Abstract
β-Casomorphins are a group of opioid peptides released during gastrointestinal digestion or food processing from the β-casein of milk protein. Consequently, milk can be divided into A1 and A2 “like” groups depending upon the presence or absence of proline or histidine at the 67th position of β-casein. A1 “like” milk is postulated to be a source of BCM-7 as histidine allows the cleavage at this position, while A2 “like” milk has proline that resists the hydrolysis. On one hand, BCM-7 has been implicated as a risk factor for cardiovascular diseases, type I diabetes, and neurological disorders. On the other hand, various physiological effects of these peptides have also been documented, i.e., secretion of mucus, increased activity of superoxide dismutase and catalase, increased levels of prolactin, and analgesic role. In addition, many evidences correlate these peptides with various immunological functions, such as development of innate immunity, lymphocyte proliferation and cellular immunity, role in autoimmune diseases, histamine release, and allergy. In conclusion, the role of β-casomorphins in physiological functions remains controversial and more research with improved diagnostic techniques is needed to unravel the mechanism and study physiological functions of β-casomorphins. Thus, health-related aspects of β-casomorphins (positive, negative, and immunological impacts) have been comprehensively reviewed in this article.
Keywords:
INTRODUCTION
Proteins are a very diverse family of large organic compounds involved in many important biological processes. Following their enzymatic hydrolysis during digestion or food processing, some peptides released remain physiologically active, i.e., bioactive, and modulate gut secretions and motility, blood pressure and have antithrombotic, antioxidant, antimicrobial, and immunomodulatory activities. On one hand, the discoveries of bioactive peptides with potential health benefits have been the subject of growing commercial interest in the context of health-promoting functional foods. While on the other, public health professionals, consumers, food producers, and food processors are becoming increasingly aware of the rapidly expanding epidemiological evidences that link the prevalence of diseases, such as cardiovascular disease, food allergy, obesity, hypertension, diabetes, and even cancer to dietary factors affecting human health adversely.[Citation1] This misperception regarding positive and negative effects of functional foods is further aggravated due to the lack of global policy for their use to various physiological threats.[Citation2] Among these, β-casomorphins (BCMs), peptide sequences present in the milk protein β-casein, have been suggested to contribute to an increased risk of cardiovascular diseases,[Citation3] type I diabetes,[Citation4] and sudden infant death syndrome.[Citation5] There are also controversial reports available that show a positive impact of these peptides like their protective role against hyperglycemia and free radical-mediated oxidative stress,[Citation6] mucin gene expression and mucus secretion,[Citation7] increased prolactin levels,[Citation8] and aid in gastric motility by expression of gastrin.[Citation9] Thus, on one hand BCMs are considered devils in the milk,[Citation10] while on the other hand, some beneficial effects of BCMs have also been documented.
β-CASOMORPHINS (BCMs)
The word casomorphin has been derived from the word caso, which means casein and the morphine from Morphus, the Greek God of sleep.[Citation11] These peptides are derived from the β-casein of milk and demonstrate opioid and pharmacological activities. These peptides bind μ-receptors, which are located in the central nervous system, gastrointestinal tract, and some immune cells.[Citation12] BCMs are 4 to 11 amino acid peptides encrypted in an inactive form that are released during either in-vivo or in-vitro digestion. Among them, the most active are BCM-7 and BCM-5, which represent fragments f60–66 and f60–64 of β-casein, respectively.[Citation13]
GENETIC VARIANTS OF β-CASEIN
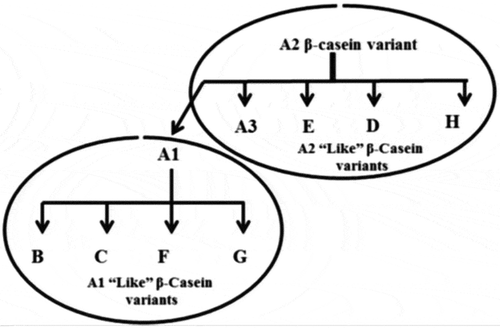
β-Casein has 209 amino acids and there are at least 12 variants of this protein that differ at different amino acid positions.[Citation14] As a result of a point mutation on exon VII of bovine β-casein gene on the sixth chromosome, a conversion from cytosine to adenine leads to replacement of proline (codon; CCT) by histidine (codon; CAT) at position 67.[Citation15] The most common are A1 and A2 forms of β-casein based on the presence of amino acid histidine or proline respectively at position 67 of β-casein. A1 “like” milk involves β-casein with A1, B, C, F, and G alleles with the His67 at residue 67 (-Tyr60-Pro61-Phe62-Pro63-Gly64-Pro65-Ile66-His67-) but variants at other positions of amino acids. A2 “like” milk (-Tyr60-Pro61-Phe62-Pro63-Gly64-Pro65-Ile66-Pro67-) has β-casein with A2, A3, D, H1, H2, and I alleles with a proline residue at 67 but variants at other positions of amino acids as shown in .[Citation16,Citation17] There are many DNA-based techniques, such as single stranded conformational polymorphism (SSCP),[Citation18] Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP)[Citation19] allele-specific PCR (AS-PCR),[Citation17] real time PCR TaqMan,[Citation20] and PCR-amplification created restriction site (PCR-ACRS)[Citation21−Citation23] to screen these alleles.
RELEASE OF β-CASOMORPHINS
Milk
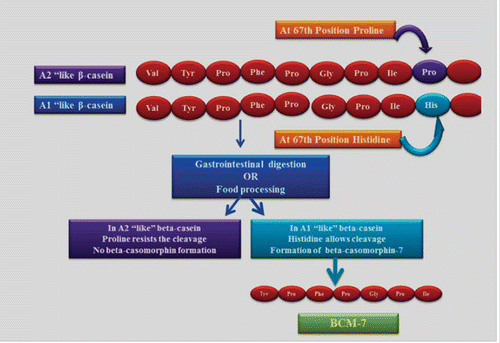
Based on the A1/A2 hypothesis, milk can be classified into two types, i.e., A1 and A2 “like” depending on the presence of proline or histidine at the 67th position of the β-casein protein. It may seem surprising but this tiny difference in protein is postulated to produce a major effect in terms of BCM release. A1 “like” milk, i.e., milk containing β-casein variants that has histidine at the 67th position of the β-casein allows the cleavage at this position by different gastrointestinal enzymes to release BCM-7. A2 “like” has β-casein with a proline at this position and hinders the cleavage at this position and hence BCM-7 is not formed as shown in . BCM-7 was released after simulated gastrointestinal digestion (SGID) by pepsin, pancreatic elastase, and leucine amino peptidase.[Citation24] Lotfi[Citation25] produced BCMs from bovine β-casein by mild acid hydrolysis (pH 2.5, 37°C) with pepsin (1:100) and trypsin. De Noni[Citation26] investigated the release of BCM-5 and BCM-7 during SGID of bovine β-casein variants (A1, A2, and B) from unprocessed milks using pepsin digestion at pH 2.0, 3.0, and 4.0 followed by hydrolysis with Corolase PPTM (mixture of pancreatic enzymes). It was found that B variant released the highest amount of BCM-7 (5–176 mmol/mol casein), followed by the A1 variant. BCM-7 was not released from variant A2 during any steps of the SGID process. De Noni and Stefano[Citation27] quantified BCM-7 levels after SGID with pepsin and Corolase PPTM in fermented milks (1.23 ± 0.04 mg/kg), powdered milk-derivatives (17.68 ± 0.12 mg/kg), cheeses (15.22 ± 0.13 mg/kg), and infant formulas (90.21 ± 0.03 mg/kg).
Infant Formulas (IFs)
Infant formula is a food that purports to be or is represented for special dietary use solely as a food for infants by reason of its simulation of human milk or its suitability as a complete or partial substitute for human milk. The most commonly used IFs contain purified cow’s milk whey and casein as protein sources. IFs contain β-caseins of either variant and have left a probability for release of BCMs from these infant diets. In this regard, Hernández-Ledesma et al.[Citation28] have recovered BCM-7 from IF when subjected to peptic hydrolysis at pH 3.5 followed by digestion with Corolase PPTM. In another study, the same authors did not find the presence of BCM-7 in an IF digested with pepsin (pH 3.5) and porcine pancreatin, however, BCM-9 was detected in the same IF.[Citation29] Jarmolowska et al.[Citation30] reported the presence of BCM-5 in peptide extracts of an IF and in its pepsin–trypsin hydrolysate. The amounts of BCM-5 in the IF extracts and its hydrolysate were 0.67 and 74.46 nmol/mL, respectively. However, no BCM-7 was found in the extracts either prior to or after SGID. De Noni[Citation26] did not find BCM-5 or BCM-7 in the peptic (pH 2.0 or 3.5) digests of milk-based IFs following SGID. These products contained both A1 and A2 variants of β-casein and it was only upon further digestion with Corolase PPTM (mixture of pancreatic enzymes) that BCM-7 levels ranging from 0.02 to 0.37 nmol/mL were detectable.
Fermented Milk
Fermented milk products, also known as cultured dairy products, are foods that have been fermented with lactic acid bacteria, such as Lactobacillus, Lactococcus, etc. Formation of BCMs from fermented milk products are unlikely, since lactic acid bacteria contain X-prolyl-diaminopeptidyl peptidase.[Citation31] These enzymes have specificity for proline residues while BCMs are also proline-rich peptides and can be degraded easily. However, many of these studies have been carried out on X-prolyl-diaminopeptidyl peptidase deficient bacteria and the release of BCMs from the fermented milk products was established.[Citation32−Citation34] BCM immunoreactive (irBCM) material was first identified in cow’s milk after incubation with lactic acid bacteria (LAB).[Citation35] Using Liquid Chromatography–Mass Spectrometry (LC-MS) Matar and Goulet[Citation36] reported formation of BCM-4 by Lactobacillus helveticus L89 from synthetic BCM-7 (37°C for 120 min) and pasteurized milk (65°C for 30 min). Schieber and Brückner[Citation32] have also found BCMs by HPLC-MS on storage of yogurt at 4°C for 3 weeks that was prepared from skimmed milk by fermentation (44°C for 3 h) using L. delbruekii ssp. bulgaricus Lb1466 strains. BCM-11 and BCM-4 were recovered from ultra-heat treatment (UHT) (142°C for 3–4 s) milk fermented with the probiotic Lactobacillus GG strain with SGID digestion of milk completed with pepsin and trypsin. These peptides were not present in the fermented milk prior to pepsin and trypsin digestion.[Citation33] However, Kahala et al.[Citation37] did not find BCM release from several Finnish fermented milk products, yogurts, produced with a mixed starter culture of L. bulgaricus and S. thermophilus. The reason may be due to the presence of only A2 milk, which is not a source of BCMs.
ABSORPTION OF BCMs
Generally, proteins are absorbed in the intestine in the form of amino acids and small peptides, i.e., up to tripeptides.[Citation38] Although active BCMs are of 5–7 amino acids long, their absorption and transport across the intestinal epithelial cells have been confirmed by different in-vivo and in-vitro models as shown in . Umbach et al.[Citation39] demonstrated the presence of irBCM-7 (precursor of BCM-7) in the plasma of newborn calves after milk intake. Singh et al.[Citation40] compared the level of irBCM-7 in plasma of 2- and 4-week-old pups and adult dogs after the intake of bovine casein-based formula. The authors speculated that the intestinal mucosa of the newborn is more permeable to the relatively large peptides due to their immature tight junction through which peptides cross, thereby escaping hydrolysis. Although human and bovine immunoreactive material irBCM was found in the blood of human infants,[Citation41] recently Wasilewska et al.[Citation42] detected BCM-5 in the blood of human infants. However, detection of irBCM-7 antibodies in plasma raises many doubts about cross reactivity of antibody with some other epitopes of antigen present in plasma that was not studied by these co-workers. Mahé et al.[Citation43] suggested that brush border peptidases seem to be the limiting step of morphiceptin transfer with dipeptidyl peptidase IV (DPP IV) enzyme playing a major role. Transepithelial transport of BCM-5 and BCM-7 across human intestinal Caco-2 cells was demonstrated,[Citation44−Citation46] but the relative flux of BCM-5 increased by treating the layer with an inhibitor of DPP IV, which has been implicated in the hydrolysis of the peptide.[Citation44]
Table 1 Absorption and transport of BCMs
PHYSIOLOGICAL SIGNIFICANCE OF BCMs
BCMs have been postulated to be implicated in many illnesses, including heart disease, type 1 diabetes, and sudden infant death syndrome.[Citation3−Citation5] Metaphorically, it is considered as ‘the devil in the milk’.[Citation10] However, controversial reports are available that link these peptides with physiological aspects that are beneficial to animals. But, all the information available is either based on the epidemiological data taken from humans or related to the animal trials mostly in vitro. Therefore, a deeper research is required to find out the exact mechanism.
Functional Importance of BCM-7
Mammalian opioidergic systems consist of opioid receptors and their ligands, the opioid peptides. Depending on their location, their physiological significance appears to be related to a considerable number of neuroendocrine regulatory functions. Opioid receptors are located in the nervous, endocrine, and immune systems as well as in the intestinal tract of the mammalian organism and can interact with their endogenous as well as with exogenous opioids.[Citation12] BCMs are μ-receptor agonists that have been reported to show functional significance in many respects as shown in . Trompette et al.[Citation7] suggested the role of BCM-7 in gut immunity by inducing a strong jejunal mucus secretion. Oral administration of BCM-7 to the diabetic group of rats increased plasma insulin level, decreased glucagon level, and elevated the activity of superoxide dismutase and catalase.[Citation6] BCM-7 has a protective role against hyperglycemia and free radical-mediated oxidative stress and inhibited NF-KB-iNOS-nitric oxide signal pathway in the pancreas of diabetic rats.[Citation47] Nedvidková et al.[Citation8] observed that BCM-7 increased the prolactin level in plasma after intraperitoneal injection, and this hormone is best known for its role in lactation and as a regulator of the immune system. An analgesic role of BCM-7 by giving intraperitoneal injection of this peptide to the rats was also described.[Citation48] The effects of intraperitoneal administration of the BCM-7 on learning were investigated in rats using T-maze, active and passive avoidance tests. The substance injected with the dose of 5 mg/kg, 5 min prior to training accelerated the acquisition of food-reinforced task and increased the number of correct trials in T-maze. The data obtained supported the idea that BCM-7 attenuated manifestations of defensive motivation.[Citation49] Modulation of gene expression of the regulatory peptides from G and D cells was also demonstrated and data from in situ hybridization studies indicated that BCM-7 affected gastrin gene expression indirectly by means of the paracrine action of somatostatin.[Citation9] Maslennikova et al.[Citation50] have found that BCM-7 was responsible for ex-vivo activation (DNA synthesis) of proliferative processes in the myocardium and ectodermal and endodermal epithelium of newborn rats. Orally administered BCM-7 has been demonstrated to influence postprandial metabolism by stimulating secretion of insulin.[Citation51] Feeding of BCM-7 remarkably enhanced the thickness of gastric antrum mucosa and accelerated the growth of intestinal villi with slight improvement in the development of ileum peyer’s patches during early weaning of a piglet. It also improved total protease activity of duodenal chyme.[Citation52] BCM-7 promoted growth of rats by influencing growth-related hormones and growth factor levels in rat serum. It up-regulated growth hormone receptor (GHR) mRNA expression in order to increase sensitivity of a growth hormone in rat liver.[Citation53]
Table 2 Beneficial effects of BCM-7 and -5
Functional Importance of BCM-5
BCM-5 is formed from BCM-7 by the exopeptidases and it has been found to show many positive impacts on physiological functions as shown in . BCMs were found to be liberated in the mammary gland, transferred to the blood, and then reached endogenous opioid receptors. In this way, BCMs were suggested to participate in the endocrinic regulation of pregnancy.[Citation54] The cardiovascular system in pregnant or lactating mammals was also found to be a target for BCM action. BCM-5 exerted a positive inotropic and antiarrhythmic effect and thus had a cardioprotective function.[Citation55] Panksepp et al.[Citation56] have found that administration of BCMs reliably reduced separation induced distress vocalizations (DVs) in young domestic chicks. BCM-5 was found to be more potent than either BCM-4 or BCM-7, but each duration of action was approximately half an hour. The effects of systemic administration of BCM-7 on spontaneous alternation behavior in the Y-maze (spatial short-term memory) and step-down-type passive avoidance response (non-spatial long-term memory) established that systemic administration of BCM-5 improved the disturbance of learning and memory that resulted from cholinergic dysfunction through central mediation involving μ1-opioid receptors.[Citation57] The effects of intracerebroventricular injection of bovine BCM-5 on step-down type passive avoidance tasks resulted in induction of amnesia with high dose and ameliorated scopolamine-induced amnesia with a low dose in mice.[Citation58]
Health Complications Linked with BCMs
Extensive evidence correlates BCMs with various adverse biological responses like type-1 diabetes mellitus;[Citation4] heart diseases;[Citation59] neurological disorders, such as autism and schizophrenia;[Citation60] and sudden infant death syndrome.[Citation5]
Diabetes Type-1
Epidemiological studies showed a significant association between the intake of A1 milk and the incidence of diabetes type-1. It is suggested that type 1 diabetes mellitus is an autoimmune disease and results from the progressive destruction of insulin secreting pancreatic β-cells by autoreactive T-lymphocytes and macrophages, leading to insulin deficiency. BCM may act as an adjuvant in the autoimmune reaction involved in destruction of β-cells in prediabetic subjects.[Citation4,Citation61] Researchers[Citation62,Citation63] have found increased antibodies production against β-casein in diabetes mellitus-I. A1 β-casein has been found to be diabetogenic for a non-obese diabetic mouse compared to A2 β-casein.[Citation64] Similarly, a patent (2003/0017250 A1) showed an increased production of antibodies IgG (1+3) against A1 β-casein relative to A2 β-casein.[Citation65] For diabetes mellitus-I, BCM-7 is the only player in the potential diabetogenic pathway affected by multiple chemical species. BCM-7 suppresses immune defense mechanisms by inhibiting lymphocyte proliferation.[Citation66] This generates an immune vulnerability (in the case of DM-I) to a certain class of enteroviruses that are still being researched as they may have potential key roles in the damage done to pancreatic β-cells.[Citation67] Through BCM-7, the compromised immune system is more vulnerable to all kinds of pathogenic infections. The strong correlation between BCM-7 and DM-I continues to point researchers in the direction of BCM-7 to study its potential impacts on helping to promote DM-I development.[Citation68]
Cardiovascular diseases
Ecological studies have linked the intake of BCM-7 with cardiovascular disease mortality.[Citation4] Chin-Dusting et al.[Citation69] have found a correlation between the consumption of A1 β-casein and increased risk for cardiovascular diseases in humans. A1 β-casein consumption has been found to cause hypercholesterolemia or atherosclerosis establishing its association with heart disease incidence in animal studies. The relationship was also calculated between the mortality rate from ischemic heart diseases and consumption of milk proteins and milk components.[Citation3] The rabbits fed with β-casein A1 milk had higher cholesterol levels and percent surface area of aorta covered by fatty streaks compared to A2 β-casein.[Citation70]
Psychomotor development
Russian scientists have shown that BCM-7 enters the blood of babies fed milk formula diets. Babies have quickly metabolized the BCM-7, while others were slow metabolizers. Babies whose BCM-7 levels in the blood remained high between feeds were at a higher risk of delayed psychomotor development.[Citation41]
Sudden Infant Death Syndrome (SIDS)
BCM-7 has been suspected as a risk factor for SIDS for more than 20 years. BCMs have been found in the brainstems of children who died from SIDS.[Citation71] Polish scientists[Citation42,Citation72] showed that babies who suffered acute life threatening events (ALTE) through apnea were characterized by circulating levels of BCM-7 that was three times higher than in normal children. These same children had DPP IV levels (the enzyme that degrades BCM-7) only 58 ± 3% of those in normal children. Though the babies are breast-fed, bovine BCM-7 was still found in the blood of the infants. This suggested that bovine BCM-7 was transferred from the mother’s stomach to her infant via human milk. Other work by this group has found bovine BCM-5 in the blood of breast-fed children.
IMMUNOLOGICAL PERSPECTIVE OF BCMs
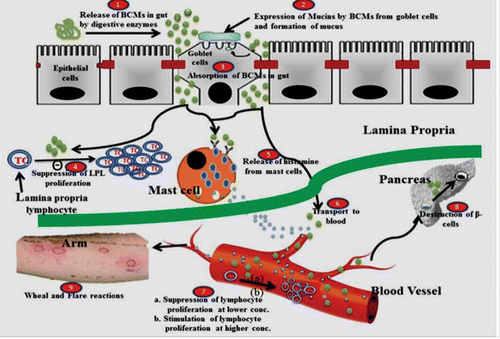
BCMs have also been suggested to show many immunological activities like chronic inflammatory responses, such as allergy, mucin production, lymphocyte proliferation, skin reactions; a proposed mechanism of action has been depicted in .
Mucus Secretion and Innate Immunity
Goblet cells are found along the epithelial lining of the intestinal and respiratory tracts and their sole function is the secretion of mucin, formation of mucus, and development of innate immunity. BCM-7 has been reported to stimulate mucin secretion in the rat jejunum through a neuron and opioid receptor activation pathway and, hence, contributes to innate immunity.[Citation7] The hypothesis that BCM-7 may also act directly on intestinal goblet cells was investigated in vitro in rat and human intestinal mucin-producing cells (DHE and HT29-MTX). In rat DHE cells, it was found that the peptide increased the expression of rat mucin rMuc2 and rMuc3. In human HT29-MTX cells, it increased MUC5AC mRNA levels and the secretion of mucin.[Citation73] A firmly bound mucous layer is extremely important for animal health as evidenced by studies of mice lacking the MUC2 gene, the major mucin of the colon mucus. These mice have bacteria in direct contact with the epithelial layer that results in inflammation and cancer development.[Citation74] Therefore, it was suggested that BCM-7 may contribute significantly to mucin production via a direct effect on intestinal goblet cells and the activation of μ-opioid receptors. Thus, dairy products containing BCM-7 may improve intestinal protection, support innate immunity, and may also have dietary and health applications.[Citation7] This bioactive peptide acts directly on the intestinal epithelium by activation of specific receptors. This interaction likely leads to improved intestinal protection and may have implications for improved intestinal strength.[Citation73] The effect of BCM-7, -6, and -4 on intestinal mucus secretion was investigated on isolated perfused rat jejunum in vitro. Luminal administration of BCM-7 but not BCM-6 and BCM-4 provoked a mucus discharge that was inhibited by naloxone, a specific opiate receptor antagonist. DAMGO (μ-receptor agonist) also evoked a potent mucus discharge. Thus, BCM-7 after absorption may modulate intestinal mucus discharge. Milk opioid-derived peptides may thus be involved in defense against noxious agents and may add up in innate immunity.[Citation7]
A hypothesis was also put forward by Bartley and McGlashan,[Citation75] based on the idea that BCM-7 causes mucin expression, mucus secretion, and release in the gut. They assumed that A1 milk may increase mucus production in the respiratory tract in the subjects who have increased intestinal permeability. Specifically, BCM-7 may act via μ-opioid receptors of goblet cells in the respiratory tract to up-regulate MUC5AC gene expression and increases mucus secretion and induction of symptoms like asthma or rhinitis. But before this correlation reaches the final conclusion, many prerequisites have to be taken under consideration like consumption of A1 as opposed to A2 milk by those subjects, and further, BCM-7 has to be absorbed in the gut, circulate in the blood, and should inflame other tissues. If it supports the hypothesis then only it can explain why some of the patients with asthma or rhinitis symptoms improve on a dairy-free diet and may oppose the concept of development of innate immunity. The effective concentration dose has also to be taken under consideration before reaching any conclusions.
Lymphocyte Proliferation and Cellular Immunity
After lymphocytes encounter an antigen or foreign protein, they begin a process by which they rapidly reproduce themselves called lymphocyte proliferation; the purpose is to increase the availability of these lymphocytes, which can then recognize and fight the invading antigens. BCMs modulate the human mucosal immune system and have an important role in immunity, which was investigated by incubating lamina propria lymphocyte (LPL) with these peptides in vitro. A significant suppressive effect was observed at concentrations as low as 10 mM and the effect was reversed by addition of naloxone (opiate receptor antagonist).[Citation51,Citation66] The immunomodulating role of BCM-7 and -10 on human peripheral blood lymphocytes proliferation was also examined by incubating the cells with these peptides and suppression of lymphocyte proliferation was observed at lower concentrations, but higher concentrations lead to stimulation.[Citation11] Further, BCMs have been found in the intestinal aspirates of human volunteers who had ingested bovine milk.[Citation76] Migliore-Samour and Jolles[Citation77] suggested that because of the high affinity of BCMs for μ-opiate receptors may exploit their endorphin-like activity on the development of T lymphocyte function and cellular immunity. Likewise, proline-rich polypeptide extracted from ovine colostrum had been previously reported as having both stimulatory and suppressive effects on the specific immune function. Since BCMs are also proline rich peptides, therefore, they may express immunoregulatory effects in cellular immunity.[Citation78] Further studies are required to decide the effective dose of BCMs with more emphasis on in vivo trials as these cells have an important role in cellular immunity.[Citation11]
Milk Allergy
Food allergy is an adverse immune response to a food protein and occurs when the body’s immune system fallaciously recognizes a protein as harmful. Some proteins or peptides are resistant to digestion and stimulate B-cells to produce IgE antibodies. These antibodies bind with receptors on mast cells or basophils and their crosslinking with the same protein (antigen) causes their degranulation to release inflammatory active compounds like histamine. The role of BCMs in the allergy was taken from the idea that morphine and other opiate alkaloids inducing selective release of histamine from the mast cells in vitro and in vivo and their effect may be associated with anaphylactoid reactions. In this regard, the role of BCM-7 in allergy was analyzed by incubating the peripheral leukocytes of healthy adult volunteers with this peptide in vitro leading to their degranulation to release histamine in a concentration dependent manner. Intradermal injection of this peptide induced a wheal and flare reaction in the skin similar to histamine or codeine. Pretreatment with the H1 antagonist terfenadine or cetirizine significantly inhibited the skin responses to BCM-7. The intradermal injection of an opiate receptor antagonist, naloxone, inhibited in vitro histamine release and skin reactions only in a 100-fold excess over BCM-7.[Citation79] Kurek and Malaczynska[Citation80] also studied the effect of BCM-7 in guinea pigs and rat peritoneal cells and found the release of histamine from the peritoneal cells and induction of wheal formation and bronchial obstruction in sensitized guinea pigs respectively. BCM-7 was found to cause wheal and flare reactions in healthy children, similar to that caused by histamine and codeine. The area of these reactions was concentration dependent. These findings suggested that this peptide can be regarded as a noncytotoxic, direct histamine releaser in humans, however, clinical relevance of these findings deserves further studies.
A correlation between serum DPP IV activity and BCM content of mother’s milk between healthy and allergic groups of infants was studied. In the allergic group, the high level of BCM in mother’s milk corresponds to the low DPP IV activity in infant’s sera. The lower BCM-5 and BCM-7 content of mothers’ milk of the allergic group seems that BCMs can pass from the intestine to the blood and may have prolonged half-life due to a lower DPP IV activity.[Citation42,Citation72] Recently, in our laboratory, Reddi et al.[Citation81] observed significant release of histamines and mast cell specific tryptase on incubation of bone marrow derived cells with BCM-5.
Milk Intolerances
Many people who drink A2 milk do so because they find it is easier to digest. However, A2 milk does contain lactose, which is often stated as the most important milk intolerance issue. The likely explanation for this apparent contradiction is two-fold. First, BCM-7 that is released from A1β-casein slows down the passage of food through the digestive system (as do other opioids) providing longer time for lactose fermentation. Second, many people are intolerant specifically to the BCM-7. However, this hypothesis needs intensive research to prove this fact beyond a doubt.
A2 Corporation Ltd.[Citation68] was established in New Zealand in 2000 to test cows for A1 and A2 genetic variants of β-casein and market milk with only the A2 variant. The easiest way to use the desirable β-casein A2 genotypes is selective distribution of bull semen. It will allow developing herds of cows producing milk with the A2 variant only. Since 2003, A2 milk has been sold in New Zealand and Australia as a premium brand, offering a natural choice in protein quality. The company has started marketing A2 milk in Asia and the United States, also.
INDUSTRIAL APPLICATIONS OF BCMs
Genetic engineering techniques have been exploited to produce BCMs and its analogues by enzymatic or chemical cleavage of the microbial fusion protein to release these peptides.[Citation82] The purpose of the production of these recombinant BCMs is to increase animal performance, e.g., weight gain or milk yield. As yet, no success has been found in human nutrition. Nevertheless, some modified BCMs have been produced by pharmaceutical companies in order to have higher analgesic potency, altered side-effects and longer duration of action. A considerable increase in analgesic or antidiarrheal activity in dogs was obtained by substitution of L- with D-amino acids,[Citation83] examples of chemically modified potent opioid peptides include morphiceptin and casokefamide. Modifications to the natural BCMs includes the increased affinity of the resulting analogues for opioid receptors, altered pharmacokinetics, particularly their inactivation by proteolytic/peptideolytic enzymes; substituted BCMs have been shown to be more resistant to enzymatic attack and exhibit higher opioid potency than the natural peptide.[Citation84]
CONCLUSIONS
β-Casomorphins originating from milk can be kept under class IV of food properties related to health as proposed by Rahman and McCarthy,[Citation85] but its efficacy to promote health and well-being is a matter of debate. On one hand, opioid peptides have been associated with various physiological disorders, while on the other hand, these peptides are potential modulators of various regulatory processes in the body. The negative data is either epidemiological from humans or animal trials and some pieces of evidence in relation to human illnesses or functional significance are not strong enough that need deep investigations. Such discrepancies in functional properties are problematic for consumers and stakeholders too. Therefore, it is necessary to continue research in exploring the role of BCMs in human health and risk assessment using in-vivo experiments with improved diagnostic techniques to verify the presence of BCMs in the blood of animals fed with the alternative β-casein variants. Thus, it is indispensable to fully understand the physiological significance of milk-protein derived opioid peptides and their release during digestion to avoid confusion in the minds of consumers for safe use of A1 and A2 “like” milk.
REFERENCES
- European Food Safety Authority (EFSA). Review of the potential health impact of β-casomorphins and related peptides. Scientific Report no. 231, 2009; 1–107.
- Sadiq, M.B.; Tauseef, M.S. Selected functional foods for potential in diseases treatment and their regulatory issues. International Journal of Food Properties 2011. DOI:10.1080/10942912.2010.551313.
- McLachlan, C.N.S. Beta-casein A1, ischaemic heart disease mortality, and other illnesses. Medical Hypotheses 2001, 56, 262–272.
- Elliott, R.B.; Harris, D.P.; Hill, J.P.; Hill, J.P. Type I insulin dependent diabetes mellitus and cow milk: Casein variant consumption. Diabetologia 1999, 42 (3), 292–296.
- Sun, Z.; Zhang, Z.; Wang, X.; Cade, R.; Elmer, Z.; Fregly, M. Relation of beta-casomorphin to apnea in sudden infant death syndrome. Peptides 2003, 24, 937–943.
- Yin, H.; Miao, J.; Zhang, Y. Protective effect of casomorphin-7 on type 1 diabetes rats induced with streptozotocin. Peptides 2010, 31, 1725–1729.
- Trompette, A.; Claustre, J.; Caillon, F.; Jourdan, G.; Chayvialle, J.A.; Plaisancie, P. Milk bioactive peptides and beta-casomorphins induce mucus release in rat jejunum. Journal of Nutrition 2003, 133 (11), 3499–3503.
- Nedvídková, J.; Kasafírek, E.; Dlabac, V. Effect of beta-casomorphin and its analogue on serum prolactin in the rat. Experimental and Clinical Endocrinology 1985, 85 (2), 249–252.
- Zong, Y.; Wei-Hua, C.; Zhang, Y.; Xiang, S. Effects of intra-gastric beta-casomorphin-7 on somatostatin and gastrin gene expression in rat gastric mucosa. World Journal of Gastroenterology 2007, 13 (14), 2094–2099.
- Woodford, K. Devil in the Milk: Illness, Health, and Politics of A1 and A2 Milk; Chelsea Green Publishing Company: Vermont, USA, 1990.
- Meisel, H.; Bockelmann, W. Bioactive peptides encrypted in milk proteins: Proteolytic activation and thropho-functional properties. Antonie van Leeuwenhoek 1999, 76 (1), 207–215.
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Current Pharmaceutical Design 2003, 9, 1331–1344.
- Kostyra, E.; Sienkiewicz-Sz apka, E.; Jarmoowska, B.; Krawczuk, S.; Kostyra, H. Opioid peptides derived from milk proteins. Polish Journal of Food and Nutritional Science 2004, 13 (54), 25–35.
- Farrell, H.M.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cow’s milk. Journal of Dairy Science 2004, 87, 1641–1674.
- Groves, M.L. Some minor components of casein and other phosphoproteins in milk. Journal of Dairy Science 1969, 52 (8), 1155–1165.
- Kaminski, S.; Cieslinska, A.; Kostyra, E. Polymorphism of bovine beta-casein and its potential effect on human health. Journal of Applied Genetics 2007, 48 (3), 189–198.
- Keating, A.F.; Smith, T.J.; Ross, R.P.; Cairns, M.T. A note on the evaluation on a beta-casein variant in bovine breeds by allele-specific PCR and relevance to beta-casomorphin. Irish Journal of Agricultural and Food Research 2008, 47, 99–104.
- Barroso, A.; Dunner, S.; Canon, J. Technical Note: Use of PCR-single strand conformation polymorphism analysis for detection of bovine beta-casein variants A1, A2, A3, and B. Journal of Animal Science 1999, 77, 2629–2632.
- Miluchová, M.; Trakovická, A.; Gábor, M. Analysis of polymorphism of beta-casein of Slovak Pinzgau cattle by PCR-RFLP for alleles A1 and A2. Lucrări Ştiinţifice Zootehnie şi Biotehnologii 2009, 42 (2), 288–292.
- Manga, I.; Dvořak, J. TaqMan allelic discrimination assay for A1 and A2 alleles of the bovine CSN2 gene. Czech Journal of Animal Science 2010, 55 (8), 307–312.
- Olenski, K.; Kaminski, S.; Szyda, J.; Cieslinska, A. Polymorphism of the beta-casein gene and its associations with breeding value for production traits of Holstein–Friesian bulls. Livestock Science 2010, 131, 137–140.
- Hanusová, E.; Huba, J.; Oravcová, M.; Polák, P.; Vrtková, I. Genetic variants of beta-casein in Holstein dairy cattle in Slovakia. Slovak Journal of Animal Science 2010, 43 (2), 63–66.
- Raies, M.; Kapila, R.; Shandilya, U.; Kapila, S. Detection of A1 and A2 genetic variants of beta-caein in Indian crossbred cattle by PCR-ACRS. Milchwissenschaft 2012, 67 (4), 396–398.
- Jinsmaa, M.; Yoshikawa, M. Enzymatic release of neocasomorphin and β-casomorphin from bovine β-casein. Peptides 1999, 20, 957–962.
- Lotfi, B. Optimization study for the production of an opioid-like preparation from bovine casein by mild acidic hydrolysis. International Dairy Journal 2004, 14, 535–539.
- De Noni, I. Release of beta-casomorphins 5 and 7 during simulated gastro-intestinal digestion of bovine beta-casein variants and milk-based infant formulas. Food Chemistry 2008, 110 (4), 897–903.
- De Noni, I.; Stefano, C. Occurrence of β-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastro-intestinal digestion. Food Chemistry 2010, 119 (2), 560–566.
- Hernández-Ledesma, B.; Amigo, L.; Ramos, M.; Recio, I. Release of angiotensin converting enzyme-inhibitory peptides by simulated gastrointestinal digestion of infant formulas. International Dairy Journal 2004, 14 (10), 889–898.
- Hernández-Ledesma, B.; Quirós, A.; Amigo, L.; Recio, I. Identification of bioactive peptides after digestion of human milk and infant formula with pepsin and pancreatin. International Dairy Journal 2007, 17 (1), 42–49.
- Jarmolowska, B.; Szlapka-Sienkiewicz, E.; Kostyra, E., Kostyra, H.; Mierzejewska, D.; Marcinkiewicz-Darmochwal, K. Opioid activity of Humana formula for newborns. Journal of the Science of Food and Agriculture 2007, 87 (12), 2247–2250.
- Law, J.; Handrickman, A. Proteolytic enzymes of lactic acid bacteria. International Dairy Journal 1997, 7, 1–11.
- Schieber, A.; Brückner, H. Characterization of oligo-and polypeptides isolated from yoghurt. European Food Research Technology 2000, 210 (5), 310–313.
- Shihata, A.; Shah, N.P. Proteolytic profiles of yogurt and probiotic bacteria. International Dairy Journal 2000, 10 (5–6), 401–408.
- Rokka, S.; Tuominen, J.; Korhonen, T.; Rokka, T.; Syväoja, E.L.; Tuominen, J.; Korhonen, H. Release of bioactive peptides by enzymatic proteolysis of Lactobacillus GG fermented UHT milk. Milchwissenschaft 1997, 52, 675–678.
- Hamel, U.; Kielwein, G.; Teschemacher, H.; Beta-casomorphin immunoreactive materials in cows’ milk incubated with various bacterial species. Journal of Dairy Research 1985, 52 (1), 139–148.
- Matar, C.; Goulet, J. ß-Casomorphin 4 from milk fermented by a mutant of Lactobacillus helveticus. International Dairy Journal 1996, 6 (4), 383–397.
- Kahala, M.; Pahkala, E.; Pihlanto-Leppaelae, A. Peptides in fermented Finnish milk products. Agricultural and Food Science in Finland 1993, 2 (5), 379–386.
- Webb, K.E.; Matthews, J.C.; DiRienzo, D.B. Peptide absorption: A review of current concepts and future perspectives. Journal of Animal Science 1992, 70 (10), 3248–3257.
- Umbach, M.; Teschemacher, H.; Praetorius, K.; Hirschhauser, R.; Bostedt, H. Demonstration of a beta-casomorphin immunoreactive material in the plasma of newborn calves after milk intake. Regulatory Peptides 1985, 12 (3), 223–230.
- Singh, M.; Rosen, C.L.; Chang, K.; Chang, K.; Haddad, G.G. Plasma beta-casomorphin-7 immunoreactive peptide increases after milk intake in newborn but not in adult dogs. Pediatric Research 1989, 26 (1), 34.
- Kost, N.V.; Sokolov, O.Y.; Kurasova, O.B.; Dmitriev, A.D.; Tarakanova, J.N.; Gabaeva, M. Beta-casomorphins-7 in infants on different type of feeding and different levels of psychomotor development. Peptides 2009, 30 (10), 1854–1860.
- Wasilewska, J.; Kaczmarski, M.; Kostyra, E.; Iwan, M.J. Cow’s-milk-induced infant apnea with increased serum content of bovine beta-casomorphin-5. Pediatric Gastroenterology and Clinical Nutrition 2011, 52 (6), 772–775.
- Mahe, S.; Tome, D.; Dumontier, A.M.; Desjeux, J.F. Absorption of intact morphiceptin by diisopropylfluorophosphate-treated rabbit ileum. Peptides 1989, 10 (1), 45–52.
- Shimizu, M.; Tsunogai, M.; Arai, S. Transepithelial transport of oligopeptides in the human intestinal cell, Caco-2. Peptides 1997, 18 (5), 681–687.
- Iwan, M.; Jarmolowska, B.; Bielikowicz, K.; Kostyra, E.; Kostyra, H.; Kaczmarski, M. Transport of μ-opioid receptor agonists and antagonist peptides across Caco-2 monolayer. Peptides 2008, 29 (6), 1042–1047.
- Sienkiewicz-Szlapka, E.; Jarmolowska, B.; Krawczuk, S.; Kostyra, E.; Kostyra, H.; Bielikowicz, K. Transport of bovine milkderived opioid peptides across a Caco-2 monolayer. International Dairy Journal 2009, 19, 252–257.
- Yin, H.; Miao, J.; Ma, C.; Sun, G.; Zhang, Y. β-Casomorphin-7 cause decreasing in oxidative stress and inhibiting NF-κB-iNOS-NO signal pathway in pancreas of diabetes rats. Journal of Food Science 2012, 772, 278–282.
- Dubynin, V.A.; Malinovskaya, I.V.; Belyaeva, Y.A.; Bibby, N.J.; Wasmuth, H.E. Delayed effect of exorphins on learning of albino rat pups. Biology Bulletin 2008, 35 (1),43–49.
- Marklakova, A.S.; Nazarenko, I.V.; Dubynin, V.; Nezavibat’ko, A.V.N.; Alfeeva, L.A.; Kamenskĭ, A.A. The effect of beta-casomorphin-7 on the level of food and defense motivations in different types of learning. Zhurnal Vysshei Nervnoi Deiatelnosti Imeni I.P. Pavlova 1995, 45 (6), 1143–1150.
- Maslennikova, N.V.; Sazonova, E.N.; Timoshin, S.S. Effect of-casomorphin-7 on DNA synthesis in cell populations of newborn albino rats. Bulletin of Experimental Biology and Medicine 2008, 145 (2), 210–212.
- Schusdziarra, V. Effect of beta-casomorphins and analogs on insulin release in dogs. Endocrinology 1983, 112 (3), 885–889.
- Pan, C.; Zou, S.; Chen, W.; Rossi, J.; Zolovick, A.J. Effect of feeding β-casomorphin-7 on the digestive tract development in early weaning piglets. Chinese Journal of Veterinary Science 2006, 21, 231–235.
- Qin, Y.; Cong-zhen, L.U.O.; Xin, M.; Dong, Z.; Wang, Y. Effect of β-casomorphin-7 on growth, growth-related hormone and GHR mRNA expression in rats. Acta Nutrimenta Sinica 2004, 2, 212–234.
- Teschemacher, H.; Koch, G.; Brantl, V. Milk protein derived atypical peptides and related compounds with opioid antagonist activity (review). In: β-Casomorphins and Related Peptides: Recent Developments; Brantl, V.; Teschemacher, H.; Eds.; VCH-Weinheim, Germany, 1994; 3–17.
- Mentz, P.; Neubert, K.; Liebmann, C.; Hoffmann, S.; Schrader, U. and Barth, A. Pharmacological effects of β-casomorphins on the cardiac function. In: β-Casomorphins and Related Peptides; Weinheim: VCH, Germanyl 1990; 133–139.
- Panksepp, J.; Normansell, L.; Siviy, S.; Rossi, J.; Zolovick, A.J. Casomorphins reduce separation distress in chicks. Peptides 1984, 5, 829–831.
- Sakaguchi, M.; Koseki, M.; Wakamatsu, M.; Matsumura, E. Effects of systemic administration of β casomorphin-5 on learning and memory in mice.European Journal of Pharmacology 2006, 530 (1–2), 81–87.
- Sakaguchi, M.; Koseki, M.; Wakamatsu, M.; Matsumura, E. Effects of beta-casomorphin-5 on passive avoidance response in mice. Bioscience, Biotechnology and Biochemistry 2003, 67 (11), 2501–2504.
- Laugesen, M.; Elliott, R. Ischaemic heart disease, type 1 diabetes, and cow milk A1 ß-casein. Journal of the New Zealand Medical Association 2003, 116 (1168), 121–132.
- Cade, R.; Privette, R.; Fregly, M.; Rowland, N.; Sun, Z.; Zele, V. Autism and schizophrenia: Intestinal disorders. Nutritional Neuroscience 2000, 3, 57–72.
- Schranz, D.B.; Lernmark, A. Immunology in diabetes: An update. Diabetes/Metabolism Reviews 1998, 14 (1), 3–29.
- Cavallo, M.; Monetini, L. Diabetes and cow’s milk. Lancet (British edition) 1996, 348 (9042), 1655.
- Padberg, S.; Schumm-Draeger, P.M. Significance of A1 and A2 antibodies against beta-casein in insulin-dependent diabetes mellitus. Deutsche Medizinische Wochenschrift 1999, 124 (50), 1518–1521.
- Elliott, R.B.; Wasmuth, H.E.; Bibby, N.J.; Hill, J.P. The role of beta-casein in the induction of insulin-dependent diabetes in the non-obese diabetic mouse and humans. In: Seminar on Milk Protein Polymorphism; IDF Special Issue No. 9702. International Dairy Federation; Brussels, Belgium, 1997; 445–453.
- Elliott, R.B.; Hill, J.P. Methods of selecting non-diabetogenic milk or milk products so selected. US Patent Application Publication No. 2003/0017250 A1; 2003.
- Elitsur, Y.; Luk, G.D. Beta-casomorphin (BCM) and human colonic lamina. Propria lymphocyte proliferation. Clinical and Experimental Immunology 1991, 85, 493–497.
- Graves, P.M.; Noris, J.M.; Pallansch, M.A.; Gerling, I.C.; Rewers, M. The role of enteroviral infections in development of IDDM. Diabetes 1997, 46, 161–168.
- A2 Corporation. The science behind A2. 2005. www.a2corporation.com/body/about_a2milk/science_review.html.
- Chin-Dusting, J.; Shennan, J.; Jones, E.; Williams, C.; Kingwell, B.; Dart, A. Effect of dietary supplementation with beta-casein A1 or A2 on markers of disease development in individuals at high risk of cardiovascular disease. British Journal of Nutrition 2006, 95 (1), 136–146.
- Tailford, K.A.; Berry, C.L.; Thomas, A.C.; Campbell, J.H. A casein variant in cow’s milk is atherogenic. Atherosclerosis 2003, 170 (1), 13–19.
- Pasi, A.; Mahler, H.; Lansel, N.; Bernasconi, C.; Messiha, F.S. Beta-casomorphin-immunoreactivity in the brain stem of the human infant. Research Communications in Chemical Pathology and Pharmacology 1993, 80 (3), 305–322.
- Wasilewska, J.; Sienkiewicz, S.E.; Kuzbida, E.; Jarmolowska, B.; Kaczmarski, M.; Kostyra, E. The exogenous opioid peptides and DPP IV serum activity in infants with apnoea expressed as apparent life threatening events (ALTE). Neuropeptides 2011, 43 (3), 189–195.
- Zoghbi, S.; Trompette, A.; Claustre, J.; El Homsi, M.; Garzon, J.; Jourdan, G.; Scoazec, J.; Pascale, P. β-Casomorphin-7 regulates the secretion and expression of gastrointestinal mucins through a μ-opioid pathway. American Journal of Physiology: Gastrointestinal and Liver Physiology 2006, 290, G1105–G1113.
- Johansson, M.E.V.; Phillipson, J.; Petersson, A.; Velcich, L.; Holm. Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proceedings of the Academy of Natural Sciences 2008, 105, 15064–15069.
- Bartley, J.; McGlashan, S.R. Does milk increase mucus production? Medical Hypotheses 2010, 74, 732–734.
- Svedberg, J.; de Haas, J.; Liemenstoff, G.; Paul, P.; Teschemacher, H. Demonstration of beta-casomorphin immunoreactive materials in in vitro digests of bovine milk and in small intestine contents after bovine milk ingestion in adult humans. Peptides 1985, 6, 825–830.
- Migliore-Samour, D.; Jolles, P. Casein, a prohormone with an immunomodulating role for the newbom? Expenentia 1988, 44, 188–193.
- Zimecki, M.; Janusz, M.; Staroscik, K.; Wieczorek, Z.; Lisowski, J.; Wieczore, Z. Effect of a proline-rich polypeptide on donor cells in graft-versus-host reaction. Immunology 1982, 47, 141–145.
- Kurek, M.; Przybilla, B.; Hermann, K.; Ring, J. A naturally occurring opioid peptide from cow’s milk, beta-casomorphine-7, is a direct histamine releaser in man. International Archives of Allergy and Immunology 1992, 97, 115–120.
- Kurek, M.; Malaczynska, T. Food allergy, atopic dermatitis-nutritive casein formula elicits pseudoallergic skin reactions by prick testing. International Archives of Allergy and Immunology 1999, 118 (2), 228–229.
- Reddi, S.; Kapila, R.; Dang, A.K.; Kapila, S. Evaluation of allergenic response of milk bioactive peptides using mouse mast cell. Milchwessienschaft 2012, 67 (2), 117–232.
- Carnie, J.; Minter, S.; Oliver, S.; Perry, F.; Metzlaff, M. Nutritional compositions containing β-casomorphins. UK Patent Application GB 2214810; 1989.
- Erll, G.; Hahn, A.; Brantl, V.; Daniel, H. β-Casomorphins and intestinal net fluid transport in vivo. In: β-Casomorphins and Related Peptides: Recent Developments; Brantl, V.; Teschemacher, H.; Eds.; Weinheim: VCH, 1994; 143–149.
- Daniel, H.; Wessendorf, A.; Vohwinkel, M.; Brantl, V. Effect of D-Ala2,4,Tyr5-β-casomorphin-5-amide on gastro-intestinal functions. In: β-Casomorphins and Related Peptides; Nyberg, F.; Brantl, V.; Eds.; Fyris-Tryck AB: Uppsala, 1990; 95–104.
- Rahman, S.M.; McCarthy, J.O. A classification of food properties. International Journal of Food properties 1999, 2 (2), 93–99.