Abstract
Two Clavaria mushrooms were investigated as sources of antioxidant compounds, essential trace elements Selenium and Zinc, and as efficient antioxidants in elimination/inhibition of different radical species. Using HPLC method, pistillarin-rare siderphore compound characterized with high antioxidant activity, was detected in both extracts. A higher content of pistillarin has been measured in C. pistillaris extract. The same extract contained the higher content of Selenium, 3.051μg/g. Both extracts showed important antioxidant activity. For C. pistillaris extract lower IC50 value has been measured and higher antioxidant activity against O2− radical has been detected. Although the content of pistillarin was higher in the case of C. pistillaris, at the concentration of 0.2 mg/ml C. fennica extract showed just some higher antioxidant activity against ·OH radicals, 82 and 87%, respectively.
INTRODUCTION
Many centuries ago traditional Asian cultures recognized the significance of every day mushroom consumption. European countries discovered importance of mushrooms in human diet long after Asians. In contrast to Europeans, in many Asian countries there is the diversity in use and cultivation of different mushroom species. In Europe all cultivation is limited only to Agaricus bisporus, Pleurotus ostreatus, Lentinula edodes, and consumption of just few wild mushroom species such are Tuber species, Boletus edulis, Cantharellus cibarius, and Craterellus cornucopiodes has been observed. To provide the higher usage, and to encourage the cultivation of these incredible natural resources, more information, more knowledge, and according to that more research about different unexplored mushroom species that are growing in the European regions are needed.
Mushrooms have been used primarily considering their nutritional values and second, considering their medicinal properties. In the traditional Chinese medicine it is believed that food and medicine share the same origin and the only difference could be their application and use. Recent studies have proven that consuming of mushrooms may have a health benefits on humans. Furthermore, mushrooms have been also reported as a therapeutic food useful in preventing diseases such as hypertension, hypercholesterolemia, and cancer. These functional characteristics are derived from the mushrooms chemical composition. It is well known that mushrooms contain almost all essential amino acids, high level of proteins (they can compete with meat), low levels of fats (among dominant are essential unsaturated), high level of medicinal important carbon-hydrates (such as β-glucans), and high levels of very important trace elements, for example essential Zinc and Selenium.[Citation1,Citation2]
Some health benefits of edible mushroom consumption are considered to be linked to the antioxidant compounds. There are many researches showing that mushrooms provide an important antioxidant support.[Citation3−Citation5] Also, many studies have identified network of antioxidant compounds among important are phenolic, or some specific, but equally important, such as glutathione or ergothioneine.[Citation6] Trace elements of Selenium and Zinc, present in mushrooms and their extracts, also play an important role in antioxidant defence systems. They are not antioxidant compounds, but are important elements of antioxidant systems. Selenium exerts its biological effects as a constituent of seleno proteins involved in a wide variety of process in the body, including antioxidant defence and redox metabolism, thyroid metabolism, immune function, reproductive function, and many others, with implications of clinical importance in many diseases such as cancer and autoimmune thyroid diseases.[Citation7,Citation8] Zinc antioxidant properties belong to structures like Zn-metallothionein, or antioxidant enzyme Cu-Zn superoxide dismutase. Deficiency of Zinc leads to oxidative damage of lipids, protein, DNA oxidation, or diseases such as cancer and atherosclerosis. Zinc possesses many different mechanisms of antioxidant functioning, among importance is the replacement of redox active metals, Copper and Iron, in membrane binding sites and thus inhibits the oxidation of liposomes.[Citation9]
Considering the demand on research of natural antioxidants/antioxidants sources, and required interest for edible mushrooms as a functional food, antioxidant compounds, antioxidant capacity, and content of essential Zinc and Selenium of two selected wild edible Clavaria mushrooms were investigated. Antioxidant properties of these species could be very important, as it has been reported that Clavaria mushrooms content pistillarin, a highly antioxidant catecholate compound characterized with high iron chelating properties.[Citation10]
MATERIALS AND METHODS
Chemicals
1,1-Diphenyl-2-picryl-hydrazyl-hydrate (DPPH), Folin-Ciocalteu reagent, (±) -catechin, gallic acid, hypoxanthine, and xanthine oxidase was purchased from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). DEPMPO (5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide) was purchased from Alexis Biochemical (Laursen, Switzerland). H2O2 was purchased from Renal (Budapest, Hungary). All other chemicals and reagents were of analytical reagent grade.
Plant Material
Wild growing mushrooms Clavaria pistillaris (also known as Clavariadelphus pistillaris) and Clavaria fennica (also known as Ramaria fennica) were collected from the same location, near village Mune in Istra region in Croatia, in 2009 at the same time of summer. In the scientific literature, there is very little information about these two mushroom species. According to Boa, his book presented global list of wild fungi (used as food, said to be edible or with medicinal properties),[Citation11] C. pistillaris has been clearly reported as a food. Edibility of C. fennica in scientific research is still unclear and this will be defined as a mushroom with unknown edibility. Mushrooms fruiting bodies were cleaned to remove any residual compost. Fresh mushrooms were air-dried and then stored in air-tight plastic bags at the room temperature.
Sample Preparation
All dried mushroom samples were ground in a blender before the extraction. Mean particle size of ground material has been determined using sieve set (Erweka, Germany). Particle size of ground C. pistillaris was 0.303 ± 0.0001 mm and of C. fennica 0.301 ± 0.0003 mm. This way prepared mushroom sample (10.0 g) was extracted using 50% ethanol (50.0 ml). The extraction process was carried out using ultrasonic bath (B-220, Branson and SmithKline Company, USA). Extraction temperature was set at 45°C, and the extraction time was 40 min. After extraction, the obtained extract was filtrated and extraction solvent was removed by rotary evaporator (Devarot, Elektromedicina, Ljubljana, Slovenia) under vacuum. Extracts were dried at 60°C to the constant mass and placed into glass bottles and stored to prevent oxidative damage until analysis.
Determination of Antioxidant Compounds
The content of total phenolic compounds in dry mushroom extracts was determined by Folin-Ciocalteu procedure[Citation12] using gallic acid as a standard. Content of total phenolic compounds has been expressed as mg of gallic acid equivalent (GAE) per g of dry mushroom extract. The total flavonoids (TF) content has been determined by aluminium chloride colorimetric assay,[Citation13] using catehin as a standard. It has been expressed as mg of catehin equivalents (CE) per g of dry mushroom extract.
To determine the content of rare component pistillarin LC/MS and HPLC/DAD analysis was performed. The dry extracts (30 mg) were dissolved in 1.5 ml of methanol/water (70/30 vol), using ultrasonic bath. The samples were filtered over 50 μ filter and injected in LC/MS or HPLC/DAD system. LC/MS analysis was performed on an Agilent MSD TOF coupled to an Agilent 1200 series HPLC, using the same column and gradient program were as those, described further, for HPLC–DAD analysis. Mass spectra were acquired using an Agilent ESI-MSD TOF. Drying gas (N2) flow was 12 L/min; nebulizer pressure was 45 psig; drying gas temperature was 350°C. For ESI analysis, the parameters were: capillary voltage, 4000 V; fragmentor, 140 V; skimmer, 60 V; Oct RF V 250 V, for positive and negative modes. The mass range was from 100–2000 m/z. HPLC analysis of the extracts was performed using an Agilent 1200 series HPLC with RR Zorbax Eclipse Plus C18 column (150 × 4.6 mm, 1.8 μm). Mobile phase A was 0.2% formic acid in water, and mobile phase B was acetonitrile. The injection volume was 1 μL, and elution at 0.95 mL/min with gradient program (0–20 min 5–16% B, 20–28 min 16–40% B, 28–32 min 40–70% B, 32–36 min 70–99% B, 36–45 min 99% B, 45–46 min 99–5% B). UV-VIS detection was carried out. Quantification was based on the measured integration area applying the calibration equation.
Determination of Trace Elements Zinc and Selenium
For the analysis of trace elements, Selenium and Zinc, the mushroom extracts were digested in concentrated HNO3 and afterwards the elements were quantified by inductively coupled plasma mass spectroscopy (ICP/MS, PerkinElmer 9000, USA).
DPPH Assay
The free radical scavenging activity of mushroom extracts was determined using simple and fast spectrophotometric method as described by Espin.[Citation14] To prepare different concentrations of investigated samples (0.01, 0.02 and 0.05 mg/ml) dry extracts were dissolved in extraction solvent (50% ethanol). Radical scavenging capacity (%RSC) was calculated by following equation (Eq. (1)):
Antioxidant activity was also expressed as IC50 which represent the concentration of test (extract solution) required for obtaining the 50% of RSC.
Scavenging Activity on ·OH and O2·− Radicals
For the analysis of mushroom extract scavenging capacity on ·OH and O2·− radicals, as a highly sensitive technique, electron paramagnetic resonant spectroscopy (EPR) has been applied. The ability of investigated samples to remove radicals for both ·OH and O2·− has been evaluated in the same manner: By the difference between the amplitudes of the EPR signals of trapped radicals in radical-generating systems, with and without the addition of the investigated extract. Results are presented as relative inhibition (Eq. (2)), which represents the relative decrease of radical production:
In Eq. (2) PArgs is second peak amplitude (radical–generating system), PArgs+e is second peak amplitude (radical-/-generating system + extract) and PIrgs is second peak intensity (radical-generating system).
The ability of mushroom extracts to scavenge ·OH radicals was tested using Fenton reaction as a generating system, while the ability of mushroom extract to scavenge O2·− radicals was tested using generating system hypoxanthine/xanthine-oxidase (HX/XO). The final investigated concentration of extracts was 0.2 mg/ml. Samples with no extract added were used as a control.[Citation15] Antioxidant activity of ascorbate, at the same concentration as in the case of investigated extracts, was determined against both radical species in order to compare relative inhibition of extracts and the inhibition effects of known antioxidant compound. EPR spectra were recorded at a room temperature using Varian E104-A EPR spectrometer operating at X-band (9.51 GHz) with the settings reported by Zivkovic et al.[Citation14] Samples were drawn into 10 cm long gas-permeable Teflon tubes (wall thickness 0.025 mm and internal diameter 0.6mm; Zeus industries, Raritan, USA). Measurements were performed by using quartz capillaries in which Teflon tubes were placed. Recordings were preceded 2 min after the beginning of reaction, with 4 min of recording time.[Citation15]
RESULTS AND DISCUSSION
Determination of Antioxidant Compounds
For preparation of dry C. pistillaris and C. fennica mushroom extracts, 50% ethanol as extraction solvent, has been used. Ethanol is non-toxic, so extracts obtained using this type of solvent could be further considered as safe for human use and consumption. Also, as it’s polar solvent it could be considered as efficient for polar antioxidant (phenols/flavonoids) extraction. Obtained extraction yield of investigated mushroom extracts has been presented in the . Almost double the extraction yield has been obtained in the case of C. pistillaris extraction process.
Table 1 Extraction yield and content of analyzed components (TP, TF, and pistillarin) in obtained C. pistillaris and C. fenica dry extracts
Many compounds present in the investigated material can be responsible for its antioxidant activity. In most investigations considering mushrooms, or products obtained from the mushrooms,[Citation3,Citation4,Citation16] phenolic compounds are reported as key compounds responsible for antioxidant activity. In the case of C. pistillaris and C. fenica dry mushroom extracts, antioxidant activity could be linked to phenolic compounds, or some specific chemical structure like rare siderphore pisillarin. In investigated mushroom extracts these compounds, responsible for antioxidant activity, were detected and quantified. According to the fact that chemical structure is the base of antioxidant activity, antioxidant properties of detected compounds could be explained by the presence of conjugated ring structures and/or the number and arrangement of hydroxyl groups, or some other specific structural characteristic. Content of total phenols (TP) and content of TF, as a group of phenolics compounds with strongest antioxidant properties, has been determined in analyzed mushroom extracts and presented in the table below ().
Much higher content of total phenolic compounds has been determined in C. pistillaris (72.48 mg/g) in comparison to content in C. fennica dry extract (42.27 mg/g). The difference in content of TF was similar as in the case of total phenolic compounds. Much higher contents of flavonoid compounds have been detected in C. pisillaris dry extract, 36.51 mg/g. The higher content of investigated compounds could implicate the higher antioxidant activity of edible C. pistillaris mushroom and their dry extract. The content of TP in investigated mushroom extracts was higher than reported for different mushroom species by Elmastas et al.,[Citation17] Puttaraju et al.,[Citation18] and Chey et al.,[Citation16] except in case of Hygrocybe sp., whose total phenol content in methanolic extract was similar to content determined in C. fennica dry extract.
Usually phenolic compounds are considered as responsible for antioxidant activity but, as reported by Perez Jimenez and Saura-Calixto,[Citation19] the presence of non-antioxidant compounds especially amino acids and uronic acid in the test solutions, or some other compounds, may produce more antioxidant capacity and may interfere with polyphenols present in food matrix, producing higher antioxidant capacity to that produced by the total polyphenols alone.
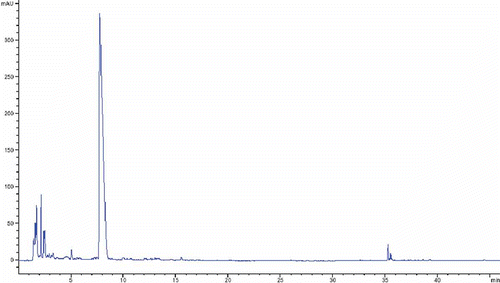
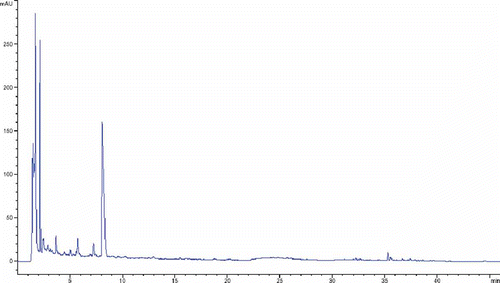
The tentative identification of other antioxidant compounds, like pistillarin, the rare siderphore compound (N1,N8-bis(2,3-dihydroxybenzoyl) spermidine)) (), was performed by LC/MS and HPLC/DAD. Precisely mass measurements of molecular ions of analytes were performed with a time-of-flight (TOF) mass spectrometer, in positive and in negative polarity mode. The dominant species observed were [M+H]+ in positive, and [M-H]- in the negative mode, as well as [2M-H]- ions for some of them, confirming molecular mass. Additionally, spectral data from a UV/VIS photodiode array detector afforded definite determination of many structures. UV/VIS maximums, characteristic for various classes of compound, were compared with the literature data.[Citation20,Citation21] The molecular mass of detected compound was 417.1907 and retention time (tR) was 8.1 min. Pistillarin was detected and further quantified in both investigated mushroom extracts. Specific aromatic ring structure, presence and arrangement of 4 hydroxyl groups, could be a reason for strong antioxidant properties of this compound. Like in the case of TP and flavonoids, the much higher content of pistillarin in C. pistillaris extract, around 3.5 times higher, could contribute to higher antioxidant activity. For both mushroom extracts characteristic chromatograms are presented below ( and ).
Determination of Trace Elements Zinc and Selenium
In C. pistillaris, dry mushroom extract contents of the essential trace element Selenium was 3.05 μg/g, while in C. fennica, mushroom with unknown edibility, this content was lower, 1.03 μg/g. Opposite to the content of Selenium much lower content of trace element Zinc has been determined in C. pistillaris dry extract, 27.7 μg/g, while in C. fennica dry extract this content was 76.07 μg/g. All amounts of investigated trace elements per 1 g of prepared extracts are below the recommended daily amount, but could contribute to the total recommended daily intake. Calculated on dry mushroom weight (DW) content of Zinc in dry C. pistillaris mushrooms was 852.48 μg/100 g, while content of Selenium was 90.28 μg/100 g. Content of Selenium in 100 g of dry C. pistillaris mushrooms was in a range of recommended daily amount of Selenium (50–100 μg), so 100 g of these edible dry mushrooms could satisfy the daily human need for this essential trace element.
DPPH Assay
The first test to evaluate the antioxidant activity of investigated materials in most research scientific papers is DPPH test. Investigation of DPPH· scavenging ability is a widely used method to evaluate antioxidant activity as it is very fast and quite simple compared to some other methods. So, at the extract concentration of 0.02 mg/ml C. pistillaris reaches RSC (%) of 96.32%, while C. fennica gives much lower scavenging capacity, 23.45%. Similar situation was at the 0.01 mg/ml concentration of extract, where RSC for C. pistillaris was 47.70%, while for C. fennica this value reached only 13.45%. RSC was found to exhibit 50% of inhibition value (IC50 value) at the extract concentration of 0.0104 ± 0.001 mg/ml for C. pistillaris. The 50% of inhibition value was achieved at the around three times higher concentration in the case of C. fennica dry mushroom extract (0.0320 ± 0.002 mg/ml). The fact that there is much stronger inhibition of DPPH radical in a case of C. pistillaris dry extract could be explained with much higher content of TP, TF, and pistillarin in this edible mushroom extract.
Scavenging Activity on ·OH and O2·− Radicals
Using more precise determination method (EPR), scavenging of other radical species (·OH and O2·−) in investigated samples was also analyzed. Among others ·OH radical is considered as the most dangerous and powerful free radical species. It is extremely reactive and has been marked as a free radical capable of damaging biomolecules of the living cells.[Citation22] So, it is a main cause of negative effects induced by free radical in living/biological systems. This radical is the main culprit of lipid peroxidation process. Superoxide anion, O2·−, is a reactive oxygen species that is produced by a number of enzyme systems in autooxidation reactions by non-enzymatic electron transfers that reduce molecular oxygen.[Citation23] Although superoxide anion is a week oxidant it gives rise to the generation of ·OH radical, as well as singlet oxygen. It has been implicated in the initialling oxidation reactions associated with aging, and in several other physiological processes due the transformation in mentioned more reactive species. Both, ·OH and O2·−, are contributing to the total oxidative stress process. Because of this analysis of RSC of investigated dry mushroom extracts against both radical species has been provided.
·OH radical has been produced by Fenton reaction and its amount has been determined by amplitude of the EPR signal which originates from the spin trap adduct formed by trapping of the radical. The same experiment has been repeated after addition of investigated extract which should lead to decrease of EPR signal, since the extract, if it possesses antioxidant properties, should remove amount of produced radicals. At the extract concentration of 0.2 mg/ml relative inhibition of ·OH radical was similar for investigated extracts, 82.0 ± 3% for C. fennica and 87.0 ± 5% for C. pistillaris. Scavenging ability of this radical exists for both mushroom extract in similar extents and both are good inhibitors of this radical species. Although the content of pistillarin was higher in the case of C. pistillaris, at the concentration of 0.2 mg/ml C. fennica extract just showed some higher antioxidant activity against ·OH radicals, implicating that this compound might not be a good scavenger of ·OH radical species, or that in the case of C. fennica extract there are some other powerful antioxidant compounds responsible for scavenging of this radical species. There is limited information on mushroom extracts radical scavenging activity against this radical species, so obtained results could be compared only with the results of this study previously obtained and published. This activity was similar to earlier reported for Boletus sp.[Citation24] and higher that reported for ethanolic extract of Daedaleopsis confragosa.[Citation1]
Other radical species, O2·− radicals, has been produced using HX/XO as generating system. Scavenging activity on O2·− radical, at the investigated extract concentration of 0.2 mg/ml, is present in both extracts. At the investigated extract concentration C. fennica dry mushroom extract achieves relative inhibition of 36.0 ± 5%, while some higher inhibition, 45.0 ± 1%, at the same concentration, reaches dry extract of C. pistillaris mushroom. The radical scavenging activity against this radical species was similar to radical scavenging activity of different mushroom species extracts reported by Elmastas et al.,[Citation17] but lower then previously reported for extract of Boletus sp.[Citation24] At the same concentration, the ascorbate, well known antioxidant compound, achieves some higher relative inhibition of ·OH, 100%, in comparison to relative inhibition of both investigated mushroom extracts (87.0 and 82.0%). The ascorbate relative inhibition of O2·− was, in contrarily to previous, much higher (85.0%).
CONCLUSION
Investigations on health benefit compounds from new natural sources can bring new elements into the everyday human diet, new products in the food or pharmaceutical industry. Results of this study contribute to expansion of the knowledge on valuable compounds in Clavaria sp. mushrooms and their extracts. In the investigated mushroom extracts, phenolic compounds, flavonoids, rare siderphore compound pistillarin, and trace elements Zinc and Selenium were analyzed and quantified. C. pistillaris dry extract contains a higher amount of phenolic compounds, flavonoids, and much higher content of pistillarin in comparison to C. fennica extract. Also, the content of essential trace element Selenium was higher in C. pistilaris extract; in fact 100 g DW of this edible mushroom could satisfy the recommended daily amount of this trace element. High extraction yield, together with all previous results, could be an important factor for encouraging the cultivation, further use, and production of edible C. pistillaris mushroom and extracts for use as a component in the food industry or as an ingredient of dietary supplements.
REFERENCES
- Vidović, S.; Mujić, I.; Zeković, Z.; Lepojević, Ž.; Radojković, M.; Živković, J. The antioxidant properties of polypore mushroom Daedaleopsis confragosa. Central European Journal of Biology 2011, 6, 575–582.
- Turhan, S.; Zararsiz, A.; Karabacak, H. Determinaton of elemental levels in selected wild mushroom species in Turkey using non-destructive analytical techniques. International Journal of Food Properties 2010, 13, 723–731.
- Sarikurkuc, C.; Tepe, B.; Yamac, M. Evaluation of the antioxidant activity of four edible mushrooms from Central Anatolia, Eskishir-Turkey: Lactarius deterrimus, Suillus collitinus, Boletus edulis, Xerocomus chrysenteron. Bioresource Technology 2008, 99, 6651–6655.
- Mau, J.L.; Lin, H.C.; Song, S.F. Antioxidant properties of several specialty mushrooms. Food Research International 2002, 35, 519–526.
- Singla, R; Gangulia, A; Ghosh, M. Antioxidant activities and polyphenolic properties of raw and osmotically dehydrated dried mushroom (Agaricus bisporus) snack food. International Journal of Food Properties 2010, 13, 1290–1299.
- Rodrigez Estrada, A.E.; Lee, H.J.; Beelman, R.B.; Jimenez-Gasco, M.; Royse, D. Enhancement of the antioxidants ergothioneine and selenium in Pleurotus eryngii var. Basidomata through cultural practices. World Journal of Microbiology and Biotechnology 2009, 25, 1597–1607.
- Brenneisen, P.; Steinbrenner, H.; Seis, H. Selenium, oxidative stress, and health aspects. Molecular Aspects of Medicine 2005, 26, 256–267.
- Thomson, C. Essentials of Human Nutrition, 3rd. Ed; Mann, J.; Truswell, S.; Eds.; Oxford University Press: London, 2007, 151–159.
- Powell, S.R. The antioxidant properties of Zinc. Journal of Nutrition 2000, 130, 1447S–1454S.
- Pradines, B.; Rolain, J.M.; Ramiandrasoa, F.; Fusai, T.; Mosnier, J.; Rogier, C.; Daries, W.; Baret, E.; Kunesch, G.; Le Bras, J.; Parzy, D. Iron chelators as antimalarial agents: In vitro activity of decatecholate against Plasmodium falciparum. Journal of Antimicrobial Chemotherapy 2002, 50, 177–187.
- Boa, E. Wild edible fungi: A global overview of their use and importance to people. In: Non-Wood Forest Products No. 17; Food and Agricultural Organization of the United Nations: Rome, Italy, 2004; 131–133.
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Markham, K.R. Methods in plant biochemistry, Harborne, J.B.; Dey, P.M.; Eds; Academic Press: London, 1989, 193–237.
- Espin, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. Journal of Agriculture and Food Chemistry 2000, 48, 648–656.
- Živković, J.; Zeković, Z.; Mujić, I.; Gođevac, D.; Mojović, M.; Mujić, A.; Spasojević, I. EPR spin-trapping and spin-probing spectroscopy in assessing antioxidant properties: Example on extract of catkin, leaves, and spiny burs of Castanea sativa. Food Biophysics 2009, 4, 126–133.
- Chey, Y.F.; Wong, Y.J.; Lee, J.S. Nutritional quality and antioxidant activity of selected edible wild mushrooms. Food Science and Technology International 2008, 14, 375–384.
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of antioxidant activity and antioxidant compound in wild edible mushrooms. Food Composition and Analysis 2007, 20, 337–345.
- Puttaraju, G.N.; Venkateshajah, U.S.; Dharmesh, M.S.; Urs, N.M.S.; Somasundram, R. Antioxidant activity of indogenous edible mushrooms. Journal of Agriculture and Food Chemistry 2006, 54, 9764–9772.
- Perez-Jimenez, J.; Saura-Calixto, F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Research International 2006, 39, 791–800.
- Capon, R.J.; Stewart, M.; Ratnajaker, R.; Lacev, E.; Gill, J.H. Citromycetins and bilains A-C: New aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. Journal of Natural Products 2007, 70, 1746–1752.
- Davoli, P.; Weber, R.W.S. Simple method for reversed-phase high-performance liquid chromatographic analysis of fungal pigments in fruit-bodies of Boletales (Fungi). Journal of Chromatography 2002, 964, 129–135.
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutrition Reviews 1997, 55, S44–S52.
- Gulcin, I.; Elmastas, M.; Aboul-Enein, Y.H. Determination of antioxidant and radical scavenging activity of basil (Ocimum basilicum L. Family Laminaceae) assayed by different methodologies. Phytotherapy Research 2007, 21, 354–361.
- Vidović, S.; Zeković, Z.; Mujić, I.; Lepojević, Ž.; Tumbas, V.; Mujić, A. Antioxidant properties of selected Boletus mushrooms. Food Biophysics 2010, 5, 49–58.