Abstract
In this work, different types of honey were characterized based on their palynological and biochemical characteristics. A mellisopalynological analysis was performed to authenticate the botanical origin of the honey samples. According to this method, the honey types were classified in: acacia (n = 10), linden (n = 10), and rape (n = 10). This article also reports the phenols content, the flavonoids content, and the antioxidant activity of honey samples. The highest level of antioxidant activity was recorded for linden honeys and the lowest for acacia honeys. The multivariate analysis demonstrated to be an important tool in classification and discrimination between different honeys concerning the palynological and biochemical properties.
INTRODUCTION
Honey is a complex food product consisting of major compounds, including monosaccharide (glucose and fructose), and minor compounds, such as proteins (including enzymes), vitamins, minerals, and various bioactive components. Honey is produced by bees from the collected nectar and its physico-chemical, rheological, palynological, sensory, and biological composition depends on the nectar source.[Citation1,Citation2] Honey composition may also depend on the geographical origin, season, processing, packaging, and storage conditions.[Citation1,Citation3–Citation5]
Several studies have been conducted in order to evaluate the characterization of honeys from different floral origins (e.g., acacia, lime, chestnut, blackberry, eucalyptus, sun-flower) from different countries.[Citation6–Citation11] Melissopalynology represents the most common technique to evaluate the contribution of different plants in the honey produced by bees and let to elaborate the pollen spectrum of the product. The pollen spectrum depends on the floral, agricultural, and forest conditions where honey is produced. The interpretation of this pollen spectrum must take into consideration the different production capacities of plants in relation to those resources that have a considerable beekeeping importance, especially nectar and pollen.[Citation12] Usually, honeys are nominated as monofloral when at least 45% of pollen grains come from the considered plant. For honey samples having under-represented pollen grains (i.e., Robinia pseudoacacia, Tilia, Rosmarinus, Citrus, Thymus, and Lavandula), botanical classification may be achieved with a pollen frequency percentage of only 10–20%. However, for honey samples having over-represented pollen grains (i.e., Brassica napus, Eucalyptus, Castanea, and Myosotis), botanical origin may be achieved with a pollen frequency percentage of 70–90% [Citation13,Citation14].
Recently, much attention has been devoted to natural antioxidants and their association with health benefits.[Citation15–Citation18] Honey serves as an antioxidant source due to its bioactive components, such as phenols (phenolic acids and flavonoids), which are efficient in reducing heart disease, cancer, immune system decline, autism disease, gastrointestinal disorders, asthma, chronic wounds, skin ulcers, and cataracts. The antioxidant potential of phenolic compounds is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donators, and singlet oxygen quenchers.[Citation19] The phenolic compounds in foods are increasingly of interest because they retard oxidative degradation of lipids and improve the nutrition and quality of food.[Citation20] Several studies were carried out in order to evaluate the antioxidant capacity of honey samples and its correlation with phenolic compounds.[Citation5,Citation21–Citation28]
In Romania, due to the geographical and climatic conditions that provide an appropriate environment for apiculture, honey production has been well developed. Romania is 19th in worldwide honey production with 39,000 beekeepers, and honey production in a normal year is roughly 20,000 tons. The main Romanian honeys are acacia, lime, rape, sun-flower, and polyfloral. The aim of this study was to increase knowledge of the Romanian unifloral honeys based on their botanical origin, the antioxidant activity, and some biochemical components.
MATERIAL AND METHODS
Materials
A total of 30 honey samples from different floristic regions of Romania were collected directly from beekeepers, who guaranteed their floral origin, during the 2009–2010 honey harvests. The samples were stored in plastic containers at 4°C prior to analysis. All of the analyses were carried out in duplicate.
CHEMICALS
Folin-Ciocâlteu reagent, gallic acid, quercetin, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma Aldrich and all other reagents were of analytical grade.
Melissopalynological Analysis
The pollen analysis was carried out according to Louveaux’s method,[Citation13] with some modifications, using an Olympus Bx 50 Microscope. Ten grams of honey with about 40 ml of distilled water were mixed, then centrifuged at 4500 rpm (3383 g) for 15 min, and the supernatant liquid was drew carefully. The residue was dissolved again and centrifuged for another 15 min. The slides were prepared with the entire sediment. A minimum of 800 pollen grains were counted to compose the pollen spectrum of each honey. The individual occurrence of each pollen type was expressed as percentage.
Total Phenolic Content
For the determination of total phenol content, the method of Folin-Ciocâlteu proposed by Singleton and Rossi[Citation28] and adapted by Singleton et al.[Citation29] was conducted. It is a spectrophotometric method based on the oxidation of phenolic compounds of phosphomolybdic and phosphotungstic acids, forming a blue complex measured at 765 nm. Ten grams of honey were diluted in 30 ml of distilled water. The honey solution was diluted to a concentration of 0.11 g/ml. In 10 ml of water was added 1 ml of this solution, 1 ml Folin-Ciocâlteu reagent, and after 2 min of stirring, 5 ml of Na2CO3 (20%) was also added. After refilling with distilled water up to a volume of 25 ml, the solutions were kept for 1 h in a dark place. For calibration curve, a stock solution of gallic acid (0.50 mg/ml) was prepared for further dilutions (0.01–0.50 mg/ml). The linearity obtained was 0.998 (R2). The absorbance was measured using a JENWAY 6505 UV-VIS Spectrophotometer at 765 nm. Results were expressed as mg of gallic acid equivalents in 100 g of honey (mg GAE/100 g).
Total Flavonoid Content
The estimation of the total flavonoid content was evaluated using the Dowd method adapted by Arvouet-Grand et al.[Citation30] Ten grams of fresh honey were diluted in 30 ml of distilled water. To determine the flavonoid content, 2 ml of the honey solution (0.33 g/ml) were added to 0.5 ml of AlCl3 (2%) and refilled with distilled water up to a volume of 25 ml. After 30 min in a dark place the absorbance of honey samples was measured at 425 nm. For calibration curve, a stock solution of quercetin (0.1 mg/ml) was prepared for further dilutions (0.002–2 mg/ml). The linearity obtained was 0.998 (R2). The results were expressed as mg of quercetin equivalents in 100 g of honey (mg QE/100 g).
Free Radical Scavenging Activity
Radical scavenging activity (RSA) was determined using the stable radical 1,1-diphenyl-2-picrylhydrazyl (DPPH), according to the method reported by Chen et al.[Citation31] and adapted by Meda et al.[Citation20] Methanol was used for honey samples preparation (0.1 g/ml). From these solutions, 0.3 ml were mixed with 2.7 ml of DPPH solution (0.06 mM). The solutions took a violet color whose intensity was decreasing with the presence of antioxidant components. Afterwards the solutions were kept in a dark place for 30 min. Finally, the absorbance of the samples was measured at 517 nm. The antioxidant activity of each sample was calculated as the percentage RSA with the formula:
Multivariate Analysis
Multivariate statistical techniques (Spearman Rank Correlation analysis, Principal Component Analysis (PCA), and Cluster analysis) were performed using STATGRAPHICS Centurion XVI software and SPSS Statistic 17.0 software for Windows. These chemometric analyses were carried out in order to relate the botanical origin and to classify the different honey types. PCA is used to determine which variables discriminate between three naturally occurring groups (among pollen types and biochemical components), while Spearman rank correlation is an additional procedure for assessing the relationship between variables. These statistical analyses allow us to see if there are any correlations between the botanical origin and bioactive compounds of analyzed honey samples.
RESULTS AND DISCUSSION
This study provides not only the palynological spectra, botanical classification, and the antioxidant capacity of honey samples, but also the correlations between these valuable characteristics.
Melissopalynological Analysis
Some families are highlighted by a notable representation in the pollen spectrum of honeys, principally: Brassicaceae, Rosaceae, Fabaceae, Fagaceae, Plantaginaceae, Tiliaceae, and Asteraceae. A total of 55 pollen types corresponding to 32 families were identified in the analyzed samples. There is a wide variety of pollen types in different honey samples, but only about 20 taxa were the main sources for bees’ food. The best represented pollen types in the honeys were: Brassica napus type, Rumex, Prunus, Robinia pseudoacacia, Plantago, Crataegus monogyna type, Filipendula, Rubus, and Tilia (). Some of these pollen types were also reported in honeys for neighboring areas, such as Bulgaria and Croatia.[Citation8,Citation32]
Table 1 Principal pollen types in samples (the percentage in the pollen spectra is expressed as a number of the samples in which the pollen type reaches the frequency)
According to the botanical classification of honeys, 10 samples were classified as acacia honey, 10 were classified as linden honey, and 10 as rape honey. In acacia samples, Robinia pseudoacacia pollen was frequently the secondary pollen (with percentages ranging between 5.8 and 30.1%); only in one sample, was this pollen dominant (being 57.9% of the pollen spectrum of the sample). In linden honeys, Tilia pollen was present with percentages between 28.3–88.3% and for nine samples was a dominant pollen (>45% of pollen spectrum). For rape honeys, Brassica napus type was present with percentages ranging between 52.1 and 93% of the pollen spectra being over-represented pollen. The high occurrence of Brassica napus type in all the samples must be noted. The secondary pollens were Tilia in one rape honey, Brassica napus type, Filipendula type, and Prunus type in acacia honeys and Brassica napus type, Helianthus annuus type, and Anthriscus type in linden honeys.
Concerning the palynological characteristics of the honeys, it is worth mentioning the frequency of some Rosaceae as Filipendula or Fragaria in acacia honeys, some Apiaceae, such as Daucus carota, Anthriscus, or Bifora radians in linden honeys. Onobrychis viciifolia was an important pollen in one rape honey. Also, Rumex, as a present pollen (<1% of the pollen spectrum), has been identified in 28 samples, making it a very frequent pollen. This frequency was also reported for Italian acacia honeys from the Varese region.[Citation33]
From a palynological approach, the pollen richness depends on the pollen production of the plant, the meteorological conditions, the distance from the beehive to the flower area, pollen’s diameter, and honey extraction.[Citation1] The pollen richness in samples varied between 525 and 19525 pollen grains per gram of honey with an average of 3700. There was a significant variation among the unifloral honeys: acacia honey had an average of 1875 pollen grains per gram of honey (minimum of 525 and maximum of 5150 pollen grains per gram of honey), linden honey recorded an average of 1852 (minimum of 875 and maximum of 3750 pollen grains), while rape honey had an average of 7372 pollen grains per gram of sample (minimum of 1975 and maximum of 19525). Rape honeys had the highest pollen content while Robinia and Tilia honeys have under-represented pollen so the percentages of the respective pollen in unifloral honeys are generally low to very low. The low quantity of pollen in some acacia and lime honey samples was also reported for these European honeys.[Citation2,Citation10]
Total Phenol Content, Total Flavonoid Content and Radical Scavenging Activity
In the second part of this study, the presence of some bioactive compounds in samples was determined. Researchers from different scientific fields investigated for many years the biological properties of honey, but recently increased interest in the application of antioxidants for humans (both in food and medical treatments) has been observed.[Citation34–Citation40] The concentration of phenolic compounds in honey depend on the floral origin, this being the major factor responsible for the biological activity of honey.[Citation41–Citation43]
The results of these determinations are presented in . Linden honeys showed the highest total phenol content ranging between 33.2 and 57.3 mg GAE/100 g honey. Linden honey was followed by rape honey with an average of 23.7 mg GAE/100 g honey. Acacia group recorded the lowest total phenol content ranging between 12.0 and 31.5 mg GAE/100 g honey. Similar values were reported for other acacia and linden honeys (between 2–39 mg GAE/100 g and 16–38 mg GAE/100 g).[Citation7] The total phenolic compounds were higher in linden samples than in acacia or rape honeys and were also reported by other authors.[Citation41–Citation43]
Table 2 Descriptive analysis of studied bioactive compounds and principal pollen types
The same linearity was obtained for flavonoids content. The acacia honey group had the lowest values regarding the total flavonoids content with an average of 1.3 mg quercetin/100 g honey, while linden honey recorded the highest total flavonoid content with an average of 3.2 mg quercetin/100 g ranging between 2.4 and 5.4 mg quercetin/100 g honey. Concerning the flavonoid content for acacia honeys, similar values were obtained for other Romanian honeys (0.91–2.42 mg QE/100 g),[Citation5] while higher values were reported for African honeys.[Citation21]
The maximum value for RSA was obtained for linden honey ranging between 19.6 and 37.9% with an average of 27.2%. As it can be observed, acacia honey had the lowest level with an average of 10.7%. These samples recorded the lowest level of antioxidant capacity. Differences between honey types were established with an ANOVA one-way analysis. The results are shown in . Linden honeys had significant highest phenol content (p < 0.05) and acacia honeys significant lowest flavonoids content (p < 0.05). Differences between acacia, linden, and rape honeys were determined in relation to the RSA (p < 0.05). Also, some significant differences in the pollen spectrum of the samples were included; it is highlighting the highest representation of Prunus and Filipendula in acacia honeys (p < 0.05).
Multivariate Analysis
To find a possible dependence between the antioxidant compounds and the antioxidant activity of honeys and the botanical origin of the samples, a Spearman rank correlation analysis has been performed (). The highest positive correlation coefficient belonged to Tilia and total phenols (0.82). This confirmed that linden honeys had the major phenol content (p < 0.05). A positive correlation between Tilia and total flavonoids, and Tilia and RSA supports the best antioxidant capacity for the samples of the linden tree (p < 0.05). On the contrary, the presence of Robinia pseudoacacia in honeys had a negative correlation with the content in phenols and flavonoids and thus a low antioxidant capacity (p < 0.05). Brassica napus had significant negative correlation with phenol content (p < 0.05) but no significant correlations were found with the flavonoids content and RSA; it may be that Brassica napus pollen is an important pollen in all of the studied samples.
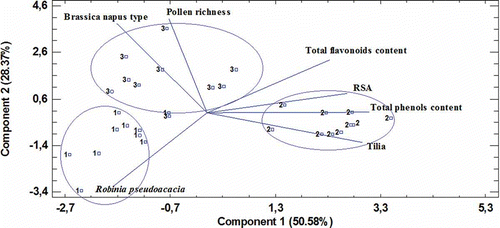
In order to classify the different honey types, a PCA was performed with variables obtained through pollen analysis and phenols content, flavonoids content, and RSA. Seven factors were extracted but only two had an eigenvalue higher than 1.0 (). These two factors let to explain a cumulative variance of 78.96%. The variables, Brassica napus and Tilia, had the highest score in the first factor while RSA had the highest score in the second one. The distribution of the samples and the factors were plotted in . Brassica napus was situated near pollen richness opposite to Tilia, total phenol content, and RSA. The unifloral honeys (acacia-1, linden-2, and rape-3 honey) were clearly differentiated.
Figure 1 Honey classification (1-acacia, 2-linden, 3-rape) by PCA considering the most representative variables.
The similarities between the samples were analyzed with a cluster analysis (). The cluster classified the samples according their botanical origin. Nine samples of acacia honey, all the rape honey, and all the linden honey were clearly grouped. Only one acacia honey sample was distributed in a separate group due to its high value of Robinia pseudoacacia (57.9%). This high content of Robinia pseudoacacia pollen is very uncommon in acacia honeys.
Table 3 Spearman rank correlations between each pair of variables
Figure 2 Cluster classification of honey types according to their palynological features (Robinia pseudoacacia, Tilia, Brassica napus).
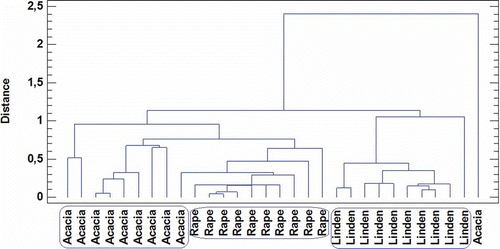
Table 4 Result of the two first components extracted in PCA and the factor score coefficients for the variables
CONCLUSIONS
This article provides information regarding the palynological characterization and antioxidant activity of the main Romanian honey types (acacia, linden, and rape unifloral honeys). The main pollen types identified in the pollen spectrum of the samples were: Brassica napus type, Tilia, Robinia pseudoacacia, Helianthus annuus, Filipendula, Fragaria, Trifolium repens type, Prunus, and Rumex. Brassica napus type occurred in all the studied samples in different percentages. Acacia honeys had a low representation of Robinia pseudoacacia pollen. Also, a low percentage of Tilia pollen was found in some linden honeys. The multivariate techniques used with the variables obtained with pollen analysis and the antioxidant compounds allowed a good classification of the samples according their botanical origin.
REFERENCES
- Anklam, E. A review of analytical methods to determine the geographical and botanical origin of honey. Food Chemistry 1998, 63, 549–562.
- Persano-Oddo, L.; Piazza, M.G.; Sabatini, A.G.; Accorti, M. Characterisation of unifloral honeys. Apidologie 1995, 26, 453–465.
- Da Costa Leite, J.M.; Trugo, L.C.; Costa, L.S.M.; Quinteiro, L.M.C.; Barth, O.M.; Dutra, V.M.L.; De Maria, C.A.B. Determination of oligosaccharides in Brazilian honeys of different botanical origins. Food Chemistry 2000, 70, 93–98.
- Azeredo, L.D.C.; Azeredo, M.A.A.; De Souza, S.R.; Dutra, V.M. Protein contents and physicochemical properties in honey samples of Apis mellifera of different floral origins. Food Chemistry 2003, 80 (2), 249–254.
- Mărghitaş, L.A.; Dezmirean, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chemistry 2009, 112, 863–867.
- Terrab, A.; Díez, M.J.; Heredia, F.J. Palynological, physico-chemical and colour characterization of Moroccan honeys: III. Other unifloral honey types. International Journal of Food Science and Technology 2003, 38, 395–402.
- La‐Serna, I.R.; Méndez-Pérez, I.; Gómez-Ferreras, C. Pollen spectra of different unifloral honeys from La Palma (Canary Islands, Spain). Grana 2002, 41 (1), 48–57.
- Atanassova, J.; Kondova, V. Pollen and chemical-physical analysis of unifloral honeys from different regions of Bulgaria. Phytologia Balcanica 2004, 10, 45–50.
- Downey, G.; Hussey, K.; Kelly, D.J.; Walshe, F.T.; Martin, P.G. Preliminary contribution to the characterization of artisanal honey produced on the island of Ireland by palynological and physico-chemical data. Food Chemistry 2005, 91, 347–354.
- Persano-Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, 38–81.
- Seijo, M.C.; Escuredo, O.; Fernández-González, M. Fungal diversity in honeys from northwest Spain and their relationship to the ecological origin of the product. Grana 2011, 50, 55–62.
- Dobre, I.; Alexe, P.; Escuredo, O.; Seijo, M.C. Palynological study of selected honeys from Romania. Grana 2013 , 52 (2), 113–121.
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Commision Internationale de BotaniqueApicole de L’UISB. Les methodes of melissopalynologie. Apidologie 1970, 1 (2), 211–227.
- Von der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, 18–25.
- Arnous, A.; Makris, D.P.; Kefalas, P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. Journal of Agricultural and Food Chemistry 2001, 49, 5736–5742.
- Huda-Faujan, N.; Noriham, A.; Norrakiah, A.S.; Babji, A.S. Antioxidant activity of plants methanolic extracts containing phenolic compounds. African Journal of Biotechnology 2009, 8 (3), 484–489.
- Alvarez-Suárez, J.M.; Giampieri, F.; González-Paramás, A.M.; Damiani, E.; Astolfi, P.; Martínez-Sánchez, G.; Bompadre, S.; Quiles, J.L.; Santos-Buelga, C.; Battino, M. Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chemistry Toxicology 2012, 50 (5), 1508–1516.
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.; Pihlaja, K.; Kujala, S.T.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. Journal of Agricultural and Food Chemistry 1999, 47, 3954–3962.
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Food for Human Nutrition 2004, 59 (3), 113–122.
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chemistry 2005, 91, 571–577.
- Perez, E.; Rodriguez-Malaver, A.J.;Vit, P. Antioxidant capacity of Venezuelan honey in wistar rat homogenates. Journal of Medicinal Food 2006, 9, 510–516.
- Bertoncelj, J.; Dobersek, U.; Jamnikand, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chemistry 2007, 105 (2), 822–828.
- Vela, L.; De Lorenzo, C.; Pérez, R.A. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. Journal of Agricultural and Food Chemistry 2007, 87, 1069–1075.
- Zalibera, M.A.; Staško, A.; Šlebodov, V.; Jančovičová, T.; Čermáková, V. Antioxidant and radical-scavenging activities of Slovak honeys—An electron paramagnetic resonance study. Food Chemistry 2008, 110, 512–521.
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chemistry 2009, 114 (4), 1438–1443.
- Frankel, S.M.; Robbinson, G.E.; Berenbaum, M.R. Antioxidant capacity and correlated characteristics of 14 unifloral honeys. Journal of Apicultural Research 1998, 37, 27–31.
- Dobre, I.; Gadei, G.; Patrascu, L.; Elisei, M.A.; Segal, R. The antioxidant activity of selected Romanian honeys. The Annals of the University Dunarea de Jos of Galati-Food Technology Fascicule 2010, 34 (2), 67–73.
- Singleton, V.L.; Rossi, J.A. Colorymetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965, 16 (3), 144–158.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology 1999, 299, 152–178.
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardisation d’un extrait de propolis et identification des principaux constituants. Journal of Pharmacie Belgique 1994, 49 (6), 462–468.
- Chen, L.; Mehta, A.; Berenbaum, M.; Zangerl, A.R.; Engeseth, N.J. Honeys from different floral sources as inhibitors of enzymatic browning in fruit and vegetable homogenates. Journal of Agricultural and Food Chemistry 2000, 48 (10), 4997–5000.
- Sabo, M.; Potocnjak, M.; Banjari, I.; Petrovic, D. Pollen analysis of honey from Varazdin Country, Croatia. Turkish Journal of Botany 2011, 35, 581–586.
- Ricciardelli-D’Albore, G. I mieli DOC di castagno (Castanea sativa Miller) e di acacia (Robinia pseudacacia L.) della provincia di Varese (Lombardia). Estr. Annuale di Facultad Agraria1988, XLII, 35–49.
- Herken, E.H.; Guzel, S. Total antioxidant capacity and total phenol contents of selected commercial fruit juices in Turkey. International Journal of Food Properties 2010, 13 (6), 1373–1379.
- Cano, A.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activity in different leaves of three lettuce varieties. International Journal of Food Properties 2005, 8, 521–528.
- Strazzulo, G.; De Giulio, A.; Tommonaro, G.; La Pastina, C.; Poli, A.; Nicolaus, B.; De Prisco, R.; Saturnino, C. Antioxidative activity and lycopene and β-carotene contents in different cultivars of tomato (Lycopersicon esculentum). International Journal of Food Properties 2007, 10, 321–329.
- Çelik, S.E.; Özyürek, M.; Altun, M.; Bektasoglu, B.; Güçlü, K.; Berker, K.I.; Özgökçe, F.; Apak, R. Antioxidant capacities of herbal plants used in the manufacture of van herby cheese: ‘OtluPeynir’. International Journal of Food Properties 2008, 11, 747–761.
- Szabo, M.R.; Radu, D.; Gavrilas, S.; Chambre, D.; Iditoiu, C. Antioxidant and antimicrobial properties of selected spice extracts. International Journal of Food Properties 2010, 13, 535–545.
- Tohma, H.S.; Gulçin, I. Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). International Journal of Food Properties 2010, 13, 657–671.
- Singla, R.; Ganguli, A.; Ghosh, M. Antioxidant activities and polyphenolic properties of raw and osmotically dehydrated dried mushroom (Agaricus bisporous) snack food. International Journal of Food Properties 2010, 13 (6), 1290–1299.
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutrition Research 2002, 22 (9), 1041–1047.
- Kücük, M.; Kolayli, S.; Karaoglu, S.; Ulusoy, E.; Baltaci, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chemistry 2007, 100 (2), 526–534.
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chemistry 2004, 85, 513–518.
