Abstract
The aim of this study was to determine the antioxidant activity, total phenolics, and total flavonoid content of six freeze dried wild edible plants, namely Helminthostachys zeylanica, Schismatoglottis ahmadii, Heckeria umbelatum, Lasia spinosa, Gonostegia hirta, and Aniseia martinicense from Sabah, Malaysia. All leaves had higher levels of total phenolics, flavonoids, and antioxidant activity than stems and flowers for all extracts, except the stem of Gonostegia hirta. Integrated antioxidant activity index showed the leaf of Heckeria umbellatum and Aniseia martinicense possessed the highest antioxidant activity for all extracts. Principal component analysis identified that the phenolic group present in the hot water and methanolic extracts was the main factor for higher values observed in oxygen radical absorbance capacity and ferric reducing antioxidant power assays. These wild edible plants are good natural sources of antioxidants to be incorporated as functional ingredients in the food industry.
INTRODUCTION
Borneo Island, which is conservatively estimated to contain around 15,000 plant species,[Citation1] is one of the world’s mega diversity areas. Sabah which is located at the northern part of Borneo is well known for its biodiversity and endemicity of flora. Since the beginning of human civilization, plants have always been the source of food due to their nutritious content. Wild growing plants are gathered from mature forests, young secondary forests, paddy fields, riverbanks, and open grass pastures. They are consumed daily by local indigenous communities as traditional vegetables as well as for medicinal purposes.
Free radicals are defined as any molecules or atoms with one or more unpaired electron that are produced in normal and/or pathological cell metabolism.[Citation2] Oxidation is essential to many living organisms for the production of energy to fuel biological processes. However, the uncontrolled production of oxygen-derived free radicals such as superoxide radical, hydrogen peroxide, hydroxyl radical, and nitric oxide may potentially initiate degenerative processes in the human body.[Citation3,Citation4] These oxidative damages caused by reactive oxygen species (ROS) on lipids, proteins, and nucleic acids may trigger various chronic disease, such as coronary heart diseases, atherosclerosis, cancer, as well as premature aging.[Citation5]
The effects of ROS, especially in damaged tissues, can be reduced by the presence of antioxidant compounds. Many species of fruits, vegetables, herbs, cereals, sprouts, and seeds have been investigated for antioxidant activities during the past decade.[Citation6−Citation8] Phenolics are one of the groups of non-essential dietary components that have been associated with the inhibition of atherosclerosis and cancer.[Citation9] Flavonoids had been proven to display a wide range of pharmacological and biochemical effects, such as antimicrobial, antithrombotic, antimutagenic, and anticarcinogenic activities.[Citation10,Citation11]
Ever expanding knowledge of the role of physiologically active food components has changed the daily diet for health benefits. Consumer’s demand for functional food has advanced beyond the treatment of primary deficiency syndromes to encompass the reduction of disease risk. Previous studies were only focused on ethno-botanical survey of the usage of wild edible plants by indigenous people in Sabah[Citation12,Citation13] while beneficial properties such as contribution as source of food antioxidants of these plants were rarely scientifically investigated. Commercial cultivated vegetables were marginalized in modern agriculture, while the wild and weedy edible species had received no special attention. Therefore, several plants species were selected for the present study to investigate on their antioxidant properties, total phenolics, and flavonoids of edible plants in different polarity of extracts. In addition, interrelationships between all the analyzed parameters were evaluated as well.
MATERIALS AND METHODS
Materials
Six species of wild edible plants that were ready for harvest had been obtained from the indigenous people who collect edible forest resources throughout the year at the local market. The morphology of the plants were noted and sent for identification and were identified as Helminthostachys zeylanica, Schismatoglottis ahmadii, Heckeria umbelatum, Lasia spinosa, Gonostegia hirta, and Aniseia martinicense. The plant samples were then cleaned and separated according to the different edible parts (leaves, stems, flowers). The edible part analyzed was determined according to usual Sabah consumer habits. Samples were frozen at –70°C before being freeze dried. The freeze dried samples were then kept in sealed plastic bags and stored under –80°C until the extraction process. Each type of plant sample was obtained in three replicates of 50 g each.
Samples Preparation
The freeze dried sample was ground, homogenized, weighed (5 g), and transferred into flasks. Methanol (80%) was added in the ratio of 1:20 and the mixture was sonicated at room temperature in a sonicator bath (Branson 2510, USA) for 30 min at 42 kHz, 130 W before shaking for 4 h at room temperature. The extract was then separated from the sample residue by filtration through Whatman No.1 and No. 42 filter paper. The remaining sample residue was re-extracted twice with 80% methanol in the ratio of 1:20. The extracts were then pooled, filtered, and concentrated under reduced pressure at 40°C using a rotary evaporator (Buchi R-114, Switzerland). The same procedure was applied for 80% ethanol and 70% acetone and water as extraction solvent. Decoction was performed with 5 g of freeze dried, ground samples boiled in 100 mL of water for 20 min. The mixture was then cooled down to room temperature before being filtered through Whatman No.1 and No. 42 filter paper.
Total Phenolics Determination
Phenolics content in extracts were determined with Folin-Ciocalteu reagent according to the method of Singleton.[Citation14] A 200 μL of diluted extract was transferred into test tube and 1 mL of Folin-Ciocalteu reagent (diluted 10 folds) was added and mixed. The mixture was allowed to stand at room temperature for 4 min. A total of 800 μL of of 75 g/L sodium carbonate was added to the mixture and mixed gently. After standing at room temperature for 2 h, the absorbance of the mixture was read at 765 nm using a Lambda 35 UV-Vis spectrophotometer (Perkin Elmer, USA). The standard calibration curve was established by using gallic acid. Total content of phenolics in the sample was calculated and expressed in milligram of gallic acid equivalent/dried weight of sample (mg GAE/g of DW sample).
Total Flavonoids Determination
The total flavonoid compounds of each extract were determined[Citation15] and expressed as milligrams of catechin (CA) equivalents per gram of dried weight sample (mg CAE/g DW sample). Deionized water (1.25 mL) and 75 μL of 5% (g/mL) sodium nitrate were added to 200 μL of each sample. A 150 μL of 10% aluminium chloride was added 6 min later. After 5 min, 0.5 mL of 1 M NaOH was added, and the total volume was made up to 2.5 mL with deionized water. The solution was mixed well again, and the absorbance was measured against a blank at 510 nm with a Lambda 35 UV-Vis Spectrophotometer (Perkin Elmer, USA). Absorbance of the developed red color was measured at 735 nm. The content of total flavonoid in each extracts was determined using a standard curve prepared with CA at varied concentrations (0, 50, 100, 200, 400, and 800 μg /mL).
Ferric Reducing Antioxidant Power (FRAP)
The FRAP assay was carried out as described by Benzie and Strain.[Citation16] Briefly, 900μl of FRAP reagent, prepared freshly and warmed at 37°C, was mixed with 90 μL of distilled water and 30μL of test sample or methanol as appropriate for reagent blank. The final dilution of the test sample in the reaction mixture was 1/34. The FRAP reagent contained 2.5 mL of a 10 mmol/L TPTZ solution in 40 mmol/L HCl plus 2.5 mL of 20 mmol/L FeCl3·6H2O and 25 mL of 0.3 mol/L acetate buffer pH 3.6. Readings at the absorption maximum (593 nm) were taken after 30 min incubation at 37°C using a Lambda 35 UV-Vis Spectrophotometer (Perkin Elmer, USA). Trolox concentrations in the range of 10–1000 μmol/L were used for calibration. All extracts were diluted and analyzed in duplicate. FRAP values were expressed as μmol Trolox equivalents per gram dried weight sample (μmol TE/g DW sample).
ABTS Radical Scavenging Determination
The ABTS+ solution was prepared by reaction of 5 mL of a 7 mM aqueous 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) solution and 88 μL 0f 140 mM (2.45 mM final concentration) potassium persulfate (K2S2O8) solution and allowing the mixure to stand in the dark at room temperature for 12–16 h before use.[Citation17] After storage in the dark for 12–16 h, the radical cation solution was further diluted in 95% ethanol until the initial absorbance of 0.7 ± 0.02 at 734 nm (at 30°C) was reached. Extracts have to be diluted such that, they produced 20–80% inhibition of blank absorbance. An aliquot (20 μL) of sample, ethanol (blank), or standard (Trolox) was added to ABTS+ working solution (2 mL). Absorbance was measured at 734 nm 6 min after mixing at 30°C. A standard reference curve was constructed by plotting percent inhibition values against concentration of Trolox. The radical scavenging activity of extracts was quantified as μmol Trolox equivalents per gram dried weight sample (μmol TE/g DW sample).
Oxygen Radical Absorbance Capacity (ORAC)
ORAC was measured following procedures previously described.[Citation18] This assay measures the ability of extracts in test materials to inhibit the decline in fluorescence that is induced by a peroxyl radical generator, AAPH. The reaction mixture contained 150 μL of 8.16 × 10−5 mM fluorescein, 25 μL of extract and 25 μL of 153 mM AAPH. Phosphate buffer (7.5 mM, pH 7.4) was used as a blank and Trolox (a water soluble α-tocopherol analogue) as a standard (6.25–75 μM) during each run. The final volume of 200 μL was used in a black walled 96 well plates (BRAND, Germany). Fluorescien and extracts were preincubated at 37°C for 15 min. The reaction was started by addition of AAPH rapidly by using multichannel pipette. Fluorescence was measured and recorded every 30 s at emission of 515 nm and excitation of 490 nm using a SpectraMaxM2 microplate reader (Molecular Devices, USA) until the fluorescence of the last reading declined to <10% of the first reading. The ORAC value refers to the net protection area under the quenching curve of fluorescien in the presence of an antioxidant. The final results (ORAC value) were calculated and expressed using Trolox equivalents per gram of dry weight of sample (μM TE/g DW sample).
DPPH Free Radical Scavenging Activity
Antioxidant activity was performed spectrophotometrically as described by Brands-William.[Citation19] An aliquot of 1.95 mL of 6 × 10−5 mol/L 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical (Sigma) was added to a test tube with 0.5 mL of extracts. Methanol was used as blank to zero the absorbance. The reaction mixture was shaken vigorously at room temperature. Absorbance at 515 nm was recorded at different time interval (0, 1, 5, 10, 15, 20, 25, 30, 60, 90, and 120 min) by using a Lambda 35 UV-Vis Spectrophotometer (Perkin Elmer, USA). Five different concentrations of each extract studied have been assayed in order to check the linearity of response and to established the antioxidant activity values in the adequate linear range.
Statistical Analysis
Total phenolics, total flavonoids, yield, and antioxidant activities values were determined in three replicates for each sample tested, and the mean values ± standard deviation (SD) were reported. The Duncan test was applied to indicate the samples between which there were differences, where differences at p < 0.05 were considered to be significant. An overall antioxidant potency composite index was determined by assigning all assays an equal weight, assigning an index value of one to the best score for each test, and then calculating an index score for all other samples within the test as follows: Integrated antioxidant activity index = [(sample value-minimum value)/maximum value-minimum value)];[Citation20] the added value of all four tests for each sample was then taken as integrated antioxidant capacity index. All assays were given equal weight and were calculated on a normalized basis for each sample. Principal component analysis (PCA) was carried out on antioxidative data for the methanolic and hot water extracts to understand the covariance and identify relationships between the variables. The correlation matrix was then used to standardize the variables, which are not measured on the same scale. The Varimax method was also performed to produce orthogonal transformations to the reduced factors to identify the high and low correlations.
RESULTS AND DISCUSSION
Yield, Total Phenolics, and Total Flavonoids Determination
shows the yield, total phenolics, and total flavonoids of wild edible plants. Helminthostachys zeylanica (stem) gave the highest yield of methanolic, ethanolic, acetone, and cold water extracts (P < 0.05), followed by the methanolic extract of the leaf of Heckeria umbellatum. Highest extraction’s yield was obtained for the hot water extract of plant samples. This variation might be due to the release of more phenolic constituents that bind with the cell wall after the hot water ruptures the cell membranes and extract a greater amount of endocellular materials. Phenolic compounds are deposited mainly in the cell wall in plant cell, where lignin and other compounds (ferulic acid esters, flavonoids) accumulate in the vacuoles.[Citation21] Moreover, compounds with structures containing –OH and –COOH functional groups such as phenolic acids, lignans, flavonoids are easily extracted by the polar solvent in samples.
The total phenolics content ranged from 0.69 mg GAE/g DW sample to 19.65 mg GAE/g DW sample of all the plant extracts and is within the current values found in literatures (). Among all the samples, the leaf of Heckeria umbellatum had the highest phenolics content (19.39 mg GAE/g DW sample) for the methanolic extract followed by the leaf of Aniseia martinicense (9.73 mg GAE/DW sample), whereas the stem of Schimatoglottis ahmadii had the lowest phenolics content (1.29 mg GAE/g DW sample). The Heckeria umbellatum (leaf) and Aniseia martinicense (leaf) contained higher phenolics as compared to broccoli floret (4.61–7.68 mg GAE/g DW) studied by Borowski et al.[Citation22] The total phenolic content varied significantly among the studied plant species () and were much higher in hot water extracts than organic solvent extracts. This can be shown by the hot water extract of the leaf Aniseia martinicense which showed the highest content (18.40 mg GAE/g DW sample) whereas methanolic and ethanolic extract of Aniseia martinicense (leaf) showed the lower content of phenolics content (9.73 and 10.61 mg GAE/g DW sample). Visioli et al.[Citation23] had showed the consumption of plant foods high in phenolics can reduce the risk of heart disease by slowing the progression of atherosclerosis due to their antioxidative properties.
On a dry weight basis, the highest total flavonoids of the studied plant extracts were from the hot water extract of the leaf and stem of Aniseia martinicense (7.94 and 8.37 mg CA/g DW sample). On the other hand, the leaf of Gonostegia hirta, Heckeria
Table 1 Yield, total phenolics, and total flavonoids of wild edible plants
Antioxidant Activities of Wild Edible Plants
A single antioxidant assay cannot provide a complete picture of the antioxidant capacity of a food in vitro due to the complexity of the antioxidant defense system and involvement of many different types of free radicals in the body. Thus, several different antioxidant assays were used in this study. Herein, the authors had attempted to analyze the assays from the chemistry principal upon which they are based. In general, the antioxidants can be classified into two mechanism categories: Single electron transfer (SET) which can be measured by FRAP (reducing power: measuring the conversion of Fe3+/ferricyanide complex to the ferrous form) and ABTS test as well as hydrogen atom transfer (HAT) that
Table 2 Antioxidant activity of wild edible plants
shows the values obtained for antioxidant activities by four antioxidant tests in wild edible plants. All the plant species in the ORAC assay showed appreciable antioxidant activity with the methanolic extract of the leaf of Heckeria umbellatum (598.78 μmol TE/g DW sample) showing the greatest antioxidant capacity and among the other samples. The antioxidant capacity for methanolic extract of Aniseia martinicense’s leaf measured with the ORAC assay was also high (388.26 μmol TE/g DW sample). However, the leaf and stem of Schismatoglottis ahmadii were identified as the plant with the lowest antioxidant capacity with the value 55.69 and 41.85 μmol TE/g DW sample respectively ().
In the FRAP assay, the strongest antioxidant activities were also detected in the hot water extract for leaf of Aniseia martinicens (200.56 μmol TE/g DW sample) and followed by Heckeria umbellatum’s (119.97 μmol TE/g DW sample) for methanolic extract. This antioxidant activity was more than twice the values of the other plant species () for methanolic extracts. The higher reducing power of the extracts might be due to the presence of reductones that have electron transferring ability as described by Berker et al.[Citation24] which could react with free radicals to stabilize and terminate radical chain reactions. With respect to FRAP assay, values obtained from the stem and flower of plant species were lower than the green leaf of plants.
Methanolic extract of Heckeria umbellatum (leaf) and Aniseia mattinicense (leaf) showed significantly (P < 0.05) higher antioxidant activity compared to the other plant extracts. Ethanolic and acetone extract of Aniseia martinicense (leaf) did not show significant (P > 0.05) difference in antioxidant activity when expressed in terms of ABTS test, but is significantly (P < 0.05) lower compared to hot water extract. The order of ABTS activity of respective hot water extract is as follow: Lasia spinosa > Gonostegia hirta (leaf) > Gonostegia hirta (stem) > Heckeria umbellatum (flower) > Aniseia martinicense (leaf) > Aniseia martinicense (stem) > Heckeria umbellatum (leaf) > Heckeria umbellatum (stem) > Schimatoglottis ahmadii (stem) > Schimatoglottis ahmadii (leaf) > Helminthostachys zeylatica (leaf) > Helminthostachys zeylanica (stem).
From the DPPH test, the EC50 which is expressed as the amount of antioxidant needed to decrease the radical concentration by 50% is inversely related to the antioxidant capacity of extract. Lower EC50 indicated higher antioxidant activity in the plant. For the reason of comparing with other assays, antiradical power (1/EC50) was used. The larger the antiradical power, the more efficient the extract. The radical scavenging activity of the five different extracts of six plant species on DPPH revealed hot water and ethanolic extracts showed high antiradical power compared to the other three extracts. This may be due to the antioxidative compounds extracted by hot water and ethanol involved both SET and HAT mechanisms. The antioxidant activity measured with DPPH method was higher in the leaf and stem of Gonostegia hirta and the leaf of Aniseia martinicense (). The exception was the ethanolic extract of the leaf of Gonostegia hirta with a significant (P < 0.05) higher antiradical power (12.63) than the other plants species, whereas Helminthostachys zeylanica (stem) and Schismatoglottis ahmadii (stem) have the lowest antiradical power among all the extracts.
The antioxidant activity varies considerably from species to species. Of all samples, the leaf of Heckeria umbellatum and Aniseia martinicense have a relatively high antioxidant capacities determined by the four antioxidant assays, whereas the stem and leaf of Schismatoglottis ahmadii were relatively low. It is interesting to note that the analysis of different plant parts (leaf, stem, and flower) resulted in various levels of antioxidant activity. Significant (P < 0.05) differences in phenolic contents were observed between all the plant species leaf and stem. Similar findings were reported by Kahkönen et al.[Citation25] who showed variation in phenolic content between different parts of different trees.
Antioxidant Activity Rank Order of the Vegetables
Due to the complex chemical reaction of the four antioxidant activities for determining plant antioxidant capacity, the values unit may vary substantially. Besides the variability of the unit, the rank of the sample might be influenced by different tests applied. For example, for the methanolic extract of Heckeria umbellatum (leaf), a high value was measured for the ORAC test, but had shown moderate activity for the DPPH test. Therefore, in order to observe the trend and rank the plant antioxidant capacities, a statistical method was developed to obtain a dynamic picture of the ranking. Sun and Tanumihadjo[Citation26] had created a statistical tool namely, the relative antioxidant capacity index (RACI) by integrating the antioxidant values generated from different in vitro methods. Tabart et al.[Citation27] proposed a weighted mean of the values obtained by the tests. Knowing the importance of integration of the activities values, a simple statistical approach was determined by assigning all assays an equal weight and were calculated on a normalized basis for each sample. The added value of all four tests for each sample was then taken as integrated antioxidant capacity index. The key advantage of this integrated approach is that normalization has no units and has consistent agreement with chemical methods.
Of the plants tested in five different extracts, Heckeria umbellatum (leaf), Aniseia martinicense (leaf), Gonostegia hirta (leaf), Gonotegia hirta (stem), and Lasia spinosa were consistently among the top five when analyzed by the antioxidant assays and were also among the highest with respect to total phenol content. However, sample ranking was not affected by the antioxidant assay used. The Heckeria umbellatum (leaf) and Aniseia martinicense (leaf) sample had the highest antioxidant capacity in all assays followed Gonostegia hirta (leaf) and Lasia spinosa.
Table 3 Principal component loadings of variables for methanolic and hot water extracts
In this study, it was observed that there are variations between the antioxidant activities in the same plants. These differences among the plant species can be explained by the maturity of the plant at harvest time which would affect the level of antioxidants present in these plants.[Citation28] Flavonoid compounds which are known as secondary natural metabolites are the major phytochemicals responsible for the antioxidant activity.[Citation29] Apparently, the biosynthesis of these natural products is profoundly influenced by a number of factors, such as weather conditions and harvest periods. This similar phenomenon was observed for total phenolics as well. Another possible reason that can be envisaged for these results are the variability that is intrinsic to plants such as genetics (sub-species and breeding) and extrinsic to plants such as meteorological factor (light intensity and temperature).[Citation30,Citation31]
Interrelationship of Antioxidant Activities, Total Phenolics, and Total Flavonoids
The loadings and percentage of variance for the first two principal components for methanolic and hot water extracts are shown in . These two extracts have been chosen to analyze the interrelationship of antioxidant activities, total phenolics, and total flavonoids are based on the highest antioxidative activities in each organic solvent and aqueous extracts’ group. For methanolic and hot water extracts after the PCA, the dimensionality of data was reduced from six partially correlated variables (total phenolics, total flavonoids, DPPH scavenging activity, reducing power, oxygen radical absorbance activity, and ABTS scavenging activity) to underlying variables principle component (PC) with almost 10.87 and 20.21% loss of variation, respectively. The first principal component (PC1) explained 75.23 and 63.78 % of the total variation for methanolic extract and hot water extracts and was related to antioxidant capacity and the phenolics content (). The second principal component (PC2) explained 13.91% (methanolic extract) and 16.01% (hot water extract) of the total variation and was mainly related to radical scavenging activity. For methanolic extract, total phenolics, ORAC and FRAP are highly loaded which indicate that the three properties are closely related while PC2 implies a high degree of relationship with DPPH test and moderate relationship with flavonoids content and ABTS test. From the loadings of the variables, the most influential features on the PC1 are total phenolics and total flavonoids for hot water extract that were found related with OARC and FRAP, while PC2 was closely related for DPPH and ABTS test. Evidently, PC1 is generally more correlated with the variable then PC2. This is to be expected because PCs are extracted successively, each one accounting for as much of the remaining variance as possible. The interrelationship of phenolics and antioxidant activities are important to group the variables based on the correlation and further understand how these variables contribute to the antioxidant activity of the plant extracts.
This result was not unexpected since it is well known that phenolics compounds, which have hydroxyl groups, are major contributors to ORAC and FRAP activity and the polar phenolics were selectively extracted in methanolic extracts of the plant species. Ou et al.[Citation32] reported that the ORAC values of beet, purple onion, and white onion has strong positive correlation with the FRAP values. The reducing properties are associated with the presence of compounds, which exert their action by breaking the free radical chain by donating a hydrogen atom or a single electron.[Citation33] The electron donation group, especially hydroxyl group located at o- or p-positions of the compounds, makes the compound polar and therefore reducing power is increased.[Citation34] Similar findings by Patthamakanokporn et al.,[Citation35] found a strong level of correlation (r2 = 0.821) between the values of total phenolics and ORAC antioxidant activity of various fruits. However, the phenolic content has a relatively small effect on the DPPH radical scavenging activity of the both extract (). It should be taken into consideration that the antioxidant activity of the extract might be attributed to other non-phenolics compounds, which are soluble in water and alcohol.[Citation36] However, the ability of the hot water extracts to scavenge DPPH and ABTS free radical scavenging activity were highly correlated on the PC2 (). This relationship can be explained by the mechanism of both assays are based on the measurement of the scavenging ability of the antioxidant towards DPPH or ABTS radicals. Due to the nature of the functional groups being extracted the correlation of antioxidant assays for hot water and methanolic extract showed similar patterns. They have the ability to react with the free radical either by scavenging or reducing. Thus, isolation and identification the compounds that are responsible for the antioxidant activity should be aimed for future study.
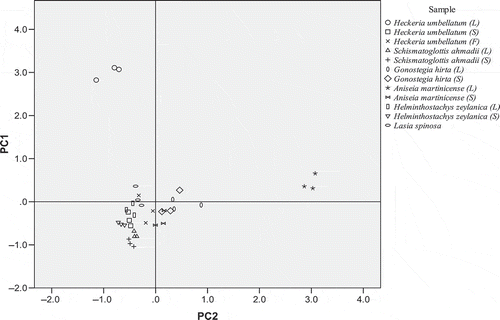
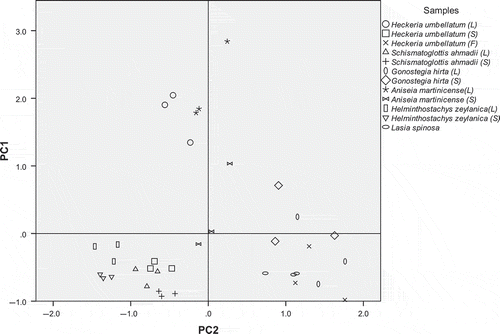
Loading of all the plant species and individual PC scores were combined into one score plot of PC1 and PC2 (). Samples with similar values for the variables explained by the PC appeared closely related, and those with large positive or negative scores are extremes in some variables. PCA score plot provided an overview of the similarities and differences between samples. For methanolic extract, three groups of samples were seen in score plots for PCs 1 and 2 with the replicates for each sample type being clustered tightly together (). Two distinct groups were formed with the leaf of Aniseia martinicense and Heckeria umbellatum were clearly separated from these groups. Moving along PC1 from bottom to top in the graph, most of the samples with intermediate activity were grouped together while Heckeria umbellatum (leaf) with the highest antioxidant activity were separated and is quite far from the others. On the contrary, Aniseia martinicense (leaf) distinguishes itself on a different vector direction might be due to its different compounds that react with the antioxidant test. On the other hand, the leaf Heckeria umbellatum and Aniseia martinicense for hot water extract share the same vector direction for PC1 which may indicate the antioxidative compounds extracted by hot water are similar and give high antioxidant activities (). While the Gonostegia hirta (leaf and stem) and Heckeria umbellatum (flower) discriminated from the other plant species owing to its high content of flavonoids and radical scavenging activity.
CONCLUSION
Wild edible plant extracts tested consisted of phenolics and flavonoids that demonstrated various antioxidant activities which are protective against ROS. Green leaves showed higher antioxidative activities, flavonoids, and phenolics content compared with the stem and flower. Consequently, the leaf of Heckeria umbellatum and Aniseia martinicense have great potential to be used in the development of functional ingredients with potent antioxidant properties for commercial exploration. Hot water extracts emerge as the most potential extract to obtain highest antioxidant compounds with great antioxidant activities. These findings are able to provide scientific evidence for their folk uses in the treatment of diseases related to the production of ROS and oxidative stress.
ACKNOWLEDGMENT
The authors would like to acknowledge Mr. John Baptist Sugau from Forest Research Centre, Sabah Forestry Department, Mr. Johnny Gisil from UMS, Dr. George Staples from Singapore Botanic Garden, and Dr. Andrew McDonald from the University of Texas-Pan American for their assistance in the identification of plant species, Dr. Ho Chung Mun and Dr. Ramzah Dambul from UMS for assistance in statistical analysis.
FUNDING
The authors wish to thank the Malaysian Ministry of Higher Education and Universiti Malaysia Sabah (UMS) for the financial support.
REFERENCES
- WWF. Borneo plants. 2011. http://www.w3.org/1999/xhtml
- Gutteridge, J.M.; Mitchell, J. Redox imbalance in the critically ill. British Medical Bulletin 1999, 55, 49–75.
- Bokov, A.; Chaudhuri, A.; Richardson, A. The role of oxidative damage and stress in aging. Mechanisms of Ageing and Development 2004, 125 (10–11), 811–826.
- Richu, S.; Abhijit, G.; Moushumi, G. Antioxidant activities and polyphenolic properties of raw and osmotically dehydrated dried mushroom (Agaricus bisporous) snack food. International Journal of Food Properties 2010, 13 (6), 1290–1299.
- Madhavi, D.L.; Deshpande, S.S.; Salunkhe, K. Food Antioxidants: Technological, Toxicological, Health perspective, Marcel Dekker: New York, 1996.
- Katalinic, V.; Smole Mozina, S.; Generalic, I; Skroza, D; Ljubenkov, I.; Klancnik. A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. International Journal of Food Properties 2013, 16 (1), 45–60.
- Negi, P.S; Jayaprakasha, G.K.; Jena, B.S. Evaluation of antioxidant and antimutagenic activities of the extracts from the fruit rinds of Garcinia cowa. International Journal of Food Properties 2010, 13, 1256–1265.
- Chon, S.U.; Heo, B.G.; Park, Y.S., Kim, D.K.; Gorinstein, S. Total phenolics level, antioxidant activities, and cytotoxicity of young sprouts of some traditional Korean salads plants. Plant Foods Human Nutrition 2009, 64, 25–31.
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs, S.M.N.; Somasundaram, R. Antioxidant activity of indigenous edible mushrooms. Journal of Agricultural and Food Chemistry 2006, 54 (26), 9764–9772.
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Human Nutrition 2004, 59, 113–122.
- Ozkul, Y.; Silici, S.; Eroglu, E. The anticarcinogenic effect of propolis in human lymphocytes culture. Phytomedicine 2005, 12 (10), 742–747.
- Kulip, J. A survey of indigenous plants used for food and medicine by the Kadazandusun ethnic in Tambunan, Sabah East Malaysia. Fourth Biennial Conference of the Borneo Research Council, Universiti Brunei Darussalam, Brunei Darussalam, June 10–15 1996.
- Lee, Y.F.; Gibot, A. Indigenous Edible Plants of Sabah. Forest Research Centre: Sabah, 2000.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.; Lester, P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology 1999, 299, 152–178.
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry 1999, 64 (4), 555–559.
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP Assay. Analytical Biochemistry 1996, 239 (1), 70–76.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine 1999, 26 (9–10), 1231–1237.
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-Well Format. Journal of Agricultural and Food Chemistry 2002, 50 (16), 4437–4444.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology 1995, 28 (1), 25–30.
- Livingstone, D. Data pretreatment and variable selection. In: A Practical Guide to Scientific Data Analysis; Livingstone, D.; Ed.; John Wiley and Sons Ltd: West Sussex, 2009, pp. 60.
- Bengoechea, M.L.; Sancho, A.I.; Bartolome, B.; Estrella, I.; Gomez-Cordoves, C.; Hernandez, M.T. Phenolic composition of industrially manufactured purees and concentrates from peach and apple fruits. Journal of Agricultural and Food Chemistry 1997, 45 (10), 4071–4075.
- Borowski, J.; Szajdek, A.; Borowska, E.; Ciska, E.; Zieliński, H. Content of selected bioactive components and antioxidant properties of broccoli (Brassica oleracea L. ). European Food Research and Technology 2008, 226 (3), 459–465.
- Visioli, F.B.L.; Galli, C. Diet and prevention of coronary heart disease: The potential role of phytochemicals. Cardiovascular Research 2000, 47 (3), 419–425.
- Berker, K.I.; Güçlü, K.; Tor, I.; Apak, R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72 (3), 1157–1165.
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S. Antioxidant activity of plant extracts containing phenolic compounds. Journal of Agricultural and Food Chemistry 1999, 47 (10), 3954–3962.
- Sun, T.; Tanumihardjo, S.A. An integrated approach to evaluate food antioxidant capacity. Journal of Food Science 2007, 72 (9), R159–R165.
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chemistry 2009, 113 (4), 1226–1233.
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Science and Technology 2007, 40 (1), 1–11.
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. Journal of Agricultural and Food Chemistry 1998, 46 (7), 2686–2693.
- Maranz, S.; Wiesman, Z. Influence of climate on the tocopherol content of shea butter. Journal of Agricultural and Food Chemistry 2004, 52 (10), 2934–2937.
- Myung-Min, O.; Rajashekar, C.B. Antioxidant content of edible sprouts: Effects of environmental shocks. Journal of the Science of Food and Agriculture 2009, 89 (13), 2221–2227.
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. Journal of Agricultural and Food Chemistry 2002, 50 (11), 3122–3128.
- Wong, S.P.; Leong, L.P.; William, J.H.K. Antioxidant activities of aqueous extracts of selected plants. Food Chemistry 2006, 99 (4), 775–783.
- Duan, S.; Weng, X.C.; Dong, X.W.; Liu, Y.P.; Li, H.P.; Jin, J.R. Antioxidant properties of butylatedhydroxytoluene refluxed in ferric chloride solution. Food Chemistry 1998, 61 (1–2), 101–105.
- Patthamakanokporn, O.; Puwastien, P.; Nitithamyong, A.; Sirichakwal, P.P. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. Journal of Food Composition and Analysis 2008, 21 (3), 241–248.
- Odabasoglu, F.; Aslan, A.; Cakir, A.; Suleyman, H.; Karagoz, Y.; Bayir, Y. Antioxidant activity, reducing power, and total phenolic content of some lichen species. Fitoterapia 2005, 76 (2), 216–219.