Etherton , P.M. , Hecker , K.D. , Bonanome , A. , Coval , S.M. , Binkoski , A.E. , Hilpert , K.F. , Griel , A.E. and Etherton , T.D. 2002 . Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer . American Journal of Medicine , 113 ( Suppl. 9B ) : 71S – 88S .
- Esmaeili , A. , Moazami , N. and Rustaiyan , A. 2012 . Biotransformation of germacranolide from Onopordon leptolepies by Aspergillus niger . Pakistan Journal of Pharmaceutical Sciences , 25 ( 1 ) : 155 – 159 .
- Esmaeili , A. , Saremnia , B. , Koohian , A. and Rezazadeh , S. 2011 . Mechanism of nanocapsules of Matricaria recutita extract formation by the emulsion–diffusion process . Superlattices and Microstructures , 50 : 340 – 349 .
- Esmaeili , A. , Hoseiny Zarea , A. , Sharafian , S. , Safaiyan , S. and Rustaiyan , A. 2009 . Biotransformation of one monoterpene by sporulated surface cultures of Aspergillus niger and Penicillium sp . Herba Polonica , 55 ( 1 ) : 78 – 83 .
- Esmaeili , A. , Saad , N. , Safaiyan , S. and Rustaiyan , A. 2009 . Biotransformation of (−)-menthol by Mucor ramannianu and study of the pathways involved . Herba Polonica , 55 ( 2 ) : 51 – 58 .
- Esmaeili , A. and Saremnia , B. 2012 . Preparation of extract-loaded nanocapsules from Onopordon leptolepis DC . Industrial Crops and Products , 37 : 259 – 263 .
- Esmaeili , A. and Hashemi , E. 2011 . Biotransformation of myrcene by Pseudomonas aeruginosa . Chemistry Central Journal , 5 : 26 – 34 .
- Wood , J.B. 1969 . Microbial fermentation of lower terpenoids . Process Biochemistry , 2 : 50 – 52 .
- Hohenberg , P. and Kohn , W. 1964 . Inhomogeneous electron gas . Physical Review , 136 : B864 – B871 .
- Kohn , W. and Sham , L.J. 1965 . Self-consistent equations including exchange and correlation effects . Physical Review , 140 : A1133 – A1138 .
- Esmaeili , A. and Khodadadi , A. 2012, 14 (7), 14–18 . Antioxidant activity of a solution of thymol in ethanol. , Zahedan University of Medical Sciences and Health Services .
- Esmaeili , A. , Khodadadi , A. and Shila Safaiyan , S. 2012 . Biotransformation of thymol by Aspergillus niger . Chemistry of Natural Compounds , 47 ( 6 ) : 966 – 968 .
- Rojano , B. , Jairo Saez , J. , Schinella , G. , Quijano , J. , Vélez , E. , Gil , A. and Notario , R. 2008 . Experimental and theoretical determination of the antioxidant properties of isoespintanol (2-isopropyl-3,6-dimethoxy-5-methylphenol) . Journal of Molecular Structure , 877 : 1 – 6 .
- Madsen , H.L. , Nielsen , B.R. , Bertelsen , G. and Skibsted , L.H. 1996 . Screening of antioxidative activity of spices . A comparison between assays based on ESR spin trapping and electrochemical measurement of oxygen consumption. Food Chemistry , 57 : 331 – 337 .
- Stephens , P.J. , Devlin , F.J. , Chabalowski , C.F. and Frisch , M.J. 1994 . Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields . Journal of Physical Chemistry , 98 ( 45 ) : 11623 – 11627 .
- Krishnan , R. , Frisch , M.J. and Pople , J.A. 1980 . Some deficiencies of MINDO/3 . Journal of Physical Chemistry , 72 : 46 – 54 .
- Scott , A.P. and Radom , L. 1996 . ZPE or other methods might employ other factors . Journal of Physical Chemistry , 100 : 16502 – 11513 .
- Mandic , A.I. , Đilas , S.M. , Ćetković , G.S. , Čanadanović-Brunet , J.M. and Tumbas , V.T. 2008 . Polyphenolic composition and antioxidant activities of grape seed extract . International Journal of Food Properties , 4 ( 11 ) : 713 – 726 .
- Ruiz-Navajas , Y. , Viuda-Martos , M. , Fernández-López , J. , Zaldivar-Cruz , J.M. , Kuri , V. and Pérez-Álvarez , J.A. 2011 . Antioxidant activity of artisanal honey from tabasco, Mexico . International Journal of Food Properties , 2 ( 14 ) : 459 – 470 .
- Chang , C. , Yang , M. , Wen , H. and Chern , J. 2002 . Estimation of total flavonoid content in propolis by two complementary colorimetric methods . Journal of Food Drug Analysis , 10 : 178 – 182 .