Abstract
Jujuboside A (JuA) is a representative saponin with significant sedative and hypnotic effects. Up to now, there were many arguments on its metabolic mechanism. In this study, a high performance liquid chromatography-tandem mass spectrometry (MS/MS) method was developed for investigating the degradation characteristics of JuA incubated with rat feces. With the method, the degradation kinetics of JuA was studied. The results showed JuA decomposed rapidly. It could decompose nearly 100% in only 4–6 h. The degradation regularity was consistent with the first dynamic process. In addition, seven metabolites were determined and identified to be the serial hydrolysis products of JuA. The results revealed that gastrointestinal microbiota played an indispensable role in the metabolic process of JuA. JuA may be a supplier of sugars under the hydrolysis effect induced by the bacterial enzymes. At the same time, its aglycone was produced as a by-product, which could be easier to be absorbed into the body to perform its specific bioactivities.
INTRODUCTION
Saponins are heterosides distributed widely in a variety of plant foods and a few marine animals.[Citation1−Citation3] Many studies have revealed that these compounds possessed various bioactivities, such as anti-cancer,[Citation4] lowering cholesterol,[Citation5] adjusting immune function,[Citation6,Citation7] etc.[Citation8] Due to these protective effects on the human body, they have drawn more and more attention from concerned researchers.[Citation9]
Jujuboside A (JuA) is a main saponin constituent isolated from the seed of Ziziphus jujuba Mill. var. spinosa (bunge) Huex H.F. Chow.,[Citation10] which is traditionally used to cure insomnia in China.[Citation11] In the prophase studies, JuA has been proved as a major pharmacological active compound with significant sedative and hypnotic effects.[Citation12−Citation14] However, its detailed metabolic mechanism is poorly understood. Above all, some researchers indicated that not JuA but its metabolites (especially the aglycon) is the real effective constituent for exerting the sedative function.[Citation15,Citation16] In our previous study, the result was consistent with the prediction.[Citation17] However, how the metabolites occurred and what was the main cause are still not available up to now.
The gastrointestinal system is responsible for the digestion and absorption of various foods. It is well known that the gastrointestinal tract is populated with abundant bacteria that contribute to normal digestive function. Before being absorbed into the body, the food components are metabolized inevitably by extensive reactions performed by these bacteria, respective enzymes, and pH conditions in the gastrointestinal tract.[Citation18] Some studies have shown that the specific metabolites appear in rat plasma after oral administration of the corresponding material, which is transformed in the intestine into metabolites.[Citation19,Citation20] As a result, more and more scientists have noticed significant meaning of the intimate connection between the gastrointestinal tract (especially its microbiota) and nutrition.[Citation21−Citation24]
In this study, a new HPLC–MS/MS approach was used for rapid detection of the JuA transformed by rats’ intestinal bacteria in vitro. In addition, the metabolic process of JuA by rats’ intestinal bacteria was determined with a time-dependent incubation study, and the metabolites were characterized.
MATERIALS AND METHODS
Chemicals and Reagents
JuA (purity over 98.0%, batch number: 110217) was purchased from Shunbo Biotech Co., Ltd. (Shanghai, China). General anaerobic medium (GAM) was purchased from Aoboxing Biotech Co., Ltd. (Beijing, China). Methanol and water (chromatographic pure) were purchased from J.T. Baker (J.T. Baker Chemicals, USA), which was used for all solutions and dilutions and 96% formic acid was obtained from Tedia Co., Ltd. (USA).
Animals
Eight male Wistar rats weighing 180 ± 20 g, approximately 1 or 2 months of age were purchased from the Institute of Materia Medica (Tianjin). The rats were kept on a 12-h light/dark cycle and had free access to standard diet and water, and were acclimated for 7 days before testing. All the experimental procedures were performed under the National Guidelines on the Proper Care and Use of Animals in Laboratory Research. The study was approved by the committee of the institution, and the protocol number was G100011.
Instrumentation and Chromatographic Conditions
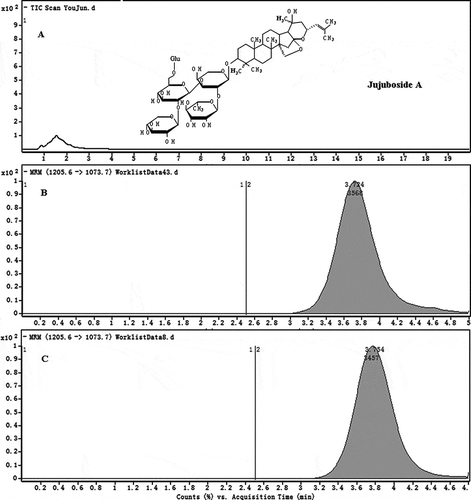
The HPLC system consisted of an Agilent 1100 Series quaternary pump (Agilent, USA). The analytical column was Waters (YMCTM ODS-AQ S-5 120A; 2.0 × 100 mm) (Waters, USA), maintained at 30°C. Chromatographic separation was achieved using a mobile phase consisting of 0.1% formic acid and methanol in a ratio of 50/50 (v/v). The flow rate was 0.3 mL·min−1. An Agilent 1100 Series quaternary mass spectrometer (Agilent, USA) was interfaced via an electrospray ionization (ESI) probe, operating in the negative ion mode with multiple reactions monitoring (MRM). The characteristic precursor [M-H]− to product ions transitions was 1205.6 → 1073.7 for JuA. The instrumental parameters were optimized for maximal generation of the protonated analyte molecules ([M-H]−) and characteristic fragment ions of JuA: heater temperature: 350°C; ion source voltage: 4000V; curtain gas: 8.0 L·min−1; nebulizer gas pressure: 35 p.s.i. The ESI-MS/MS operating parameters were set at 60V as collision energy and ion energy voltage.
Preparation of Standard Solutions and Calibration Curve
First, 6.4 g of the anaerobic medium was added to 200 ml of water and autoclaved at 115°C for 20 min. The fresh feces were obtained from eight healthy rats. The feces and the anaerobic medium solution were then mixed in a ratio of 1 g/15 mL (m/v), and the suspension was centrifuged at 700 g for 2 min at 4°C. Then, the clear supernatant liquid was used as the culture solution of intestinal bacteria. The stock solution of 1 mg·mL−1 JuA was diluted with methanol into gradient concentrations: 1, 2, 4, 8, 10, and 20 μg·mL−1. Next, 100 μl of solution at each concentration was charged in Eppendorf tubes, respectively, and dried with N2. Then, 100 μl of the culture solution was added to each of the Eppendorf tubes. The linearity was determined by analyses of the series of standard solutions. For calibration, the plots for the peak area (y) of the analyte versus the concentration (x) were drawn to obtain linearity.
Method Validation
The sample solutions at low, medium, and high concentration levels (1 μg·mL−1, 10 μg·mL−1, 20 μg·mL−1) were used to assess stability and precision. Inter-day variations were determined for precision of the developed method, and the stability of JuA in sample solutions at −20°C was investigated. The accuracy was validated with the three concentration levels of JuA (4.8 μg·mL−1, 8.8 μg·mL−1, 12.8 μg·mL−1).
Degradation Study
The stock solution of JuA was diluted with methanol into gradient concentrations: 5, 10, and 20 μg·mL−1. Next, 100 μl of solution at these concentrations was charged in Eppendorf tubes, respectively, and dried with N2. Then, 100 μl of the culture solution of intestinal bacteria was added. The sample solution was vortexed for 5 min. Then, the tubes were filled with CO2, and cultivated under the constant temperature of 37°C. Series samples were collected after 0, 15 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 3 h, and 4 h, respectively. The sample solutions were diluted with methanol/water (50/50, v/v) and filtered by 0.45 μm filters. The degradation characters of JuA were studied by detecting the changes of its concentrations.
Conditions for Qualitative Analysis
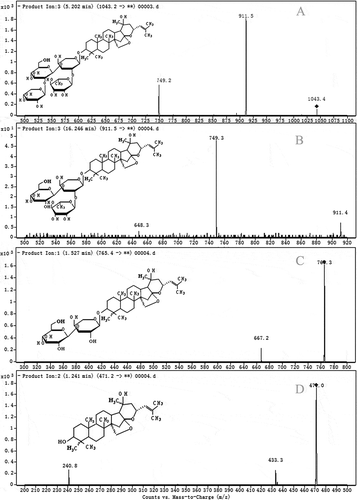
The metabolites were searched and identified with the aid of the product ion scan and the protonated molecules [M-H]−. The conditions for transitions of the metabolites were shown in .
Table 1 MS/MS detection parameters for the metabolites of JuA
Statistical Analysis
For multiple comparisons, data were analyzed by one-way analysis of variance (ANOVA) using SPSS 11.0.1 (SPSS. Inc., Chicago, IL, USA). P-values of less than 0.05 implied significance.
RESULTS AND DISCUSSION
Matrix Effect
Absolute matrix effect (M.E.%) was used to evaluate the MS signal suppression and enhancement effects. It was calculated by comparing the peak areas of JuA dissolved in culture solution (A) and with chromatographic mobile phase (B) at the same concentrations (A/B × 100%). The absolute matrix effects of the method in three levels (1, 10, and 20 μg·mL−1) were in the range of 85–101%, which demonstrated that there was no significant matrix effect and the method was feasible.
Assay Method Validation
The calibration curve range was 1–20 μg·mL−1 for JuA with excellent linearity. A typical calibration equation was Y = 448.71X + 160.41 (R2 = 0.9993, n = 3). A typical LC–MS/MS chromatogram of the sample was shown in . Stability results indicated that the analyte at three concentrations in the solution was stable at −20°C in 8 h. The method showed high precision and accuracy. The RSD% values of the three concentrations were all less than 5.0% in inter-day precision (n = 3). summarized the accuracy for JuA of three concentration levels, respectively. It showed that the method was accurate.
Table 2 Accuracy of JuA in sample solutions (n = 3)
Degradation Kinetics
The result showed that JuA decomposed rapidly from the beginning, and down to 20% of original concentration in only 2 h. The degradation characteristics of JuA were identical in different concentrations. The degradation curves of JuA concentration logarithm (y) versus degradation time (x) at 5, 10, and 20 μg·mL−1 were shown in . The three curves were consistent with the first dynamic process though the degradation rates were somewhat different.
Figure 2 Logarithmic degradation curves of JuA in different concentrations incubated with rat’s feces (—) and negative control (- - - -). (n = 3). (a) 5 μg·mL−1; (b) 10 μg·mL−1; (c) 20 μg·mL−1.
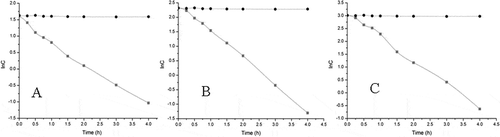
According to the statistical methods, one-factor analysis of variance was carried out to evaluate the degradation rate constants of logarithm of concentration (Klnc). P was far less than 0.05 indicated Klnc of the three concentration levels had significant deviation, which meant the degradation rate of JuA was related to the primal dosage concentration. It was obvious that JuA showed great instability in the environment of intestinal bacteria. In only 4–6 h, JuA could decompose nearly 100%. As a result, it could be deduced that JuA might not be the meaningful component for nutrition, and the metabolites were the real effective material absorbed into the body.
Identification of the Metabolites
Just as shown in , JuA is a dammarane-type triterpenoid glycoside with a glycone of five bonded sugar groups (two glucoses, one arabinose, one xylose, and one rhamnose). These sugars are linked to each other and bound to the aglycone with various specific valence bonds, such as β-D-glc-β-D-glc, β-D-xyl-β-D-glc, and α-L-ara-β-D-glc. From the typical product ions in MS spectrum, seven metabolites (MJ1, MJ2, MJ3, MJ4, MJ5, MJ6, and MJ7) of JuA were detected expressly, and their molecular weights were 1043.2, 911.5, 898.4, 765.4, 750.4, 603.7, and 471.2, respectively. According to their molecular weights and typical fragment ions, the metabolites could be identified as a series of the hydrolysis products from JuA. MJ1 was from JuA loss of a glucose [M-C6H12O6+H2O]−, MJ2 was the loss of a glucose and a xylose [M-C6H12O6-C5H10O5-+2H2O]−, MJ4 was the loss of a glucose, a xylose, and a rhamnose [M-C6H12O6-C5H10O5-C6H12O5+3H2O]−, and MJ7 was the aglycone from the loss of all the sugars [M-2C6H12O6-2C5H10O5--C6H12O5+5H2O]−. Of course, MJ3, MJ5, and MJ6 were all from the loss of the specific sugars correspondingly. The chromatograms of these typical metabolites (MJ1, MJ2, MJ4, and MJ7) were shown in . In addition, the quantity of MJ7 increased as the extension of the degradation process. MJ7 might be the real ultimate product of the degradation.
Enzymatic hydrolysis is a process in digestion in which macromolecules are split from food. It has been well known that there are many families of glycoside hydrolases in the gut produced by the microbiota, which play a key role in maintaining human health to exploit dietary polysaccharides and host glycans as nutrients.[Citation25,Citation26] In this case, JuA might be a supplier of sugars under the hydrolysis effect induced by the bacterial enzymes. At the same time, its aglycone is produced as a by-product, and is easier to be absorbed into the body to perform its specific bioactivities. JuA could be hydrolyzed into serial metabolites, which further revealed the multiplicity and multifunctionality of the gut bacterial hydrolyase. Many chemicals are stored with the form of inactive glycosides in the natural foods. They usually could be activated by enzyme hydrolysis to make themselves available for use. The findings in this study indicated that the gastrointestinal microbiota played an indispensable role in the process, which is meaningful to food nutrition research.
CONCLUSION
A rapid and sensitive LC–MS/MS method was developed and validated for the determination of JuA in rat intestinal bacteria. The method was successfully applied to a degradation dynamics study of JuA in vitro. The result showed that the degradation regularity was consistent with the first dynamic process. In addition, seven metabolites were determined and identified to be the serial hydrolysis products of JuA. The result revealed that gastrointestinal microbiota played an indispensable role in the digesting and processing of food nutrition.
ABBREVIATIONS
| JuA | = | Jujuboside A |
| GAM | = | General anaerobic medium |
| MRM | = | Multiple reaction monitoring |
| M.E.% | = | Absolute matrix effect |
FUNDING
This work was supported by a grant from the National Natural Science Foundation of China (No. 31000749, 31101235).
REFERENCES
- Milgate, J.; Roberts, D.C.K. The nutritional and biological significance of saponins. Nutition Reserach 1995, 15, 1223–1249.
- Bialy, Z.; Jurzysta, M.; Mella, M.; Tava, A. Triterpene saponins from the roots of Medicago hybrida. Journalof Agricultural and. Food Chemistry 2006, 54, 2520–2526.
- Tohma, H.S.; Gulçin, I. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza Glabra L.). International Journal of Food Properties 2010, 13, 657–671.
- Wang, G.; Chen, H.; Huang, M.; Wang, N.; Zhang, J.; .Zhang, Y.; Bai, G.; Fong, W.F.; Yang, M.; Yao, X. Methyl protodioscin induces G2/M cell cycle arrest and apoptosis in HepG2 liver cancer cells. Cancer Letter 2006, 241, 102–109.
- Matsuura, H. Saponins in garlic as modifiers of the risk of cardiovascular disease. Journal of Nutrition 2001, 131, 1000S–1005S.
- Kukhetpitakwong, R.; Hahnvajanawong, C.; Homchampa, P.; Leelavatcharamas, V.; Satra, J.; Khunkitti, W. Immunological adjuvant activities of saponin extracts from the pods of Acacia concinna. International Immunopharmacology 2006, 6, 1729–1735.
- Uemura, T.; Hirai, S.; Mizoguchi, N.; Goto, T.; Lee, J.Y.; Taketani, K.; Nakano, Y.; Shono, J.; Hoshino, S.; Tsuge, N.; Narukami, T.; Takahashi, N.; Kawada, T. Diosgenin present in fenugreek improves glucose metabolism by promoting adipocyte differentiation and inhibiting inflammation in adipose tissues. Molecular Nutrition & Food Research 2010, 54, 1596–1608.
- Chan, J.Y.; Koon, J.C.; Liu, X.; Detmar, M.; Kong, S.K.; Fung, K.P. Polyphyllin D, a steroidal saponin from Paris polyphylla, inhibits endothelial cell functions in vitro and angiogenesis in zebrafish embryos in vivo. Journal of Ethnopharmacology 2011, 137, 64–69.
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714.
- Zhang, M.C.; Zhang, Y.Q.; Xie, J.B. Simultaneous determination of Jujuboside A, B and betulinic acid in semen Ziziphi spinosae by high performance liquid chromatography-evaporative light scattering detection. Journal of Pharmaceutical and Biomedical Analysis 2008, 48, 1467–1470.
- Xie, J.B.; Guo, L.; Pang, G.C.; Wu, X.; Zhang, M.C. Modulation effect of Semen Ziziphi Spinosae extracts on IL-1β, IL-4, IL-6, IL-10, TNF-α and IFN-γ in mouse serum. Natural Product Research 2011, 25, 464–467.
- You, Z.L.; Xia, Q.; Liang, F.R.; Tang, Y.J.; Xu, C.L.; Huang, J., Zhao, L.; Zhang, W.Z.; He, J.J. Effects on the expression of GABA(A) receptor subunits by jujuboside A treatment in rat hippocampal neurons. Journal of Ethnopharmacology 2010, 128, 419–423.
- Wang, X.; Yang, B.; Zhang, A.; Sun, H.; Yan, G. Potential drug targets on insomnia and intervention effects of Jujuboside A through metabolic pathway analysis as revealed by UPLC/ESI-SYNAPT-HDMS coupled with pattern recognition approach. Journal of Proteomics 2012, 75, 1411–1427.
- Zhang, M.; Ning, G.; Shou, C.; Lu, Y.; Hong, D.; Zheng, X. Inhibitory effect of Jujuboside A on glutamate-mediated excitatory signal pathway in hippocampus. Planta Medica 2003, 69, 692–695.
- Chen, C.Y.C. Chemoinformatics and pharmacoinformatics approach for exploring the GABA-A agonist from Chinese herb suanzaoren. Journal of the Taiwan Institute of Chemical Engineers 2009, 40, 36–47.
- Chen, C.Y.C. Insights into the suanzaoren mechanism—From constructing the 3D structure of GABA-A receptor to its binding interaction analysis. Journal of the Chinese Institute of Chemical Engineers 2008, 39, 663–671.
- Wang, L.; Zhang, M.C.; Li, B.; Dai, K. The hypnotic effects of alcohol extracts and semi-bionic extracts of semen ziziphi spinosae. Lishizhen Medicine and Materia Medica Research 2010, 21 (cover article).
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. International Journal of Pharmaceutics 2008, 363, 1–25.
- Yang, X.N.; Wang, Y.J.; Liu, Y.S.; Tang, X. Pharmacokinetics of salvianolic acids after intravenous injection, with and without Panax quinquefolium protopanaxadiol saponins, in rats. Journal of Ethnopharmacology 2008, 117, 408–414.
- Wu, X.J.; Liu, L.; Zhang, M.; Wu, D.; Wang, Y.; Sun, Y.; Fawcett, J.P.; Gu, J.; Zhang, J. Simultaneous analysis of isomers of escin saponins in human plasma by liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetic study after oral administration. Journal of Chromatography B 2010, 878, 861–867.
- Neu, J. Gastrointestinal development and meeting the nutritional needs of premature infants. American Journal of Clinical Nutrition 2007, 85, 629S–634S.
- Delzenne, N.M.; Cani, P.D. Interaction between obesity and the gut microbiota: Relevance in nutrition. Annual Review of Nutrition 2011, 31, 15–31.
- Siggers, R.H.; Siggers, J.; Thymann, T.; Boye, M.; Sangild, P.T. Nutritional modulation of the gut microbiota and immune system in preterm neonates susceptible to necrotizing enterocolitis. Journal of Nutritional Biochemistry 2011, 22, 511–521.
- Gupta, P.; Premavalli, K.S. In-vitro studies on functional properties of selected natural dietary fibers. International Journal of Food Properties 2011, 14, 397–410.
- Zhu, Y.; Suits, M.D.L.; Thompson, A.J.; Chavan, S.; Dinev, Z.; Dumon, C.; Smith, N.; Moremen, K.W.; Xiang. Y.; Siriwardena, A.; Williams, S.J.; Gilbert, H.J.; Davies, G.J. Mechanistic insights into a Ca2+-dependent family of α-mannosidases in a human gut symbiont. Nature Chemical Biology 2010, 6, 125–132.
- Hehemann, J.H.; Correc, G.; Barbeyron, T.; Helbert, W.; Czjzek, M.; Michel, G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010, 464, 908–911.