Abstract
Strong protein gel networks may result from synergistic interactions with other proteins or food materials above that are not achievable with a single protein alone. The varying flow and viscoelastic behavior of calcium caseinate or whey protein isolate mixed with egg albumin, fish protein isolate, soy protein isolate, or wheat gluten in a model system with wheat flour and glycerol as starch and oil surrogates was determined. Temperature sweeps revealed peak tan δ values as the proteins aggregated. Single protein gels of calcium caseinate, soy protein isolate, and wheat gluten were predominantly elastic, while egg albumin and whey protein isolate gels were mostly viscous. For example, egg albumin steady shear viscosities were: 0.0145 Pa s (0.5 min) and 0.1331 Pa s (45 min), and whey protein isolate 0.0003 Pa s (0.5 min) and 0.0024 Pa s (45 min); but combined with whey protein isolate (whey protein isolate/egg albumin: 10/5 wt%), the apparent viscosity values dropped to 0.0053 Pa.s (0.5 min) and 0.0221 Pa s (45 min), respectively.
INTRODUCTION
Food proteins may be combined with other ingredients such as wheat flour and oils to create strong protein-enriched food gels. The practical relevance of protein gel systems applies from food to material science.[Citation1] When heated, dilute colloidal protein systems form solid phase network structures.[Citation2] Cross-linked entanglements induced by other physical processing such as pressure may form such gels. In some instances, such as in yogurts and structured meat products, the presence of polysaccharides in protein gels is beneficial for network formation, producing strikingly different rheological behavior upon heating.[Citation3] The critical elements that determine the texture of gels is the combination, miscibility, and homogeneity of the selected proteins.[Citation4]
Combined protein systems sometimes result in stiff entangled networks that are resistant to shear deformations, evidenced by networks with high storage modulus () values.[Citation5] Heat-induced denaturation of proteins created aggregation and disulfide/sulfhydryl interchange in an egg albumin (EA), soy protein concentrate, soy protein isolate (SPI), or whey protein concentrate gels.[Citation6] Elevating temperature in a mixed protein system increases the collision of partially unfolded aggregated molecules forming the network.[Citation2] Proteins may be assumed to be entangled networks of polymer coils, with
reflecting the resistance to deformation; the stronger the network, the higher the G′ value.[Citation5] When the values of G′ and loss modulus (G″) are close to each other and tan δ values therefore close to 1.0, there may be sufficient time for an entangled network to disentangle and rearrange within the oscillation period, as such, energy storage and energy dissipation may be balanced.[Citation7]
In whey protein blends, for instance, the ratio of the α-lactalbumin and β-lactoglobulin fractions can change the rheological property.[Citation8] One study of whey protein blends (α-lactalbumin, β-lactoglobulin, and bovine serum albumin) showed synergistic interaction and co-aggregation of molecules.[Citation9] Ngarize et al.[Citation14] reported synergistic interactions for EA combined with whey protein isolate (WPI) mixed system. For example, synergistic increases in viscoelasticity include soy protein and wheat gluten (WG),[Citation10] and soy protein and WPI.[Citation11]
The assembly structure and size of aggregates of mixed proteins affects both macroscopic and microscopic interactions; for example, protein assembly can form visible rod-like fibrils with fine branches.[Citation1] Low shear small amplitude oscillatory shear analysis (SAOSA) has been used to determine aggregation, denaturation, and gelation of heated protein systems.[Citation12] Heat denatured whey proteins containing casein increased gel strength, and changing the pH of the whey/casein system exerted a great influence on complex formation even at ambient temperatures.[Citation13] The synergies that increase network strength include heat-induced large aggregate formation.[Citation14] O'Kennedy and Kelly[Citation13] demonstrated increased gel strength from casein aggregation. Synergistic increases in viscoelasticity of skim milk and EA from heat gelation was reported; but also with antagonistic effects on foaming.[Citation15]
Previous research on protein blends (the whey proteins α-lactalbumin and β-lactoglobulin) showed non-specific mixed networks between different types of protein that affected rheological properties depending solely on the total protein concentration.[Citation8] The purpose of this work was to determine the rheological changes in incipient gels of heated mixed protein blends from different sources.
MATERIALS AND METHODS
The proteins used for the study were calcium caseinate (CC) from (American Casein Company, Burlington, NJ, USA), EA from (Primera Foods Corp., Cameron, WI, USA), fish protein isolate (FPI) from (Federal Laboratories Corporation, Alden, NY, USA), soy protein isolate (SPI) from (Pro-Fam® 974, ADM Specialty Food Ingredients Division, Decatur, IL), WG from (Wheatpro® 80, ADM), and WPI from (Provon® 190, Glanbia Nutritionals, Inc., Monroe, WI). Food grade glycerin (99.5% USP) was purchased from (Lab Chem Plus, Springfield, MA, USA). Wheat flour was purchased from ConAgra Food Ingredients, (Omaha, NE, USA). The proximate composition of the protein materials is presented in .
Table 1 Proximate concentrations of CC, WPI, EA, FPI, and SPI
The model system consisted of a mixture of 60 g protein, 30 g wheat flour, and 10 g glycerol. A two-protein system contained 30 g of each the selected protein, and a three-protein system contained 20 g of each protein selected, plus the rest of the materials. After blending the protein with wheat starch and glycerol, 40 g aliquot of the mixture was mixed with 60 g water to produce model test specimen that contained 24.0% protein (the protein combinations can be seen under material columns in –).
Table 2 Viscosity and viscoelastic temperature sweep data for single-protein samples of CC, WPI, EA, FPI, WG, and SPI
Table 3 Two-way protein interactions of CC and WPI on the flow behavior of CC and other protein blends. Viscosity and viscoelastic temperature sweep data for single-protein samples of CC, WPI, EA, FPI, WG, and SPI
Table 4 Three protein interactions of CC and WPI on the flow behavior of CC and other protein blends. Viscosity and viscoelastic temperature sweep data for single-protein samples of CC, WPI, EA, FPI, WG, and SPI
The protein powders, wheat flour, glycerol, and water were weighed separately and blended manually by stirring with a spatula in a 150 mL beaker for 5 min. A total of 42 mixed protein model blend specimens were created from combinations of WPI, CC, FPI, EA, WG, and SPI. After hand mixing the powders, water was added to the blends at 22°C, the beaker was placed into a 60°C water bath and stirred for 15 min with a Virtis-23 mixer (Gardiner, NY, USA) for incipient gels. Separate blends were made for each run, and the experiments were repeated three times.
The flow behavior and viscosity measurements were made immediately after mixing the slurry and heating to 60°C and holding for 15 min to form incipient gels. Approximately 60 mL of the blended specimen was poured into a thermosel system maintained at 60°C attached to a DV-III Ultra Brookfield Viscometer (Middleboro, MA, USA) with a UL #3-LV spindle. Correction factors were used to account for spindle/wall effects. The up/down ramp profiles was used to determine flow behavior of the mixtures. Samples were ramped up and down at 10 rpm per min between 50 and 250 rpm. Apparent viscosity, shear rate, and shear stress were recorded. The resulting data was modeled with the Hershel-Buckley model (Eq. 1). The flow behavior (‘n’, flow index) was determined by modeling the “up ramp” shear rate and shear stress with the Hershel-Buckley model using the program TableCurve 2D (ASIN Software, Inc., Chicago, IL, USA).
The viscoelasticity of all protein gel systems were tested after holding at 22°C for 60 min. After holding, SAOSA of the resulting paste was conducted using an AR2000 rheometer (TA Instruments, New Castle, DE). Specimens placed between parallel aluminum plates with a gap of 3 mm were subjected to temperature sweeps from 26 to 80°C at a frequency of 50 rad/s. The strain was set at 0.8%, which was within the linear viscoelastic range. G′, G″, tan δ, time, and temperature were recorded every 2°C. The frequency sweeps lasted between 67 to 108 min to complete for the samples, at the heating rates of 0.5–0.8°C/min. Duplicate temperature sweeps were made, new specimen was used each time, and the results were averaged.
For scanning electron microscopy (SEM), the model protein gels were prepared at 22°C and placed in dialysis membrane tubing (Spectrum Laboratories, Rancho Dominguez, CA, USA) and equilibrated with a solution of 2.5% glutaraldehyde-0.1M imidazole buffer at pH 7.2. After washing in the buffer, the cross-linked protein in the dialysis tubing was dehydrated in a graded series of ethanol solutions (50%, 80%, and absolute) and freeze-fractured. Fragments of the proteins were mounted on SEM sample stubs, coated with a thin layer of gold by DC sputtering and imaged with a Quanta 200 scanning electron microscope (FEI Co., Inc., Hillsboro, OR, USA), operated in the secondary electron imaging mode.
RESULTS
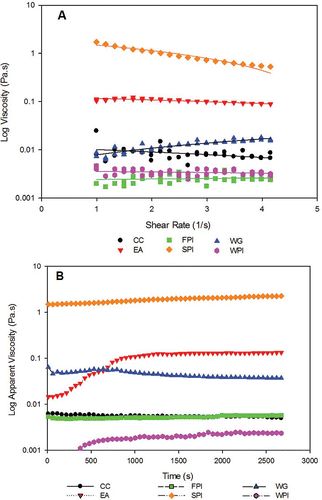
The relative viscosity values of the six proteins soft gels (<15% solids) studied are presented. The results of CC, WPI, EA, FPI, SPI, and WG are presented in . The relative viscosity (Pa.s) of the protein gels measured at 150 rpm, in ascending order are: FPI (0.0025) < WPI (0.0034) < CC (0.0079) < WG (0.0126) < EA (0.1336) < SPI (0.8798). The log apparent viscosity of the protein formulations with their time dependent relative apparent viscosity order is represented in . EA and SPI were the only gels above 0.1 Pa.s; the others were very soft gels (<0.015 Pa.s) except WG, which was steady around 0.055 Pa.s. This is in agreement with increased consistency index of EA (0.0172 Pa.s) and SPI (0.0244 Pa.s), which were significantly higher than the others (). The yieldstress for all the single protein formulations was below zero and mostly insignificant (P < 0.05). When evaluated with the Herchel-Buckley model, the flow indices (n) of the protein formulations were mostly shear thinning (<1), with viscosity decreasing with the rate of shear (pseudoplastic ˜1), except for WG, though weak in viscosity (<0.013 Pa.s) but was shear thickening (>1) (). The flow behavior of a material during processing may vary significantly because the consistency and composition of the material could be drastically altered due to mixing, heating, and shearing, creating an aerated homogenized or an entangled network over time. The majority of the protein models studied showed non-Newtonian flow behavior patterns; this is typical of many soft types of slurry from multiphase mixtures.[Citation16]
Figure 1 Relative apparent viscosity (a) and steady shear (b) profiles of the six proteins, CC, EA, FPI, SPI, WG, and WPI.
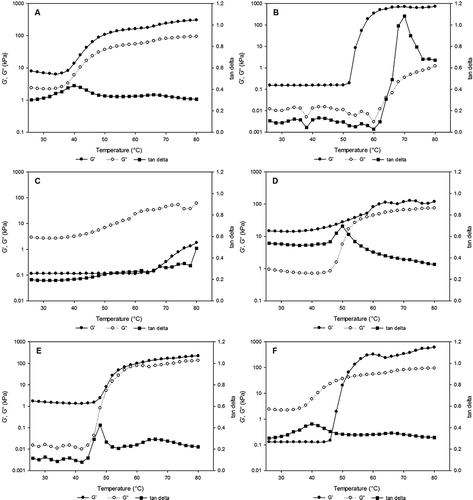
The viscoelastic response of the temperature sweeps of CC, EA, FPI, SPI, WG, or WPI protein models are shown in . The point in the transition region at which tan δ was at its maximum value was identified, and the temperature, and G′ at that point were designated Tmax, G′max (). The tan δmax exceeded 1.0 in a few samples, indicating fluid-like behavior (data not shown). In every sample tested, the G′ values at the start of the temperature sweep (
init) produced initial loss modulus values (tan δinit) between 0.2 and 0.7 (values <1.0 indicated solid-like behavior). The Tmax for the proteins ranged from 40 to 70°C. The G′ values at the end of the sweep (G′final) were also higher than the G″ values at the end, leading to tan δfinal values between 0.2 and 0.7. Most samples exhibited a transition region where G′ and G″ underwent a sharp increase, with G″ rising at a faster rate than G′ (). The storage modulus (G′) was low initially for EA (0.1 kPa) but became very high (731.3 kPa) at the final point. Other single protein model gels increased in viscoelasticity in the order WPI > CC > WG > SPI, indicating network formation (). The solid/liquid state measure (tan δ) showed FPI and SPI to be more solid-like while the others, particularly EA and WPI, were mostly liquid-like ().
The viscosity of CC gels (0.0079 Pa.s), and CC gels blended with other proteins () showed very small increase with WG (0.0086 Pa.s) and marked increase when blended with SPI (0.0693 Pa.s). The viscosity of CC gel was lowered by blending with WPI (0.0043 Pa.s), EA (0.005 Pa.s), and FPI (0.0063 Pa.s); the general effect of blending other proteins with CC on the model gel viscosity was loss in viscosity. Blending CC with the other proteins increased the flow index of CC except for FPI, which had no effects. The other protein combinations increased flow index values several fold: SPI, EA, WPI, and WG. An increase in flow behavior represents a thickening effect. The effect of blending other proteins on the flow behavior index of WPI was mostly negative except with WG which increased by 39%. Viscosity values of WPI gels (<15% solids) were significantly lower than those of EA, SPI, and WG; but higher than FPI (). When blended with EA, SPI and WG the viscosity value of WPI increased; only FPI suppressed WPI viscosity value. The case was different for the flow behavior indices of WPI blended with other proteins; only WG increased flow index, the other proteins reduced flow index.
The flow index increased only with EA and SPI, while viscosity increased significantly (P < 0.05) with SPI. WPI blends increased in consistency with EA, FPI, and SPI; WPI increased in viscosity with EA and SPI (). EA decreased in viscosity when blended with FPI, SPI, and WG. The viscosity of EA increased significantly (P < 0.05) with SPI. FPI blended with SPI increased in consistency and viscosity (). Two-protein model gels containing SPI with CC, EA, FPI, and WG all had G′init > 1.3 kPa; CC/WG was the only other two-protein combination with G′init > 0.45 kPa (). The two-protein samples of SPI with CC, EA, WG, and WPI had G′max > 20 kPa. FPI produced the lowest G′ values. The G′init and G′final values for FPI were the lowest of the six proteins, and the tan δinit value was the highest. The peak temperature (Tmax) for the two-protein mixtures was reduced and ranged from 33 to 62°C, compared to the single proteins which ranged from 40 to 70°C.
Two-way interaction of proteins in the viscoelastic models of CC model gels in showed marginal improvements in yield stress, reduction in consistency indices, increases in flow index, but no increases in viscosity, except with SPI, which increased significantly (P < 0.05) in viscosity (0.0693 Pa.s). Changes in viscoelastic properties were seen with SPI in initial G′init and G′final. No changes were observed from temperature at maximum storage modulus or in tan δ values. The combinations containing SPI and CC gave different results than those above: Tan δ values were steady at the start of the sweep, decreased sharply in the transition region (G′ rose at a faster rate than G″), and leveled off (). Samples containing SPI usually displayed the highest G′ values at the start of the temperature sweep and at the transition region. SPI had by far the largest G′init of the single-protein samples ().
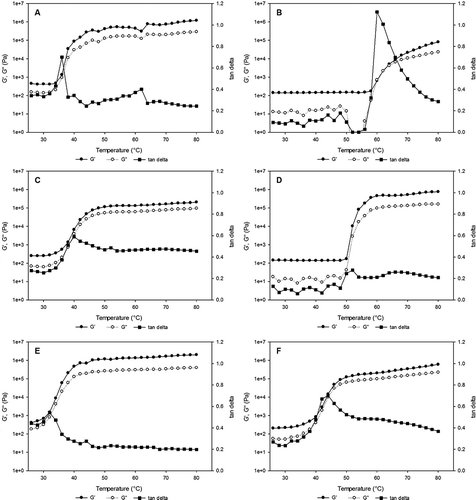
Figure 3 The effect of mixing other food proteins on the flow index of (a) CC-WPI, (b) EA-FPI, (c) CC/FPI, (d) WPI/EA, (e) CC/EA, and (f) WPI/FPI.
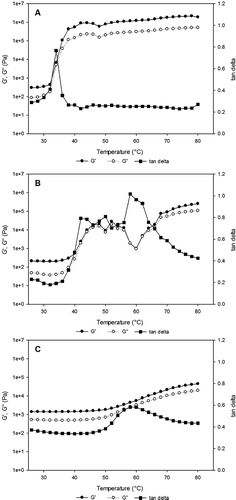
The three protein combinations were mostly weak gels (). The three-protein samples that had G′final > 1600 kPa contained both CC and WPI. The three-protein gels with G′final < 625 kPa and tan δfinal > 0.265 all contained FPI. The presence of FPI lowered the G′, G′final, and tan δfinal of samples containing both SPI and FPI (). Three-protein combination samples showed extended transition regions, exhibiting tan δ values in excess of 0.5 over a span of 30°C or more (data not shown). The CC/EA/FPI gels which had the lowest G′final of any protein combination, had tan δ > 0.8 from 56–70°C (), FPI-WG-WPI had tan δ > 0.95 from 46–66°C, and SPI-FPI-WPI had tan δ > 0.95 from 50–62°C. Typical transition patterns for three-protein combination are shown in . The combination of CC/EA/WPI had peak tan δ of 0.7 at 30°C. CC/FPI/WPI had two high tan δ transitions at 30°C (0.8) and 60°C (1.0) (). These two three-protein combinations were in contrast to FPI/SPI/WG which did not exhibit a transition region but had a peak tan δ of 0.6 (). The peak temperature (Tmax) for the proteins was reduced further and ranged from 33 to 61°C.
Figure 4 Temperature sweeps of three-protein combinations (a) CC, EA, and FPI, (b) CC, FPI, and WPI, and (c) FPI, SPI, and WG.
The steady shear flow behavior of the six proteins individually showed varying time dependencies over the 2700 s (). EA, SPI and WPI showed shear thickening behavior, with time, while CC and WG were shear thinning and FPI was time independent. The individual proteins showed time dependent changes in flow behavior at: WPI and EA at (600 s), SPI and WG (1200 s), and CC (2700 s). Of the five protein combinations with CC, only EA and FPI changed flow behavior time dependency (). CC/EA showed shear thickening at 600 s and CC/FPI showed shear thinning at 2700 s. The apparent viscosities of the CC mixtures, with the exception of CC/SPI, were frequently at or below the viscosity of CC alone (). Combining WPI with the other five proteins changed the flow behavior of WPI (). Although WPI and EA individually showed shear thickening behavior throughout 2700 s, WPI/EA blend showed time independence at 600 s, and shear thickening at 1200 s. WPI/CC, WPI/FPI, and WPI/WG were time independent. WPI/FPI was time independent up to 1200 s, but by 2700 s it was shear thinning. The apparent viscosities of the WPI mixtures were between the two individual protein values comprising the mix. The most interesting combination appears to be WPI/SPI. Both WPI and SPI individually shear thickened over time (2700 s), but when combined, they became shear thinning ().
Table 5 Steady shear behavior of CC and WPI on the flow behavior of CC and other protein blends. Viscosity temperature sweep data for single-protein samples of CC, WPI, EA, FPI, WG, and SPI
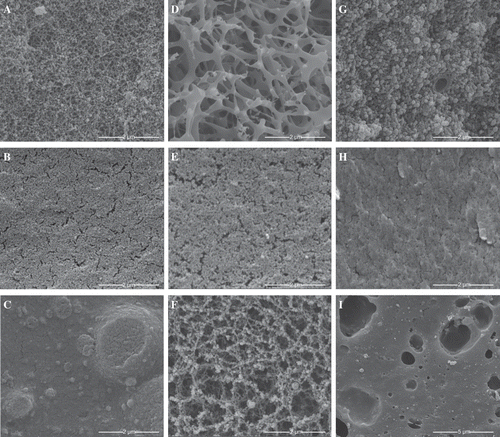
The SEM of the six-protein model gels are displayed . CC () showed a network-like structure with granular modules displaying a more open surface and WPI appear granular. WPI () appeared grainy and fused with multiple fissures. CC and WPI combined showed prominent fused granular with multiple small aggregates, and a couple of aggregates greater than 500 nm and one aggregate greater than 2.0 μm (). FPI was the most different, with an open fibrous interconnected network () with uniformly spaced network bridges 200 to 400 nm thick. When FPI was combined with CC (), the resulting gel became uniformly granular aggregates 100 to 300 nm clusters. EA was similar to WPI in granularity (); when combined with WPI, EA/WPI formed a meat-like striated texture with surface slabs (). SPI displayed a mesh-like network with granular nodes (). WG exhibited cheese-like features with vacuoles (). SPI shows chains of granules surrounded by a surface similar to those of EA or WG. CC, SPI, and WG aggregated between 40 and 60°C, as evidenced by hundredfold increase in storage modulus (G′). EA and WPI aggregated between 55 and 65°C, with G′ increasing by four orders of magnitude; FPI was basically unchanged. The G′ values of CC, SPI, and WG were greater than the G″ values throughout the temperature sweeps, signifying that elastic behavior predominated. G″ was greater than G′ for FPI, and before aggregation of EA and WPI, representative of viscous behavior. Scanning electron microscopic imaging revealed changes during heating of CC, EA, and WPI, indicating differences at the microstructural level; the other proteins did not change (data not shown). The results show that these solutions aggregate differently but predictably during heating.
Figure 5 Scanning electron micrographs of (a) CC, (b) WPI, (c) CC/WPI, (e) FPI, (e) EA, (f) SPI, (g) CC/FPI, (h) WPI/EA, and (i) WG.
In sum, the proteins were significantly different in their consistency, flow behavior, and viscosity (). In combinations, CC/EA, CC/SPI, WPI/EA, and WPI/SPI were significantly different in consistency (P < 0.05) from the others. In flow behavior index CC/WG and WPI/EA were significantly different from the others, while the viscosity of CC/SPI and WPI/SPI were significantly different from the others. These combinations offer the best mixed properties.
Table 6 Statistical analysis of mixed protein of CC and WPI on the flow behavior of CC and other protein blends. Viscosity temperature sweep data for single-protein samples of CC, WPI, EA, FPI, WG, and SPI
DISCUSSION
There is a definite relationship between the solubility of whey protein concentrates and its physical properties, which depends on the concentration, pH, temperature, and other factors.[Citation17] Solubility of proteins especially in mixed systems, is of primary importance because it affects all subsequent functionality such as gelation and viscoelastic properties.[Citation18] Unheated protein systems were high in solubility: 95 to 100% for WPI, CC, and EA; solubility decreased with heating; loss in solubility patterns were reported for heated protein systems.[Citation19] In one dry heated protein system (105 to 145°C), the heated protein solubilities determined were less than 20% in a 1% sodium dodecyl sulfate plus 1% beta-mercaptoethanol, except for casein. The order of protein products were: whey protein, milk powder, beta-lactoglobulin > EA, bovine serum albumin > soy isolate, sodium caseinate > gluten > gelatin.[Citation19]
The viscosity of a heated mixed protein system may depend on the nature of the gel system formed. For instance, in a casein-whey protein blend, heterogeneous aggregates or particulates networks resulted in low viscosity gels.[Citation20] In unheated acid-induced milk gels from mixtures of casein and whey proteins, casein contributes to gel formation, but when heated the presence of whey protein fraction beta-lactoglobulin makes the gel much stiffer, minimizing syneresis. After heating the whey protein aggregates have large impact on the structure and mechanical properties, particularly the effect of pH and temperature.[Citation21]
Though protein solubility is a function of many factors, the state and source or type of protein is intrinsically important.[Citation22] Anandharamakrishnan et al.[Citation23] suggested that denatured state of proteins is not enough to cause loss in solubility, but aggregation must occur.[Citation23] However, aggregation is heavily influenced by the pH, surface charges, the net charge of the system, and the electrostatic repulsive forces between the molecules.[Citation24] Protein solubilities are sensitive to salt and hydrogen ion concentration.[Citation25] The net charges of the proteins may produce attraction or repulsion at the same pH. As a generalized rule, proteins are more soluble in low acids or high alkali,[Citation26] and rheological behavior of products are affected by the concentration of soluble proteins, for example yogurt rheology was affected by the solubility of WPI.[Citation17] It was postulated that EA coagulates forming a solid-like network which expels the rest of the protein serum.[Citation27] FPI gels form at ambient at pH 8.5 to 11; the strength increases with concentration and temperature.[Citation28] In mixed protein systems, for example the mixture of gelatin and EA, the compatibility of the two proteins increased the gelling properties.[Citation29]
Flow Behavior
The viscosity and gelling effects of different types of proteins depends on multiple factors such as the concentration, amount of water present, ionic strength, pH, and temperature;[Citation30] as such, it would be surprising if viscosity can be related strongly to only one factor. Dumetz et al.[Citation31] stated that protein gels are hard to reproduce disordered events with short-range temperature-dependent attractive interactions.[Citation31] These findings agree with those of Ren et al.[Citation32] who showed that for soy proteins, the effect of protein concentration and temperature to be shear thinning in soy protein and micellar casein system, which increased apparent yield stress with increasing viscosity.[Citation32] WPI solutions behaved as Newtonian fluids at concentration <10%; 10 to 50% non-Newtonian.[Citation17]
The majority of the foods do not show Newtonian flow behavior. For non-Newtonian liquid the viscosity is a function of the rate of shear, meaning that for an applied rate of shear the corresponding shear stress remains constant provided the rate of shear has not changed, however, this is not true for many fluids particularly for multiphase mixtures.[Citation3] In classical models for multiple emulsions, the relative viscosities depends volume fraction of the total dispersed phase, and the ratios of the primary constituents.[Citation33] In a mixed protein system combining gelatin and WPI, unheated slurries (ambient temperature) was very weak, but increased concentration and with heating to 85°C.[Citation34]
Shear thinning was prevalent in soy protein-micellar casein systems; for soy proteins temperature and concentration increased viscosity, and apparent yield stress increased. For negatively charged mixtures, the stability decreased with concentration and temperature. In soy protein mixtures, gelation occurs at concentration greater than 7.5% and temperature above 80°C. Heat treatment did not significantly affect the viscosity of micellar casein; increasing concentration increased apparent viscosity and apparent yield stress. Most casein systems exhibited Newtonian flow.[Citation35] Interactions between soy proteins in heated gels indicates strong interaction between beta-conglycinin (7S) and soluble fractions of soybean at pH 4.5 at temperatures ranging from 60 to 95°C prevents the formation of strong networks with other proteins of soy glycinin (11S). Tang et al.[Citation30] showed that the concentration, temperature, and pH of whey protein concentrate solutions affected the flow and other physical functions.[Citation30]
Mixed soy protein and micellar casein mimicked the behavior of soy protein, with most rheological properties in-between soy protein and micellar casein networks. Mixtures greater than 7.5 to 12.5% concentration treated at over 90°C exhibited phase separation, low viscosity and low yield stress, while concentrations greater than 15% treated at 90°C showed protein aggregation and incipient network formation.[Citation35] Incompatibility is common for proteins from different classes such as casein and soy globulins, or conformations (native or denatured).[Citation36] Miscibility is an important factor that can impede interaction of casein micelles and soy proteins; miscibility depends on concentration, temperature, and molecular weight.[Citation37]
Steady Shear
Time dependent flow behavior can be investigated as a function of time throughout tests where both the degree of shear load and the measuring temperature are preset as constant values. Foods such as suspensions, emulsions, and foams are time dependent fluids and show thixotropy and rheopexy behavior.[Citation38] On the contrary, rheopexy means an increase in the structural strength during the load phase and a more or less rapid but complete decomposition of the increased structural strength during the subsequent period of rest.[Citation39] In other words, when the viscosity of a fluid slowly increases with time at a constant shear stress or shear rate, the recovery of the original viscosity is achieved over a period of time after the cessation of the applied stress.[Citation3] Twenty percent solutions of CC, EA, FPI, SPI, WG, and WPI were examined during heating by small amplitude oscillatory shear measurements, which provided an indication of protein behavior. CC, SPI, and WG aggregated between 40 and 60°C, as evidenced by hundredfold increases in storage modulus (G′). EA and WPI aggregated between 55 and 65°C, with G′ increasing by four orders of magnitude; FPI was basically unchanged. The G′ values of CC, SPI, and WG were greater than the G″ values throughout the temperature sweeps, signifying that elastic behavior predominated. G″ was greater than G′ for FPI, and before aggregation of EA and WPI, representative of viscous behavior. Possible mechanism for increased modulus may be due to partial denaturation resulting in aggregated entanglements.
Viscoelasticity
Proteins may be assumed to be an entangled network of polymer coils, with G′ reflecting the resistance to deformation; the stronger the network, the higher the G′ value.[Citation5] When the values of G′ and G″ are close to each other (tan δ around 1.0), there is sufficient time for an entangled network to disentangle and rearrange within the oscillation period, so that energy storage and energy dissipation are close to equal.[Citation7] In every sample (except SPI), there was a region in which tan δ was elevated, which indicates that heat-induced gelation of proteins were taking place.
The temperature sweep of SPI was similar to those obtained by Ahmed et al.[Citation7] of 15% SPI dispersed in water, with steadily increasing values of G′ in the 10–100 kPa range.[Citation7] They determined that the 15% concentration was enough to form a strong gel, by the protein molecules coming into contact, and interacting in a non-Newtonian manner. Strong network kinetics follows a second-order model of partially and fully unfolded protein aggregate molecules found at elevated temperatures in the collision of two proteins.[Citation2]
Yoon et al.[Citation40] reported a second-order kinetics for temperature sweeps of surimi.[Citation40] The brief decrease of G′ at 38°C in the FPI sweep () was observed in the surimi at slightly higher temperatures, and was attributed to unfolding of myosin and disruption of protein networks that were starting to form, resulting in a dip in the value for G′.[Citation40] The low values of G′ throughout the temperature sweep of FPI indicate that this protein does not aggregate easily, resulting in a weak gel.
The presence of CC in the samples lowered the point at which G′ and G″ started to increase rapidly. CC is not heat stable, with aggregation starting to occur at 45°C.[Citation41] The network patterns for WG ( and ) are consistent with those obtained by Hayta and Schofield,[Citation5] who also observed a decrease in G′ until about 50°C, and an increase thereafter (). The presence of wheat flour and glycerol in the samples apparently caused differences between the WPI and EA results, and those found by others. The G′ and G″ values for WPI started to increase rapidly at 48°C (), which was lower than the 80°C observed by Li et al. (2006) [Citation42] for 15% WPI solutions heated at 1.0°C/min. Moreover, Tang et al.[Citation30] observed no increase in G′ in 12% WPI solutions heated at 1.0°C/min, and their G′ values for 12% egg white solutions started to increase rapidly at 65°C, which was higher than the 52°C found for EA in the present study.[Citation30]
In contrast, Ngarize et al.[Citation14] did observe increases in G′ for 15% solutions of EA and WPI heated from 20 to 90°C at 2°C/min. Their G′ values were 60 times lower than those in the present study, though the ratio of G′final values for EA and WPI, 1.17, is almost identical to the 1.19 ratio found here. Research on protein blends (the whey proteins α-lactalbumin and β-lactoglobulin) suggests that their rheological properties may depend solely on total protein concentration, consistent with a non-specific mixed network between different types of protein.[Citation8]
A drop in tan δ values was observed in the SPI/CC blends implies antagonistic effect not seen in the other blends. Some blends exhibited a small increase in tan δ at the temperature expected for the components, and a much larger increase at a lower temperature. For instance, the Tmax values for EA and WG were 53.1 and 51.1°C, respectively, but the EA/WG blend displayed a small tan δ peak at 53°C and a large tan δ peak at 38°C. In contrast, the tan δ curves of the three samples with extended transition regions, FPI/WG, FPI/WG/WPI, and SPI/FPI/WPI (), appeared to be composites of the tan δ curves of their components, and bore a resemblance to unresolved chromatographic peaks. Various combinations of proteins will therefore produce strikingly different behavior upon heating, which the food industry can use to their advantage when developing new products with protein-based ingredients.
The structural forms of proteins vary depending on concentration, temperature, pH, salt concentration, and solvents; the type of aggregates formed either spherical or linear tendril-like aggregates depends on the overall charge of the solution. The assembly structure and size of aggregates affects microscopic and macroscopic interactions; a protein assembly forms rod-like fibrils with fine branches.[Citation1] Heat denatured whey proteins combined with casein to increase gel strength; pH exerted a great influence on complex formation even at ambient; increased gel strength from casein aggregation.[Citation14]
Matringe et al.[Citation15] reported synergistic increases in heat gelation properties of skim milk and EA, and antagonistic effect on foaming.[Citation15] The synergies that increase network strength are formed by heat-induced large aggregates.[Citation10] Research on protein blends (the whey proteins α-lactalbumin and β-lactoglobulin) suggests that their rheological properties may depend solely on total protein concentration, consistent with a non-specific mixed network between different types of protein.[Citation8]
Other materials such as starch may complex synergistically with proteins resulting in strong gels, compared to either polymer alone;[Citation43] the strength may come from compatible polymers or one acting as active filler; example would be disrupted starch in an WPI/Starch system.[Citation44] In mixed corn starch and WPI system gels, the corn starch fractions were more influenced by temperature and whey protein was more influenced by pH. Whey proteins formed transient network structures. Corn starch/WPI mixed gels formed networks at 15% total solids at pH 9. In higher solids gels, the degree of elasticity decreased with WPI content. Unique chemical compatibility existed at pH 9 combining the best features of elasticity of corn starch and internal stress dissipation of WPI.[Citation43]
SEM
Scanning electron microscopic imaging revealed changes during heating of CC, EA, and WPI, indicating differences at the microstructural level; the other proteins did not change. The results show that these solutions aggregate differently but predictably during heating. Knowledge of viscoelastic properties and microstructure will allow protein-enriched foods to be tailored toward specific functional properties. Altered conformational structures due to heat influence solubility of proteins.[Citation45] Solubility and surface hydrophobicity can be used as predictors of surface properties of proteins.[Citation46] There is a definite relationship of solubility of whey protein concentrates and physical properties, which depends on the concentration, pH, temperature, and other factors.[Citation17] Solubility of proteins, especially in mixed systems, is of primary importance because it affects all subsequent functionality such as gelation and viscoelastic properties.[Citation18]
In unheated acid-induced milk gels from mixtures of casein and whey proteins, casein contributes to gel formation, but when heated the presence of whey protein fraction beta-lactoglobulin makes the gel much stiffer minimizing syneresis. After heating the whey protein aggregates have large impact on the structure and mechanical properties, particularly the effect of pH and temperature on starch/whey protein mixed gels.[Citation43] Structural aggregates of heat-induced WPI are fractal-like network structures;[Citation47] microstructural differences can be observed in pore size, strand thickness, fine stranded linear filamentous association, and randomly cross-linked fibrils.[Citation48,Citation49] Maltais et al.[Citation50] showed through SEM and transmission electron microscopy that the filamentous and particulate networks in gels were related to the gel strength.[Citation50] To contrast the nature of gels, in soy protein cold-set hydrogels, particulate gel cells were more elastic while filamentous gels was more viscous.[Citation50]
CONCLUSIONS
In viscoelastic studies of protein, wheat flour, and glycerol model gels, it is evident that the proteins interacted, and that the protein-protein interactions affected and changed the flow behavior of some of the protein gels, both in time dependence and apparent viscosity measures. An understanding of the precise mechanism of interactions may aid in predicting the behavior of mixed food proteins in complex products. Flow of different proteins in starch and glycerol formulations was mostly shear thinning except for WG and combinations of EA, which were shear thickening. Relative viscosity was in decreasing order SPI, EA, WG, CC, WPI, and FPI. Shear thickening proteins may be combined with the shear thinning ones to increase their consistency. There was a strong evidence of protein-protein interaction which in some cases enhanced viscous properties. Overall, the mixed protein viscosities increased over individual protein viscosity values, and mixed protein flow indices were not affected except for a few cases where they increased over both individual values and a few that decreased below both individual values. Because multiple protein gels are complex systems, many aspects of their flow behavior must be studied to fully understand what effects shear, time, and temperature have on the gels. Temperature sweeps of protein blends revealed that most displayed a peak tan δ value as the samples aggregated. The strength of the gels formed varied greatly, with SPI producing high G′ values and FPI leading to low values. CC lowered the temperature at which G′ and G″ started to sharply increase. Some blends, such as EA-WG and those containing SPI-CC, gave results that indicate a synergistic effect.
ACKNOWLEDGEMENT
The help of Kareem Dennis, Eric Tilman, Dr. Peter Cooke, and Guoping Bao with the microscopy, and John Phillips with statistics, is appreciated.
Notes
This article not subject to United States copyright law.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/ljfp
REFERENCES
- van der Linden , E. and Venema , P. 2007 . Self-assembly and aggregation of proteins . Current Opinion in Colloid & Interface Science , 12 : 158 – 165 .
- Richardson , R.K. and Ross-Murphy , S.B. 1981 . Mechanical properties of globular proteins gels: 1 . Incipient gelation behaviour. International Journal of Biological Macromolecules , 3 : 315 – 322 .
- Tabilo-Munizaga , G. and Barbosa-Canovas , G.V. 2005 . Rheology for the food industry . Journal of Food Engineering , 67 : 147 – 156 .
- Roesch , R.R. and Corredig , M. 2005 . Heat-induced soy-whey proteins interactions: Formation of soluble and insoluble protein complexes . Journal of Agricultural and Food Chemistry , 53 : 3476 – 3482 .
- Hayta , M. and Schofield , D. 2005 . Dynamic rheological behavior of wheat glutens during heating . Journal of the Science of Food and Agriculture , 85 : 1992 – 1998 .
- Beveridge , T. , Jones , L. and Tung , M.A. 1984 . Progel and gel formation and reversibility of gelation of whey, soybean, and albumin protein gels . Journal of Agricultural and Food Chemistry , 32 : 307 – 313 .
- Ahmed , J. , Ramaswamy , H.S. and Alli , I. 2006 . Thermorheological characteristics of soybean protein isolate . Journal of Food Science , 71 : 158 – 163 .
- Kavanagh , G.M. , Clark , A.H. , Gosal , W.S. and Ross-Murphy , S.B. 2000 . Heat-induced gelation of β-lactoglobulin/α-lactalbumin blends at pH 3 and pH 7 . Macromolecules , 33 : 7029 – 7037 .
- Hines , M.E. and Foegeding , E.A. 1993 . Interactions of α-lactalbumin and bovine serum albumin with β-lactoglobulin in thermally induced gelation . Journal of Agricultural and Food Chemistry , 41 : 341 – 346 .
- Were , L. , Hettiarachchy , N.S. and Coleman , M. 1999 . Properties of cysteine-added soy protein-wheat gluten films . Journal Food Science , 64 : 514 – 518 .
- Shimada , K. and Cheftel , J.C. 1988 . Texture characteristics, protein solubility, and sulfhydryl group/disulfide bond contents of heat-induced gels of whey protein isolate . Journal of Agricultural and Food Chemistry , 36 : 1018 – 1024 .
- Baier , S.K. and McClements , D.J. 2005 . Influence of co-solvent systems on the gelation mechanism of globular protein: Thermodynamic, kinetic, and structural aspects of globular protein gelation . Comprehensive Reviews in Food Science , 4 : 43 – 54 .
- O'Kennedy , B.T. and Kelly , P.M. 2000 . Evaluation of milk protein interactions during acid gelation using a simulated yoghurt model . Milchwissenschaft , 55 : 187 – 190 .
- Ngarize , S. , Adams , A. and Howekk , N.K. 2004 . Studies on egg albumin and whey protein interactions by FT-Raman spectroscopy and rheology . Food Hydrocolloids , 18 : 49 – 59 .
- Matringe , E. , Tan , Phan , Luu , R. and Lorient , D. 1999 . Functional properties of milk-egg mixtures . Journal of Food Science , 64 : 787 – 791 .
- Tung , M. 1978 . Rheology of protein dispersions . Journal of Texture Studies , 9 : 3 – 31 .
- Patocka , G. , Cervenkova , R. , Narine , S. and Jelen , P. 2006 . Rheological behavior of dairy products as affected by soluble whey protein isolate . International Dairy Journal , 16 : 399 – 405 .
- Pelegrine , D.H.G. and Gasparetto , C.A. 2005 . Whey proteins solubility as function of temperature and pH . LWT-Food Science and Technology , 38 : 77 – 88 .
- Mohammed , Z.H. , Hill , S.E. and Mitchell , J.R. 2000 . Covalent crosslinking in heated protein systems . Journal of Food Science , 65 : 221 – 226 .
- Schorsch , C. , Wilkins , D.K. , Jones , M.G. and Norton , I.T. 2001 . Gelation of casein-whey mixtures: Effects of heating whey proteins alone or in the presence of casein micelles . Journal of Dairy Research , 68 : 471 – 481 .
- van Vliet , T. , Lakemond , C.M.M. and Visschers , R.W. 2004 . Rheology and structure of milk protein gels . Current Opinion in Colloid & Interface Science , 9 : 298 – 304 .
- Pace , C.N. , Trevino , S. , Prabhakaran , E. and Scholtz , M. 2004 . Protein structure, stability, and solubility in water and other solvents . Philosophical Transactions of the Royal Society B: Biological , 359 : 1225 – 1235 .
- Anandharamakrishnan , C. , Rielly , C.D. and Stapley , A.G.F. 2008 . Loss of solubility of ∞-lactalbumin and β-lactoglobulin during the spray drying of whey proteins . LWT-Food Science and Technology , 41 : 270 – 277 .
- Roth , C.M. , Neal , B.L. and Lenhoff . 1996 . A.M. Van der Waals interactions involving proteins . Biophysical Journal , 70 : 977 – 987 .
- Jenkins , W.T. 1998 . Three solutions of the protein solubility problem . Protein Science , 7 : 376 – 382 .
- Pelegrine , D.H.G. , Santos , Moraes and Gomes , M.T. 2008 . Whey proteins solubility curves at several temperature values . Ciencia e Natura, Universidade Federal de Santa Maria, UFSM , 30 : 17 – 25 .
- Campo-Deano , L. and Tovar , C. 2009 . The effect of egg albumen on the viscosity of crab sticks made from Alaska Pollock and Pacific Surimi . Food Hydrocolloids , 23 : 1641 – 1646 .
- Brenner , T. , Nicolai , T. and Johannsson , R. 2009 . Rheology of thermo-reversible fish protein isolate gels . Food Research International , 42 : 915 – 924 .
- Badii , F. and Fish gelatin , Howell, N. 2006 . Gelling properties and interaction with egg albumin proteins . Food Hydrocolloids , 20 : 630 – 640 .
- Tang , Q. , Munro , P.A. and McCarthy , O.J. 1993 . Rheology of whey protein concentrate solutions as a function of concentration, temperature, pH and salt concentrations . Journal of Dairy Research , 60 : 349 – 361 .
- Dumetz , A.C. , Chockla , A.M. , Kaler , E.W. and Lenhoff , A.M. 2008 . Protein phase behavior in aqueous solutions: Crystallization, liquid-luquid phase separation, gels, and aggregates . Biophysical Journal , 94 : 570 – 583 .
- Ren , C. , Tang , L. , Zhang , M. and Guo , S. 2009 . Interactions between whey soybean protein (WSP) and β-conglycinin (7S) during the formation of protein particles at elevated temperatures . Food Hydrocolloids , 23 : 936 – 941 .
- Pal , R. 2008 . Viscosity models for multiple emulsions . Food Hydrocolloids , 22 : 428 – 438 .
- Fitzsimons , S.M. , Mulvihill , D.M. and Morris , E.R. 2008 . Segregative interactions between gelatin and polymerized whey protein . Food Hydrocolloids , 22 : 485 – 491 .
- Beliciu , C.M. and Moraru , C.I. 2011 . The effect of protein concentration and heat treatment temperature on micellar casein—soy protein mixtures . Food Hydrocolloids , 25 : 1448 – 1460 .
- Polyakov , V.I. and Ya , Grinberg, V. 1997 . Tolstoguzov, V.B. Thermodynamic incompatibility of proteins. Food Hydrocolloids , 11 : 171 – 180 .
- Moraru , C.I. , Lee , T.C. , Karwe , M.V. and Kokini , J.L. 2002 . Phase behavior of a meat—starch extrudates illustrated on a state diagram . Journal Food Science , 67 : 3026 – 3032 .
- Abu-Jdayil , B. 2003 . Modelling the time-dependent rheological behavior of semisolid foodstuffs . Journal of Food Engineering , 57 : 97 – 102 .
- Razavi , S.M.A. and Karazhiyan , H. 2009 . Flow properties and thixotropy of selected hydrocolloids: Experimental and modeling studies . Food Hydrocolloids , 23 : 908 – 912 .
- Yoon , W.B. , Gunasekaran , S. and Park , J.W. 2004 . Characterization of thermo-rheological behavior of Alaska pollock and Pacific whiting surimi . Journal of Food Science , 69 : 338 – 343 .
- Singh , H. 2002 . “ Encyclopedia of Dairy Sciences ” . In Milk proteins/Functional properties , Edited by: Roginski , H. , Fuquay , J.W. and Fox , P.F. 1976 – 1982 . New York , NY : Academic Press .
- Li , J. , Ould Eleya , M.M. and Gunasekaran , S. 2006 . Gelatin of whey protein and xanthan mixture; effect of heating rate theological properties . Food Hydrocolloids , 26 : 678 – 686 .
- Shim , J. and Mulvaney , S.J. 2001 . Effect of heating temperature, pH, concentration, and starch /whey protein ration on the viscoelastic properties of corn starch/whey protein mixed gels . Journal of the Science of Food and Agriculture , 81 : 706 – 717 .
- Clark , A.H. and Ross-Murphy , S.B. 1987 . Structural and mechanical properties of biopolymer gels . Advances in Polymer Science , 83 : 57 – 192 .
- de Wit , J.N. and Klarenbeek , G. 1984 . Effects of various heat treatments on structure and solubility of whey proteins . Journal of Dairy Science , 67 : 2701 – 2710 .
- Kilara , A. and Mangino , M.E. 1991 . Relationship of solubility of whey protein concentrates to thermal properties determined by differential scanning calorimetry . Journal of Dairy Science , 56 : 1448 – 1449 .
- Panick , G. , Malessa , R. and Winter , R. 1999 . Differences between the pressure- and temperature-induced denaturation and aggregation of beta-lactoglobulin A, B, and AB monitored by FT-IR spectroscopy and small-angle X-ray scattering . Biochemistry , 38 : 6512 – 6519 .
- Ziegler , G.R. and Foegeding , E.A. 1990 . The gelation of proteins . Advances in Food Nutrition Research , 34 : 203 – 298 .
- Dumay , E.M. , Kalichevsky , M.T. and Cheftel , J.C. 1998 . Characteristics of pressure-induced gels of beta-lactoglobulin at various times after pressure release . LWT-Food Science and Technology , 31 : 10 – 19 .
- Maltais , A. , Romondetto , G.E. and Subirade , M. 2009 . Soy protein cold-set hydrogels as controlled delivery devices for nutraceutical compounds . Food Hydrocolloids , 23 : 1647 – 1653 .