Abstract
Silter cheese is a traditional hard cheese, produced in Valcamonica, Brescia, Italy. A total of 426 lactic strains isolated from Silter were analyzed to determine their probiotic characteristics. 274 out of 426 strains were found to produce bacteriocins against at least one of eight different pathogens (Salmonella enterica, Listeria monocytogenes, Salmonella derby, Salmonella thyphimurium, Salmonella napoli, Staphylococcus aureus, E. coli O157:H7, Salmonella enteritidis). In addition, 211 of 274 bactericin-producer strains adhered to Caco-2 cells and were characterized by RiboPrinter, revealing predominance of Enterococcus faecalis (26%) and Enterococcus durans-faecium (22%). These findings suggest that Silter may qualify as an important source of feasible probiotic strains.
Keywords:
INTRODUCTION
Functional foods are foods that provide health benefits beyond basic nutrition due to certain physiologically active components. A definition of functional foods was first depicted in Japan in the 1980s, referring to processed foods containing ingredients that aid specific bodily functions. Health benefits related to consumption of functional food are mostly triggered by probiotic microorganisms, such as live lactobacilli and bifidobacteria.[Citation1]
The interest in functional foods is growing rapidly, driven by several factors including the rapid advances in scientific knowledge, consumer demand for healthier foods, the aging populations, the ever-increasing health-care costs, and the food industry desire to fulfill the consumer appetite for products derived from food that can promote good health condition.[Citation2] In the category of nutraceuticals, comprised of functional foods and supplement components, the role of probiotics appears to be even more significant. The term “probiotics” was first used by Parker in 1974[Citation3] to define organisms or substances able to contribute to intestinal microbial balance.[Citation3] Schrezenmeir and de Vrese[Citation4] defined probiotics as “a preparation of or a product containing viable, microorganisms in sufficient numbers which, by implantation or colonization, alter the microflora in a compartment of the host and by that exert beneficial health effects in the host.” Probiotics are largely used in fermented dairy products and cheeses.[Citation5] The Japanese Association of Fermented Milks and Fermented Milks Drinks has established a minimum level of 1 × 107 viable lactic-acid-producing-bacteria (LAB) per ml for probiotic milk product.[Citation6] Finally, the FAO-WHO guidelines define probiotics as “live microorganisms which, when administered in adequate amount, confer health benefits to the host.”[Citation7]
The beneficial effects of probiotics are not limited to the large intestine, since they also extend to the nasal cavities and to the urogenital system.[Citation8] Probiotics related health benefits include improvement of intestinal peristalsis, decrease in cholesterol level, and enhancement of immune system activities. Adhesion to intestinal cells and competitive activities against potential pathogens or dismetabolic microbial populations are significant/beneficial features of lactic bacteria.[Citation9,Citation10] Functional foods enriched with probiotics are highly popular among different European consumers’ categories, and they are largely available on the market. In spite of their increasing economic relevance, functional foods are not specifically regulated by European legislation.[Citation11] The evolution of legislation on the matter is very slow; currently only Japan, the U.K., the U.S.A., and Scandinavian countries have achieved some considerable progress. Moreover, the labelling of functional foods is far from being informative, and only provides scanty information about nutritional value, storage, and cooking recipes.[Citation12]
Silter cheese is a PDO (protected designation of origin) hard or semi-hard cheese, produced using milk from Italian Brown and Italian Red Pied cows in the province of Brescia, northern Italy, since the end of the 16th century as found in a document written by a local chanchellor.[Citation13] Its name derives from the local dialect and refers to the shelter and dairy used by humans and animals in high Alps mountains pastures. Silter cheese is made in the shape of a cylinder of 30 to 40 cm in diameter and 8 to 12 cm in height, with a weight ranges from 6 to 15 kg. The crust is yellow, hard, and smooth. The yellow straw inner part presents many little holes and the flavor is slightly sweet and aromatic (). This characteristic derives from typical aromatic grass growing at high altitudes which is used to feed the cows. One year’s seasoning makes the holes grow in number, the flavor and aroma more intense, and the cheese ready for grating.
The probiotic properties of the micro flora found in Silter cheese were studied. LAB isolated from Silter cheese were characterized both genetically and phenotypically. The data gathered from this preliminary probiotic screening was used to define a baseline to be used for the adoption of microbiological standards in the qualitative assessment of cheese production.
MATERIALS AND METHODS
Samples
Silter is a hard/semi-hard traditional cheese produced in northern Italy since the end of the 16th century. Silter cheese production process: (a) Raw, partially skimmed milk from the two daily milkings is warmed to 35–36 °C; after (b) adding the rennet, the natural whey from previous work, and mesophilic and termophilic starters, it coagulates in around 40–45 min; (c) the curd which has formed is then broken using a traditional tool called spada or spannarola; it rests into a vat for 10–40 min; and then, using a tool called spino, it is broken down again into a rice sized pieces and cooked at 46–48°C for 10–15 min; (d) curd rests for nearly 1 h in vat at 45–46°C; after that it is transferred into a marked mould and dry salted for two days. The cheese is seasoned for at least 200 days. Following the guidelines of the European Commission Regulation (EC) No. 2081/92,[Citation14] in 2005–2007 a project aimed at the study of the microbiological features of Silter cheese was carried out, helping this cheese to be awarded with PDO designation. A total of 50 samples of Silter cheese, seasoned for 200 days, having a mean weight of 500 g, were obtained from 50 different cheese manufactured in two of the around 40 different cheese industries members of the consortium for protection of Silter cheese in Lombardy, Italy. Samples were transported to the laboratory at ≤ 4°C and on arrival their interior portion was analyzed after being cut by means of a sterile knife.
Strains Isolation
Lactic acid bacteria strains were isolated onto MRS agar (Man Rogosa Sharpe) (Oxoid).[Citation15,Citation16] The isolates obtained from MRS agar were cultured in MRS broth (Oxoid) at 37°C[Citation17] and titrating ≥ 1 × 107 colonies/ml were stored at –80°C with 20% glycerol.
Bacteriocins Production
Production of bacteriocins was assessed against eight different pathogens: Listeria monocytogenes (ATCC 19115); Salmonella enterica (IZSLER 230747); Salmonella derby (IZSLER 259473); Salmonella thyphimurium (ATCC 6994); Salmonella napoli (IZSLER 337144); Salmonella enteritidis (IZSLER 240752); Escherichia coli O157:H7 (IZSLER 675); Staphylococus aureus (IZSLER 542). The productions of bacteriocins were assessed by a well diffusion assay as described previously.[Citation16,Citation18,Citation19] Briefly, the plate wells were soaked with a suspension of lactic strain cultured in MRS broth at 37°C for 24 h, while the control plate was loaded with 10 μg/ml of Nisin (Sigma-Aldrich) (pH 8) with addition of EDTA for analysis of Gram positive pathogens. The plates were incubated at 30°C for 5 h, covered with a layer of PCA (Plate Count Agar) (Oxoid) and of PCA mixed with a broth suspension of the pathogen (grown at 37°C in Brain Heart Infusion broth) , and then incubated overnight at 37°C.
In Vitro Adhesion Assay
The lactic acid bacteria strains able to produce bacteriocins were tested by an in vitro adhesion assay on Caco-2 cells line, originally isolated from a human colon adeno-carcinoma, as described previously.[Citation20] For the adhesion assay, monolayers of cells, cultured in Dulbecco modified Eagle’s minimal essential medium, were prepared in six well tissue culture plates. Forty-eight-hours cultures (10 ml) in MRS broth of each lactic bacterium isolate were harvested and washed in physiological saline. Tenfold serial dilution in physiological saline were inoculated on differentiated Caco-2 cells monolayers, after removing the growth medium. After a contact of 1.5 h at 37°C in 5% CO2 atmosphere, the cells were washed twice using physiological saline, trypsinised and re-suspended in 4 ml of physiological saline with addition of 500 μl of foetal bovine serum. One ml of each cell suspension was mixed with 13 ml of MRS agar and the plates were incubated at 37°C in 5% CO2 for three days. The colonies grown in the plates were counted to verify bacterial adhesivity.[Citation16] A two logarithms difference in bacterial count before and after contact with Caco-2 culture cells was used to evaluate bacterial adhesivity.
Strains Characterization
All the lactic acid bacteria strains able to produce bacteriocins and to adhere to Caco-2 cells were further characterized by automated ribotyping using the RiboPrinter system (DuPont-QualiconLtD, Wilmington, Del.) as described previously.[Citation21,Citation22]
RESULTS AND DISCUSSION
A total of 426 strains of lactic-acid-producing bacteria at concentration ≥ 1 × 107 colonies/ml were isolated from the Silter cheese samples. This value has been proposed as a distinctive criterion for probiotic products.[Citation6] Noteworthy, in Silter cheese, fulfillment of this criterion is not derived from production artifacts, as the presence of lactic acid bacteria is not due to addition during production, but it is rather an intrinsic property of the traditional techniques inherited over more than four centuries of history.
Adhesion to intestinal cells and production of factors inhibiting potential pathogens or dismetabolic microbial populations are significant mechanisms by which probiotics exert beneficial effects on health. These properties can be investigated in vitro in order to assess the probiotic qualities of lactic acid producing bacteria. In this study, out of 426 bacterial isolates, a total of 242 strains (56.8%) produced bacteriocins against Listeria monocytogenes, 251 (58.9%) against Salmonella enterica, 251 (58.9%) against Salmonella derby, 244 (57.2%) against Salmonella thyphimurium, 253 (59.3%) against Salmonella napoli, 241 (56.5%) against Salmonella enteritidis, 249 (58.4%) against Escherichia coli O157:H7 and 247 (57.9%) against Staphylococcus aureus ( Production of bacteriocins against, at least, one of the eight bacterial pathogens was demonstrated by the agar well diffusion assay in 274 out of 426 (64%) isolates obtained from Silter cheese. An interesting result is that most of the 274 bacteriocins-positive isolates exhibited a broad spectrum of inhibition activity against both Gram-negative and Gram-positive bacterial pathogens. The mechanisms by which bacteriocins inhibit growth of bacterial pathogens are unclear. Abee et al.[Citation23] demonstrated that the inhibitory activity of these substances is limited to Gram-positive bacteria. On the other hand, Stevens et al.[Citation24] and Ray[Citation25] demonstrated that bacteriocins are also active against Salmonella spp. and other Gram-negative pathogens.
FIGURE 2 Bacteriocin activity in the lactic acid producing bacteria isolated from Silter cheese. Bacteriocin activity was assessed using a panel of 8 different pathogens. Abbreviations: N.D., not determinable.
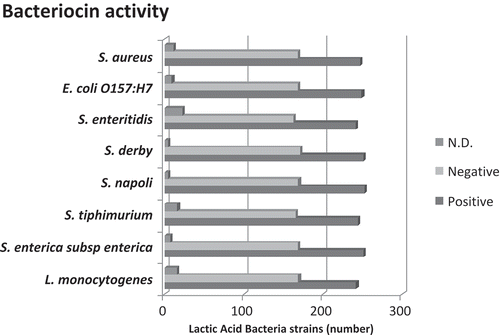
Two hundred and eleven out of 274 bacteriocins-producing isolates (77%) were also found to be able to adhere to Caco-2 cells by an in vitro adhesion assay. The ability to adhere to Caco-2 cells is a good proxy to assess the ability of the bacteria to adhere to human epithelial cells in the intestinal tract.[Citation9,Citation10] Adhesion to intestinal cells and competition with potential pathogens or dismetabolic microbial populations are believed to be an important mechanism by which lactic bacteria exert their beneficial activity.
Accordingly, a total of 211 lactic acid bacteria strains isolated from Silter cheese displayed probiotic characteristics (production of bacteriocins and ability to adhere to Caco-2 cells). These strains were further characterized by ribotyping, revealing a highly heterogeneous microflora population. The predominant strains were Enterococcus faecalis (26%) and Enterococcus durans faecium (22%), followed by Lactobacillus curvatus (8%), Lactobacillus pantheris (8%), Lactobacillus lactis (7%), Lactobacillus salivarius (7%), Lactobacillus paraplantarum (5%), Streptococcus thermophilus (5%), Enterococcus spp. (4%), Lactococcus garviae/Enterococcus (4%), Lactobacillus delbrueckii bulgaricus (2%), and Lactobacillus parabuchneri (2%). Enterococci (48% of isolated LAB in this work) are natural members of the intestinal flora in the large bowel of humans and in the intestinal tract of mammals and birds.[Citation26] They are also found in soil, plants, and water. Enterococci have emerged as a major cause of nosocomial infections, and, within this group, Enterococcus faecalis causes the majority of human enterococcal infections. These bacteria may be associated with bacteremia, systemic or local infections in the urinary tract, abdomen, wounds, and endocardium.[Citation27] However, Enterococci have also been studied for their possible use as probiotics. Administration of an E. faecalis strain has been shown to decrease diarrhea.[Citation28]
In this study, Lactobacillus spp. accounted for nearly 40% of the isolates made from Silter cheese. Lactobacilli are an important part of the human flora, but they can be pathogenic under certain conditions. Lactobacillus species are used for production of fermented food, such as yogurt, cheese, beer, wine, cider, chocolate, and animal feeds, such as silage. In recent years, interest has increased for their use as probiotics and many probiotic formulations containing Lactobacillus species. Various species of lactobacilli may be beneficial in certain infectious diarrheas or other diseases.[29]
Silter cheese has peculiar characteristics deriving from the use of local ingredients and production techniques developed over centuries of traditions and linked to the Valcamonica territories. This cheese is considered a territorial heritage and the techniques/modalities of production have recently been codified and disciplined according to PDO guidelines[14] in order to guarantee food safety and preserve the identity of this cheese. The findings of this study provide evidence that this traditional food possesses a lactic flora with functional activity. The potential probiotic activity, along with the nutritional properties and the territorial specificity, surely adds intrinsic value to Silter cheese and this could be a claim in advertising and marketing promotions, respecting the requirements of the European community legislation.[29]
In conclusion, in this study many information were gathered on the microflora of a traditional Italian processed food. Based on these findings, Silter cheese represents an important source of probiotic strains, but it is still not possible to define it as a functional food, because this should be further evaluated, by repeating and extending the investigations. It should also be assessed whether, and, in case, to which extent, virulence and drug resistance factors are harbored in the lactic microflora of Silter cheese, especially in Enterococcus genus. Enterococci need to be considered safe to confer probiotic activity to the matrix to which they belong. These aspects are not commonly considered when assessing the microbiological features of food products. The above mentioned information could be used to support and promote this category of products on both domestic and international markets.
REFERENCES
- Sanders, M.E. Overview on functional foods: Emphasis on probiotic bacteria. International Dairy Journal 1998, 8, 341–347.
- Guizani, N.; & Sablani Shyam, S. Guest Editorial. Special Issue: Functional Foods. International Journal of Food Properties 2007, 10 (2), 199.
- Parker, R.B. Probiotics, the other half of the antibiotics story. Animal Nutrition Health 1974, 29, 4–8.
- Schrezenmeir, J.; De Vrese, M. Probiotics, prebiotics, and synbiotics: Approaching a definition. American Journal of Clinical Nutrition 2001, 73, S361–S364.
- Gorbach, S.L. The discovery of Lactobacillus GG. Nutrition Today 1996, 31 (6), 2S–4S.
- David, C. Probiotic as functional foods. Nutrition in Clinical Practice 1996, 18, 497–506.
- Probiotics in food. Health and nutrition properties and guidelines for evaluation. FAO-WHO Probiotic Guidelines 2006.
- Habermann, W.; Zimmermann, K.; Skarabis, H.; Kunze, R.; Rusch, V. Reduction of acute recurrence in patient with chronic recurrent hypertrophic sinusitis by treatment with a bacterial immunostimulant Enterococcus faecalis bacteria of human origin. Arzneimttel Forschung 2002, 52, 622–627.
- Elo, S.; Saxelin, M.; Salminen, S. Attachment of Lactobacillus casei strain GG to human colon carcinoma cell line Caco-2: Comparison with other dairy strains. Letters in Applied Microbiology 1991, 13, 154–156.
- Tuomola, E.M.; Salminen, S.J. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Journal of Food Microbiology 1998, 41, 45–51.
- Commission Regulation (EC) No. 178; 2002.
- Arvanitoyannis, I.S.; Van Houwelingen-Koukaliaroglou, M. Functional foods: A survey of health claims, pros and cons, and current legislation. Critical Reviews in Food Science and Nutrition 2005, 45 (5), 385–404.
- http://www.ruralpini.it/Malga_Silter_di_Gianico.htm
- Commission Regulation (EC) No. 2081; 1992.
- Barakat, R.K.; Griffiths, M.W.; Harris, L.J. Isolation and characterization of Carnobacterium, Lactococcus, and Enterococcus spp. from cooked, modified atmosphere packaged, refrigerated, poultry meat. Journal of Food Microbiology 2000, 62 (1–2), 83–94.
- Lorenzo, J.M.; Garcìafontàn, M.C.; Cachaldora, A.; Franco, I.; Carballo, J. Study of the lactic acid bacteria throughout the manufacture of dry-cured lacòn (a Spanish traditional meat product). Effect of some additives. Food Microbiology 2010, 27, 229–235.
- Bellei, M.; Miguel, M.; Mere Del Aguila, E.M.; Silva, J.T.; Paschoalin, V.M.F. Purification of a bacteriocin produced by Enterococcus faecium and its effectiveness for preservation of fresh-cut lettuce. Journal of Microbiology and Antimicrobials 2011, 3 (5), 119–125.
- Blom, H.; Katla, T.; Hagen, F.B.; Axelsson, L. A model assay to demonstrate how intrinsic factors affect diffusion of bacteriocins. International Journal of Food Microbiology 1997, 38, 103–109.
- Hoover, D.G.; Steenson, L.R. Screening methods for detecting bacteriocin activity. In: Bacteriocin of Lactic Acid Bacteria; Montville, T.J.; Kaiser, A.L.; Eds.; Academic Press: NY, 1993; Cap 2. 1–22.
- Kimoto, H.; Kurisaki, J.; Tsuji, N.M.; Ohmomo, S.; Okamoto, T. Lactococci as probiotic strains: Adhesion to human enterocyte-like Caco-2 cells and tolerance to low pH and bile. Letters in Applied Microbiology 1999, 29, 313–316.
- Mohania, D.; Nagpal, R.; Kumar, M.; Bhardwa, J A.; Yadav, M.; Jain, S.; Marotta, F.; Singh, V.; Parkash, O.; Yadav, H. Molecular approaches for identification and characterization of lactic acid bacteria. Journal of Digestive Diseases 2008, 9, 190–198.
- Ryu, C.S.; Czajka, J.W.; Sakamoto, M.; Benno, Y. Characterization of the Lactobacillus casei group and the Lactobacillus acidophilus group by automated ribotipyping. Microbiology and Immunology 2001, 45 (4), 271–275.
- Abee, T.; Krockel, L.; Hill, C. Bacteriocins:Modes of action and potentials in food preservation and control of food poisoning. International Journal of Food Microbiology 1995, 28, 169–185.
- Stevens, M.E.; Sheldon, B.W.; Klapes, N.A.; Klaenhammer, T.R. Nisin treatment for the inactivation of Salmonella species and other Gram-negative bacteria. Applied and Environmental Microbiology 1991, 57, 3613–3615.
- Ray, B. Sublethal injury, bacteriocins, and food microbiology. ASM News 1993, 59, 285–291.
- Gilmore, M. The Enterococci: Pathogenesis, Molecular Biology, and Antibiotic Resistance. American Society for Microbiology Press: Washington, DC, 2002. 101–130.
- Murrary, B.E. Diversity among the multidrug-resistant enterococci. Emerging Infectious Diseases 1998, 4 (1), 46–65.
- Slover, C.M.; Danziger, L. Lactobacillus:A Review. Clinical Microbiology Newsletter, 30:4, 2008.
- Commission Regulation (EC) No. 1924; 2006.

