Abstract
In this study, antioxidant potentials of persimmon peel extracted with different levels of ethanol (0 [DW], 50, 70, and 99%) were screened for their antioxidant activity at various concentrations. The persimmon peel extracted by 70% ethanol inhibited 89.82% of 1,1-diphenyl-2-picrylhydrazyl radical at 0.5 mg/mL and had β-carotene bleaching inhibition activity at values of 91.26%. superoxide dismutase-like activity and reducing power had the highest in persimmon peel extracted by 70% ethanol each at 0.5 mg/mL and 1 mg/mL and the lowest persimmon peel extracted by distilled water. These results provided that persimmon peel extracted by 70% ethanol could be the most effective as a natural antioxidant in food industry.
INTRODUCTION
Reactive oxygen species (ROS) are produced by biological oxidation, which is caused by exposure to pollutants, stress, and blood-flow disorders. ROS contain unpaired electrons, including superoxide anions, hydroxyl radical, and hydrogen peroxide. ROS may damage enzymes, DNA, cell membrane, lipids, proteins, and nucleic acid in cell.[Citation1] Consequently, they may cause several diseases associated with cancer, cardiovascular disorders, atopic dermatitis, ageing, and inflammation in humans.[Citation2] As free radical scavengers, antioxidants may donate electrons to free radicals, and hence prevent them from causing damage.[Citation3] In the past, synthetic antioxidants have been effectively used to prevent the oxidation of food and cosmetics. Such antioxidants include butylated hydroxyltoluene (BHT), butylated hydroxyanisole (BHA), and tertiary butylhydroquinone (TBHQ).[Citation4] However, the use of synthesized antioxidants is limited, because it may lead to cancer and toxic effects through the modification of biological enzymes and lipids; hence, consumers generally avoid products that contain added synthetic antioxidants[Citation5] As a result, natural antioxidants that originate from plants, grains, vegetables, and fruits are preferentially used. Such antioxidants include ascorbic acid, α-tocopherol, and tocotrienols. Various studies have been conducted on the content of phenolic compounds, including flavonoids, phenolic acid, tannins, and vitamins because of their safety and nutritional value.[Citation6] In addition, studies have been conducted on the antioxidant activity and/or phytochemical properties of stem bark extract of Schotia latifolia Jacq kale extract, and kiwifruit extract.[Citation7−Citation9]
Persimmon (Diospyros kaki Thunb.) is native to Korea, China, and Japan. It is widely cultivated in East Asia, and is a fruit enjoyed by Asians. Persimmon contains sugars (such as glucose and fructose), vitamins (such as A and C), and tannin (which has an astringent taste). Tannin has physiologically active substances that contribute to antioxidant activity, antibacterial activity, lowering cholesterol, antitumor activity, and removal of heavy metal.[Citation10] Traditionally, persimmon is used in styptics, intestine-contracting agents, and cough medicines. Persimmon has two classes, sweet and astringent, with the latter being used for dried persimmon.[Citation11] More than 22% of total persimmon production in Korea is of the astringent class.[Citation12] In the past, persimmon peel, which is a by-product of dried persimmon, was discarded in large quantities and caused environmental pollution. However, in recent years, several studies have been conducted on reducing the environment pollution caused by persimmon peel. For instance, the potential of various permission peel by-products have been investigated; Kim et al.[Citation11] studied the utility of persimmon vinegar; Kim and Kim[Citation13] studied the effects of dietary persimmon powder on the quality of preserved pork; and Moon et al.[Citation14] studied a range of other products.
Previous studies have evaluated the physiological activities of persimmon peel or the method of processing. The objectives of this study were to investigate the antioxidant effect of persimmon peel extract on different solvents (including distilled water [DW] and 50, 70, and 99% ethanol), for potential use in food. The effects were analyzed by investigating total phenol and flavonoid content, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, β-carotene bleaching inhibition activity, reducing power, superoxide dismutase (SOD)-like activity, and extraction yield.
MATERIALS AND METHODS
Materials
Persimmon was obtained from the region of Gwangyang, Korea. It was dried and the peel was removed. The peel was cut into small pieces, and washed three times with tap water, and then dried using a hot air dryer (Enex-Co-600; Enex, Koyang, Korea) at 50°C for 15 h. The dried peel was pulverized with a blender (KA-2610; Jworld Tech, Ansan, Korea) for 30 s, passed through a 35-mesh sieve, and stored at –20°C until use.
Preparation of Extracts
Dried persimmon peel powder (15 g) was extracted twice using 300 mL of DW, 50, 70, and 99% ethanol overnight in a VS-8480 shaker (Vision Scientific, Bucheon, Gyeonngi) at room temperature. The extract was filtered through Whatman No. 1 filter paper, and the solvent was removed using a CCA-1110 vacuum evaporator (Rikakikai, Tokyo, Japan) at 45°C until dry. After evaporation of the ethanol, the persimmon peel ethanolic extracts were dissolved in DW (5% v/w).
Extraction Yields
Extraction yields for each solvent were calculated by subtracting the dried weight of test material residue after extraction from the weight of the original plant material.[Citation15]
Total Phenol Content
The total phenol content was determined by the Folin–Ciocalteu method.[Citation16] DW was added to 0.1 mL of extract sample (1 mg/mL), and then 0.3 mL of 2% Na2CO3 solution was added. After 3 min, the solution was mixed with 3.5 mL of the Folin–Ciocalteu reagent (diluted 3-fold with DW), and thoroughly vortexed for 10 s. After 2 h at room temperature, the absorbance was measured at 760 nm by using a Libra S22 UV-vis spectrophotometer (Biochrom, Cambridge, England). The amount of total phenolic compounds was calculated as milligrams of gallic acid equivalents (GAE) from the calibration curve of gallic acid standard solution, and expressed as mg gallic acid/g of dried plant extract.
Total Flavonoid Content
Total flavonoid content was determined using the method of Woisky and Salatino,[Citation17] with some modifications. In brief, to 0.5 mL of each persimmon peel extract (1 mg/mL), 0.5 mL of 2% AlCl3 ethanol solution was added. After 1 h at room temperature, absorbance was measured at 420 nm. Total flavonoid content (TFC) was calculated as kaempferol from a calibration curve.
DPPH Radical Scavenging Activity
The antioxidant activities of the persimmon peel extracts were measured by evaluating their free radical-scavenging effects on the DPPH radical. The value was modified based on the method of Brand-Williams et al.[Citation18] A 0.2-mL aliquot of each extract (0.1–10 mg/mL) was mixed with 7.8 mL of DPPH methanolic solution. The solution was vortex-mixed for 10 s. The absorbance at 517 nm was measured after 10 min in a Libra S22 UV-vis spectrophotometer (Biochrom, Cambridge, England). DPPH radical scavenging activity was calculated according to the following equation:
In addition, the DPPH radical scavenging activity presented by the IC50 value denotes that 50% DPPH is present in the test solution. The synthetic antioxidant reagent BHT, was used as the positive control.
β-carotene Bleaching Inhibition Activity
Evaluation of antioxidant activity, based on coupled oxidation of β-carotene and linoleic acid, was conducted as described by Taga, Miller, and Pratt,[Citation19] with some modifications. β-carotene (2 mg) was dissolved in 20 mL of chloroform. A 3 mL aliquot of the solution was added to a conical flask with 40 mg linoleic acid and 400 mg Tween 40. Chloroform was removed with a rotary evaporator at 50°C. Oxygenated DW (100 mL) was added to the β-carotene emulsion and mixed well. Aliquots (3 mL) of the oxygenated β-carotene emulsion and 0.12 mL of the water-soluble potato extract were placed in test tubes and mixed well. The tubes were immediately placed in a water bath and incubated at 50°C. Oxidation of β-carotene emulsion was monitored spectrophotometrically by measuring absorbance at 470 nm. Sample absorbance was measured at 10, 20, 30, and 40 min after the addition of oxygenated water, and then the sample was incubated at 50°C. A control consisted of 0.12 mL DW, instead of potato extract. The degradation rate of vegetable extracts was calculated according to first order kinetics:
Reducing Power
The reducing power of the plant leaf extracts was determined according to the method of Oyaizu.[Citation20] Briefly, 2.5 mL of extracts of different concentrations (0.1–10 mg/mL) was mixed with 2.5 mL of 200 mmol sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min. After 2.5 mL of 10% trichloroacetic acid was added, the mixture was centrifuged at 800 rpm for 20 min. The supernatant (2.5 mL) was mixed with 2.5 mL DW and 0.5 mL ferric chloride, and the absorbance was measured at 700 nm against a blank control in a Libra S22 UV-vis spectrophotometer (Biochrom, Cambridge, England). The higher absorbance of the reaction mixture indicated greater reducing power. In addition, the reducing power presented by the EC50 value denoting the effective concentration at which the absorbance was 0.5. The synthetic antioxidant reagent BHT, was used as the positive control.
SOD-Like Activity
SOD-like activity was measured by the method of Marklund and Marklund.[Citation21] A 0.3 mL aliquot of each sample solution was mixed with 0.3 mL of 7.2 mM pyrogallol and 4.5 mL of Tris-HCl (pH 8.5) containing 50 mM Tris (hydroxymethyl) aminomethane and 10 mM EDTA. After 10 min of incubation at 25°C, 1.5 mL of 1 N HCl was added. The absorbance of the reaction mixture was read at 420 nm. The capability of scavenging SOD was calculated using the following equation:
A decrease in the absorbance of the reaction mixture indicates increased superoxide radical anion scavenging activity.
Statistical Analysis
Analysis of variance was performed on all of the variables that were measured using the General Linear Model (GLM) procedure of the SAS statistical package.[Citation22] The Duncan’s multiple range test (P < 0.05) was used to determine differences between treatment means. The IC50 and EC50 values were calculated from linear regression analysis. The correlations between antioxidant activity assays for the extracts of persimmon peel were analyzed using the Pearson’s test in the SAS package.
RESULTS AND DISCUSSION
Extraction Yield
Extraction yields of persimmon peel in ethanol with different percentage were presented in (33.51–47.42%). PPE by 70% ethanol was the highest extraction yield at values of 47.42% and PPE by DW was the lowest extraction yield at values of 33.51%. Extraction yield affected by various factor such as extraction method, solvent, time, and temperature.
Table 1 Total phenol and flavonoid content of persimmon peel extracts (PPE) by different levels of ethanol
Total Phenol Content
Polyphenolic compounds are aromatic compounds having more than phenolic hydroxyl groups (–OH); these compounds have various structures and molecular weights and contribute to plant color. In addition, phenolic compounds are associated with antioxidant activity, playing a role as hydrogen donors, singlet oxygen quenchers, reducing agents, hydroxyl radical quenchers, and metal chelators.[Citation23]
Total phenol content of PPE for different amounts of ethanol is presented in . The phenol content was determined using the Folin–Ciocalteu method. Total phenol content ranged from 2.30 to 12.39 mg GAE/g of dried PPE for different amounts of ethanol. This result supported that of Hwang et al.[Citation24] who found that persimmon peel contained total phenols of 8.80–18.31 mg/g dried PPE. The 70% ethanol solution extracted the largest (P < 0.05) total phenol content (12.39 mg GAE/g of dried extract) from persimmon peel. A recent study showed that lyophilized water extract from kiwifruit has a total concentration of 16.67 μg GAE/mg total phenolic compounds.[Citation8] Furthermore, You[Citation25] observed that total phenol content of Yuza peel extracts was higher than that of Yuza fruit extracts. According to Mbaebie, Edeoga, and Afolayan,[Citation9] the hydroxyl groups of phenolic compounds may contribute to biophysical activities, such as antioxidant, antimicrobial, anti-inflammatory, and anticancer activities, through binding to giant molecular structures, such as proteins. In addition, the quantity of phenolics may affect the stability of radical intermediates against superoxide anion radicals that are produced by various enzymes.[Citation26]
Total Flavonoid Content
The antioxidant activity of flavonoids is associated with its basic backbone of diphenylpropanes (C6-C3-C6), with a central pyran ring.[Citation27] These compounds act as scavengers and inhibitors of free radicals, and their antioxidant activity is regulated by the terminal radical chain reaction, which is dependent on the number or position of hydroxyl groups and glycosylation of flavonoid molecules.[Citation28] In addition, a previous study stated that the antioxidant activity of flavonoids is caused by the inhibition of free radicals and the metal chelating activity of 3-hydroxy/4-keto group or 5-hydroxy/4-keto group.[Citation8]
The total flavonoid content of PPE was 43.55, 105.21, 117.02, and 95.04 mg of kaempferol eq./g in 0% [DW], 50, 70, and 99% ethanol, respectively (). The PPE of 70% ethanol was the highest (P < 0.05), while that of 0% ethanol [DW] was the lowest (P < 0.05) in TFC. This result supports data obtained for pineapple extracts, in which 70% ethanol had the highest total flavonoids (4.14 mg catechin equivalents (CEQ)/100 g fresh weight) and total phenolics (54.7 mg GAE/100 g fresh weight).[Citation29] These differences in total flavonoid content and total phenol content with respect to the ratio of solvent may be due to the polarity of the solvent in relation to the solubility of phenolic compounds. For instance, Meyer et al.[Citation30] reported that the antioxidant activity of dissolved phenolics depends on the type of extraction solvent, extraction procedure, and condition.
DPPH Radical Scavenging Activity
Free radicals cause the autoxidation of unsaturated lipids, with antioxidants potentially inhibiting biological damage to humans by initiating (or propagating) the step of lipid oxidation. Antioxidants may stabilize DPPH free radical scavengers by donating hydrogen. DPPH radical scavenging activity on antioxidants has been subject to extensive study using spectrometry, because of the convenience and sensitivity of this technique.[Citation31] An increase in antioxidant activity is shown by decreased absorbance at 517 nm and a color change from violet to yellow.[Citation32]
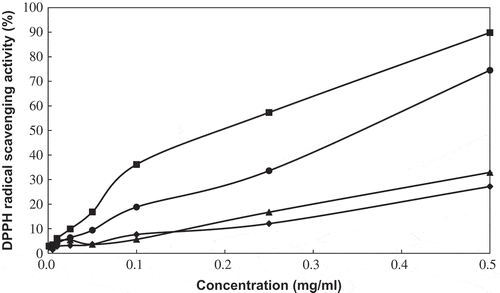
shows the DPPH radical scavenging activity of PPE (0.001–0.5 mg/mL) with respect to different percentages of ethanol. At a concentration of 0.001 mg/mL PPE, no significant difference (P > 0.05) was found among treatments, except for DW, which had the lowest DPPH inhibition ability (P < 0.05). DPPH radical inhibition activity increased following an increase in PPE concentration. However, PPE slowly increased with DW and 99% ethanol compared to the other extracts. At 0.5 mg/mL PPE, the DPPH radical scavenging activity of PPE with different percentages of ethanol was ordered as follows: 70% ethanol (89.82%) > 50% ethanol (74.50%) > 99% ethanol (32.89%) > DW (27.19%). Recently, there have been many studies on the DPPH radical scavenging activity of extracts from plants. For instance, aqueous stem bark and kiwifruit were reported to have 79.14% DPPH activity at 0.2 mg/mL and 16.7% at 30 μg/mL, respectively.[Citation8]Citation10] According to Mbaebie, Edeoga, and Afolayan,[Citation9] DPPH generally exhibits antioxidant activity when free radical inhibition activity is at 50%. IC50 equals the concentration of extracts when the inhibition percentage is 50%, while extracts with amounts higher IC50 have lower inhibition activity. The IC50 values were 981.1, 337.4, 243.7, 804.9, and 195.0 ppm in DW, 50, 70, and 99% ethanol, and BHT, respectively (). Gupta and Prakash[Citation23] reported IC50 of DPPH to be 9.62 mg/mL in T. graecum and 19.89 mg/mL in Amaranthus sp. In the current study, it was shown that PPE with 70% ethanol had the highest phenol content and the highest DPPH inhibition activity, which was related to total phenol content.
Table 2 IC50 of DPPH radical scavenging activity (ppm), EC50 of reducing power (mg/ml), and β-carotene bleaching inhibition activity (%) of persimmon peel extracts by different levels of ethanol
β-carotene Bleaching Inhibition Activity
The oxidation of linoleic acid produces peroxyl free radicals, which extract hydrogen atoms from one of the diallylic methylene groups that attack β-carotene. The β-carotene is prone to free radical-mediated oxidation, inducing discoloration from orange to white, because the molecular structure of unsaturated β-carotene is unstable on oxidation.[Citation32] However, the amount of β-carotene discoloration may be interrupted by antioxidant activity through the donation of hydrogen atoms. The β-carotene–linoleic acid model system determines primary antioxidant activity through the extent of spectrophotometric change in orange color.[Citation33]
The antioxidant activity of PPE with respect to different percentages of ethanol was evaluated using the β-carotene–linoleic acid model system, and expressed by comparison with BHT (). In this study, β-carotene bleaching inhibition activity was higher (p > 0.05) for the PPE with 70% ethanol (91.26%) compared to that with BHT (91.02%) (data not shown), and it was accompanied by strong antioxidant activity. These results may be attributed to the amount of phenolic compounds that is extracted by 70% ethanol. Shyu et al.[Citation34] reported that β-carotene bleaching was inhibited by EC50 156.08 μg of dried dill flower extract. The EC50 equals the concentration of extracts when the inhibition percentage is 50% in β-carotene bleaching activity.
Reducing Power
Reducing power is one of the indicators of antioxidant activity, and it helps determine secondary antioxidant activity by allowing the measurement of the amount of reduction through the stabilization of reactive radicals by donated electrons. The yellow color of this solution changes to a blue or green color, depending on the antioxidant activity (reducing power) of the extracts. Through the antioxidant transfer of Fe3+/ferricyanide complexes to the ferrous (Fe2+) form, it is possible to measure reducing power though absorbance at 700 nm.[Citation8] In addition, the stronger green color has higher absorbance and lower EC50, which indicates high antioxidant activity of the samples.
illustrates the reducing power of PPE (0.1–1.0 mg/mL) with different percentages of ethanol. The reducing power gradually increased with increasing concentration in all PPE treatments. This result supported that of Mbaebie et al.[Citation9] who reported a concentration-dependent increase in the reducing power of aqueous stem bark extract from Schotia latifolia Jacq. Extraction of PPE with DW did not have a major effect on the reducing power of PPE and produced the lowest values. Extraction of PPE with 70% ethanol had a major effect on its reducing power and lowest EC50 () at the same concentration as DW. This high reducing power may be attributed to mono- and dihydroxyl substitutions, which may donate hydrogen present in the aromatic ring.[Citation32] BHT is one of the synthetic antioxidants, with a reducing power range of 2.27 to 2.41 (data not shown) at concentrations of 0.1–1.0 mg/mL. The reducing power of 1 mg/mL PPE with solvent was ordered as follows: BHT > 70% ethanol > 50% ethanol > 99% ethanol > DW.
Table 3 Superoxide dismutase (SOD)-like activity of persimmon peel extracts by various levels of ethanol
Figure 2 Reducing power of PPE by different levels of ethanol. (◆) DW: PPE by DW, (•) 50%: PPE by 50% ethanol, (▪) 70%: PPE by 70% ethanol, and (▲) 99%: PPE by 99% ethanol.
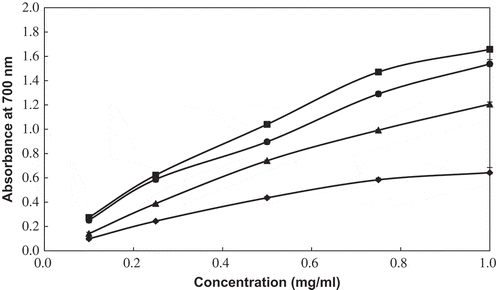
Table 4 Correlation coefficients, R, for relationships between antioxidant activity assays for the extracts of persimmon peel (PPE) (n = 48)
SOD-Like Activity
Most organisms obtain energy through the process of aerobic respiration, consequently producing superoxide radicals from oxygen. These superoxide radicals (superoxide anions) cause oxidative damage and result in diseases.[Citation35] SOD, which is a catalase, converts oxygen free radicals to peroxide at the cellular level, and the peroxide is then converted to H2O or O2 by the actions of catalase or glutathione peroxidase. SOD-like activity is used to measure the scavenging activity of superoxide anion radicals, which are produced during autoxidation of pyrogallol.
The SOD-like activities of different concentrations of PPE (100–1000 ppm) in different percentages of ethanol are shown in . An increase in PPE concentration may have a major effect on SOD-like activities. PPE in 70% ethanol containing total phenol compounds had the highest SOD-like activity. Similar results were reported by Lee, Kim, and Lee,[Citation36] who used Lespedeza bicolor extract and showed the influence of phenolic compounds and high anion radical scavenging activity. In the current study, SOD-like activity was ordered as follows: 70% ethanol > 50% ethanol > 99% ethanol > DW.
Correlations of Antioxidant Activity with Total Phenolic and Flavonoid Content
shows the correlation coefficients for the relationship of total phenol content, total flavonoid content, IC50 of DPPH, and IC50 with respect to PPE reducing power obtained by using the Pearson’s test. The lower values in IC50 of DPPH and reducing power had higher antioxidant activity. In general, the total phenol content resulted in significant (P < 0.001) negative linear correlations with IC50 of DPPH (R = –0.8218) and IC50 with respect to PPE reducing power (R = –0.9259). However, the correlation coefficient in this study was lower than that reported by Zeng et al.[Citation37] who recorded a total phenol content correlation coefficient of 0.9817 in 1/IC50 of DPPH and 0.9876 in reducing power. These results indicate that an increase in total phenol content may occur with decreasing IC50 of DPPH and IC50 with respect to PPE reducing power, supporting that PPE has high antioxidant activity. Similarly, the negative linear correlation (P < 0.001) between TFC with IC50 of DPPH (R = –0.8719) and IC50 of reducing power (R = –0.9924) shows that TFC is closely related to the antioxidant activity of PPE. Furthermore, a significant (P < 0.001) relationship of 0.9242 was found for TFC with total phenol content, with flavonoid belonging to phenolic compounds depending on the antioxidant activity of PPE.
CONCLUSION
In this study evaluated the antioxidant activity and phenolic content of PPE by different percentages solvents (DW, 50, 70, and 99% ethanol). The 70% PPE was the highest and 50% PPE was the lowest in total phenol content, total flavonoid content. The DPPH radical scavenging activity at 0.5 mg/mL PPE and SOD-like activity was ordered as follows: 70% ethanol > 50% ethanol > 99% ethanol > DW. The β-carotene bleaching inhibition activity was higher (P > 0.05) for the PPE with 70% ethanol (91.26%) compared to that with BHT (91.02%) containing strong antioxidant activity. The reducing power of 1 mg/mL PPE with solvent was ordered as follows: BHT > 70% ethanol > 50% ethanol > 99% ethanol > DW. The 70% PPE which had the highest total phenol and flavonoid content had excellent antioxidant activity comparing others. In other words, the total phenol and flavonoid content closely related with antioxidant activity. As it can be seen in these results, PPE can be used as natural antioxidants for health and 70% PPE may be the most effective natural antioxidant in the food industry.
REFERENCES
- Babior, B.M. Phagocytes and oxidative stress. American Journal of Medicine 2000, 109, 33–44.
- Aruma, O.I. Nutrition and health aspects of free radicals and antioxidant. Food and Chemical Toxicology 1994, 62, 671–683.
- Stukey, B.N. Handbook of Food Additives. In: Antioxidants as Food Stabilizers; Furia, T.E.; Ed.; CRC Press: Cleveland, OH, USA, 1972, pp. 185–224.
- Roy, L.G.; Arabshahi-Delouee, S.; Urooj, A. Antioxidant efficacy of Mulberry (Morus Indica L.) leaves extract and powder in edible oil. International Journal of Food Properties 2010, 13, 1–9.
- Branen, A.L. Toxicology and biochemistry of butylated hydroxylanisole and butylated hydroxytoluene. Journal of the American oil Chemists’ Society 1975, 52, 59–62.
- Rufino, M.S.M.; Fernandes, F.A.N.; Alves, R.E.; Brito, E.S. Free radical-scavenging behavior of some north-east Brazilian fruits in a DPPH· system. Food Chemistry 2009, 114, 293–295.
- Ayaz, F.A.; Hayırlıoglu-Ayaz, S.; Alpay-Karaoglu, S.; Grúz, J.; Valentová, K.; Ulrichová, J.; Strnad, M. Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities.Food Chemistry 2008, 107, 19–25.
- Bursal, E.; Gülçin, I. Polyphenol contents and in vitro antioxidant activities of lyphilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Research International 2011, 44, 11482–1489.
- Mbaebie, B.O.; Edeoga, H.O.; Afolayan, A.J. Phytochemical analysis and antioxidants activities of aqueous stem bark extract of Schotia latifolia Jacq. Asian Pacific Journal of Tropical Biomedicine 2012, 2, 118–124.
- Seo, J.H.; Jeong, Y.J.; Kim, K.S. Physiological characteristics of tannins isolated from astringent persimmon fruits. Korea Journal of Food Science and Technology 2000, 32, 212–217.
- Kim, S.K.; Lee, G.D.; Chung, S.K. Monitoring on fermentation of persimmon vinegar from persimmon peel. Korean Journal of Food Science and Technology 2003, 35, 642–647.
- Statics Korea. Agricultural Products Research, Food Industry Statics System. (2010).
- Kim, Y.J.; Kim, B.K. Effect of dietary persimmon peel powder on physic-chemical properties of pork. Journal for Food Science of Animal Resources 2005, 25, 39–44.
- Moon, K.D.; Kim, J.K.; Kim, J.H.; Oh, S.L. Studies on valuable components and processing of persimmon flesh and peel. Korean Journal of dietary culture 1995, 10, 321–326.
- Cha, J.Y.; Ahn, H.Y.; Eom, K.E.; Pack, B.K.; Jun, B.S.; Cho, Y.S. Antioxidative activity of Aralia elata shoot and leaf extracts. Journal of Life Sciences 2009, 19, 652–658.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. Journal of Agricultural Research 1998, 37, 99–105.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm-Wiss Technology 1995, 28, 25–30.
- Taga, M.S.; Miller, E.E.; Prett, D.E. China seeds as a source of natural lipid antioxidants. Journal of American Oil Chemical Society 1984, 61, 928–993.
- Oyaizu, M. Studies on product of browning reaction prepared from glucosamine. Japanese Journal of Nutrition 1986, 44, 307–315.
- Marklund, S.; Marklund, G. Involvement of superoxide anion radical in the oxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry 1975, 47, 468–474.
- “SAS User’s Guide: Statistics ”. In: Version 10.1, Statistical Analysis System: Raleigh, NC: Inc. 2010.
- Gupta, S.; Prakash, J. Studies on Indian green leafy vegetables for their antioxidant activity. Plant Foods for human Nutrition 2009, 64, 39–45.
- Hwang, I.W.; Kim, E.J.; Kim, Y.I.; Jeong, M.C.; Kim, S.K.; Jeoung, S.G. Physico-chemical properties and antioxidant activity of persimmon peel powder by ground size. Food Security and Rice Industry Revitalization 2010, P5–28, 330.
- You, J.M.; Park, J.B.; Seoung, K.S.; Kim, D.Y.; Hwang, I.K. Antioxidant activities and anticancer effects of YUZA. Food Science and Industry 2005, 38, 72–77.
- Gülcin, Í.; Kirecci, E.; Akkemik, E.; Topal, F.; Hisar, O. Antioxidant and antimicrobial activities of an aquatic plant: duckweed (Lemna minor L.). Turkish Journal of Biology 2010, 34, 175–188.
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, USA, 2004, pp. 421–426.
- Bouziz, M.; Grayer, R.J.; Simmonds, M.S.J.; Damak, M.; Sayad, S. Identification and antioxidant potential of flavonoids and low molecular weight phenols on olive cultivar Chemlali growing in Tunisia. Journal of Agricultural Food chemistry 2005, 53, 236–241.
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chemistry 2009, 155, 785–788.
- Meyer, A.S.; Heinonen, M.; Frankel, E.N. Antioxidant interactions of catechin, cyaniding, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chemistry 1998, 61, 71–75.
- Adnan, L.; Osman, A.; Hamid, A.A. Antioxidant activity of different extracts of red pitaya (Hylocereus Polyrhizus) seed. International Journal of Food Properties 2011, 14, 1171–1181.
- Cao, L.; Si, J.Y.; Liu, Y.; Sun, H.; Jin, W.; Li, Z.; Zhao, X.H.; Pan, L.R. Essential oil composition, antimicrobial, and antioxidant properties of Mosla chinensis Maxim. Food Chemistry 2009, 115, 801–805.
- Sulaiman, S.F.; Ooi, K.L. Antioxidant and anti food-borne bacterial activities of extracts from leaf and different fruit parts of Myristica fragrans Houtt. Food Control 2012, 25, 533–536.
- Shyu, Y.S.; Lin, J.T.; Chang, Y.T.; Chiang, C.J.; Yang, D.J. Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food Chemistry 2009, 115, 515–521.
- Meyer, A.S.; Isaksen, A. Application of enzymes as food antioxidants. Trends in Food Science and Technology 1995, 6, 300–304.
- Lee, K.S.; Kim, M.G.; Lee, K.Y. Antioxidative activity of ethanol extract from lotus (Nelumbo nucifera) leaf. Journal of the Korean Society of Food Science and Nutrition 2006, 35, 182–186.
- Zeng, L.B.; Zhang, Z.R.; Luo, Z.H.; Zhu, Z.X. Antioxidant activity and chemical constituents of essential oil and extracts of Rhizoma Homalomenae. Food Chemistry 2011, 125, 456–463.