Abstract
Higher levels of phenolics were found in steam distilled clove extract (256.5 mg of GAE/g) than its oleoresin (177.1 mg of GAE/g). Antioxidant potential using β-carotene–linoleic acid model of butylated hydroxy anisol, steam distilled and clove oleoresin were 92.39, 85.51, and 77.88%, respectively, at 200 ppm. Radical scavenging activity of butylated hydroxy anisol, extracts of steam distilled and oleoresin were found to be 91.77, 88.93, and 80.84%. Oxidative stability of ghee with butylated hydroxy anisol was highest followed by steam distilled extract and oleoresin throughout 21 days of storage at 80 ± 1°C. During deep frying, steam distilled clove extract had strong antioxidant activity as compared to others.
INTRODUCTION
Ghee is anhydrous milk fat, having a pleasing and appetizing aroma. It has a considerably longer shelf life as compared to other indigenous dairy products and also is considered as the supreme cooking and frying medium. It undergoes oxidative degradation during storage and deep-fat frying, resulting in an alteration of major quality parameters such as color, flavor, aroma, and nutritive value.[Citation1] Deep-fat frying is one of the most commonly used procedures for the preparation and manufacture of foods throughout the world.[Citation2] During frying, the ghee is exposed continuously, or repeatedly, to elevated temperature in presence of air and moisture. A number of chemical reactions including oxidation, hydrolysis, and polymerization of frying fat take place, leading to the formation of desirable and undesirable secondary products. As these reactions proceed, the functional, sensory, and nutritional quality of oil deteriorates and may reach a point where it has to be ultimately discarded.[Citation3] Development of rancidity reduces the shelf-life of product which ultimately affects consumer acceptability.[Citation4] There are numerous evidences correlating oxidized lipids with negative health implications.[Citation5]
A number of synthetic antioxidants like butylated hydroxy anisol (BHA), tert-butylhydroquinone (TBHQ), and propyl gallate (PG) are often used to retard fat oxidation in foods.[Citation6] However, the use of these synthetic antioxidants has been questioned due to their potential health risks and toxicity.[Citation7] These have also been suspected of being responsible for liver damage and carcinogenesis.[Citation8,Citation9] Therefore, the development and utilization of more effective antioxidants of natural origin are desired.[Citation10] Hence, this has directed the attention towards the use of natural antioxidants as resources of safer food in place of synthetic antioxidant. Recently, the use of natural antioxidants in the food industry has increased rapidly and consequently many related studies have been reported.[Citation11] Many herbs and spices are known to exhibit antioxidant activity in food lipids.[Citation12] Addition of herbal extracts in dairy products is a newly emerging area[Citation13] which has a vast potential.
Clove (Syzygium aromaticum) is widely cultivated in India, Madagascar, Sri Lanka, Indonesia, and China.[Citation14] It has been granted as a “generally regarded as safe” substance by the USFDA (United States Food and Drug Administration) when administered at levels not exceeding 1500 ppm in all food categories.[Citation15] The buds of clove used in folk medicine as diuretic, odontalgic, tonicardiac, aromatic condiment properties, and condiment with carminative and stimulant activity.[Citation16] Its active ingredient is eugenol (4-allyl-2-methoxyphenol) which makes up 70–90% by weight,[Citation17] besides this, eugenol acetate (>17%) and caryophyllene (>12%) are also present in clove extract. Eugenol is used in varied applications, e.g., as an antioxidant and antimicrobial agent.[Citation18]
MATERIALS AND METHODS
Collection of Raw Materials
Cream
Freshly prepared mixed cream was obtained from experimental dairy plant of National Dairy Research Institute, Karnal, India.
Clove extract
Clove oleoresin was procured from Synthite Industry Ltd., Kerala, India and steam distilled clove extract was provided by Katyani Exports, Delhi, India.
Synthetic antioxidant
BHA was procured from Sigma Aldrich, USA.
Chemicals and Reagents
BHA, β-carotene, linoleic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH) hydrate free radical, Folin–Ciocalteu’s reagent, Tween-40, gallic acid, and eugenol were obtained from Sigma Chemical Co. (USA). Chloroform from Loba Chemie Pvt Ltd. (Mumbai, India) and ethyl acetate, glacial acetic acid, iso-octane, and potassium iodide from RFCL Ltd. (New Delhi, India) were used. Sodium carbonate was obtained from Qualigens Fine Chemicals (Mumbai, India). Vacuum pump (Millipore, India), reverse phase HPLC system (Waters, 515, Singapour), C18 column (5μm, 4.5 250 mm, 100 A°, Phenomener, USA), filtration assembly with Milipore 0.45 μm filter for filtering all reagents, water, syringe filter 0.45 μm for sample filtration.
Preparation of Ghee and Addition of Antioxidants
Two sets of cream were taken during preparation of ghee. In one set of cream, steam distilled clove extract was added at the rate of 0.50% (w/w) as it was water soluble and then ghee was prepared by direct creamery method. The other set of cream was utilized for the production of ghee, BHA as well as oleoresin were added directly to the freshly prepared ghee at the rate of 0.02 and 0.50% (w/w), respectively, as they are fat soluble. The levels of clove extract utilized in the study were selected on the basis of their antioxidant activity and organoleptic characteristics. Three levels of clove extracts (0.25, 0.50, and 0.75%) were initially incorporated into ghee. Clove extract (0.50%) was ultimately selected for incorporation into ghee as it showed higher antioxidant activity than clove extract (0.25%) as measured by Rancimat, while still retaining the acceptable quality of ghee, whereas, higher rate of addition, i.e., 0.75% of the clove extract led to ghee with poor organoleptic properties.
Ghee without any added synthetic antioxidant or herb extract served as a control. Ghee samples were stored in a hot air oven at 80 ± 1°C and analyzed at regular intervals of 0th, 3rd, 6th, 9th, 12th, 15th, 18th, and 21st days and another set of samples was used for deep fat frying studies at 180 ± 5°C. All the ghee samples were evaluated for peroxide value, conjugated dienes, thiobarbituric acid value, fatty free acid (FFA), and radical-scavenging activity by DPPH assay. Accelerated stability test using Rancimat 743 was also conducted for determination of induction period (IP).
Deep Fat Frying Study
Deep fat frying experiment was carried out by simulating frying conditions, considering the method of Krishnamurthy[Citation19] as a reference. During frying, 500 g of ghee samples viz. control ghee, ghee incorporated with clove extracts as well as that containing BHA were transferred to vessel and then placed on a heating mantle whose temperature was set to be at 180 ± 5°C. Temperature of ghee was monitored using a thermometer. When the frying temperature of 180°C was reached, four wet cotton balls, each weighing 2 g, were immersed one after another. Air was incorporated using a vacuum pump at flow rate of 7.2 L/min. These cotton balls were fried for exactly 7 min followed by cooling the ghee to room temperature. The entire frying cycle was repeated for four times with similar frying conditions.
Total Phenolic Content
Total polyphenolic content of steam distilled clove extract and clove oleoresin was estimated by Folin–Ciocalteu colorimetric method of Kahkonen.[Citation20] Total phenolic content was estimated using a standard curve (400 μl of 10–100 μg/ml) of gallic acid and presented as gallic acid equivalents (GAE) per gram of clove extract.
Eugonol Content in Clove Extracts Estimated by RP-HPLC
Eugenol (4-allyl-2-methoxyphenol), a well known phenolic compound of clove, possesses many medicinal properties including antioxidant activity. It was estimated by RP-HPLC method developed by Yun.[Citation21] Thirty milligrams of clove extracts (steam distilled and oleoresin clove) were taken in round bottom flask and refluxed with 30 ml of ethanol. The sample was refluxed for different time interval from 2–5 h by using water reflux condenser. After refluxing, evaporation of the mixture was carried out on water bath till it was dry. This was then redissolved in 30 ml ethanol and filtered through PTFE (Polytetrafluoroethylene) syringe filter 0.45 μm and then directly injected in RP-HPLC system. The analysis was performed on C18 column with isocratic mobile phase (methanol and water in the ratio 60:40 v/v) at 280 nm. Flow rate was maintained at 0.8 ml/min and column temperature was set at 30°C.
β-carotene Linoleic Acid Model System
Antioxidant activity of clove extracts and BHA were evaluated by β-carotene Linoleic acid model system developed by Marco,[Citation22] and modified by Miller,[Citation23] which involved measuring β-carotene bleaching at 470 nm.
Radical Scavenging Activity by DPPH Method
The radical-scavenging activity of clove extracts (steam distilled and oleoresin) and BHA were determined according to the procedure of Blois,[Citation24] with a minor modification, by using ethanol instead of methanol during sample preparation.
Peroxide value
Peroxide value of ghee samples were determined by the method as described in IS:3508.[Citation25]
Conjugated dienes
Conjugated dienes were determined as per the method of AOAC.[Citation26]
Thiobarbituric acid (TBA) test
TBA value of ghee samples were determined by the method of Patton and Kurtz.[Citation27]
FFA value
FFA levels of ghee samples were determined by the method as described in IS:3508.[Citation25]
Accelerated oxidation test: Rancimat 743
The oxidative stability of ghee samples incorporated with antioxidants were measured by using Metrohm Rancimat, 3 g of samples were directly weighed into reaction vessels, oxidized at 120°C with 20 L/h air-flow rate.[Citation1] The oxidative stability was expressed as IP or oxidative stability index. IP is the time required to reach an endpoint of oxidation corresponding to either a level of detectable rancidity or a sudden change in the rate of oxidation.[Citation28]
Statistical Analysis
All determinations were carried out in triplicate and data was subjected to analysis of variance. In the experiments, one way and two way analysis of variance (ANOVA) with a subsequent difference (P > 0.05) in the mean values was conducted as described by Snedecor and Cochran.[Citation29]
RESULTS AND DISCUSSION
Total Phenolic Content
Steam distilled clove extract showed significantly (P < 0.05) higher phenolic content than its oleoresin counterpart. The difference in phenolic content can be ascribed to the presence of hydrophilic and lypophilic antioxidant compounds in extracts, methods of extraction, and difference in solubility. Zhou[Citation30] and Shan[Citation31] reported that phenolic content and total antioxidant activity were positively related with hydrophilic antioxidant compounds and also higher phenolic compound were obtained in steam distilled clove extract. Guan et al.[Citation32] reported that clove oil obtained by steam distillation contained highest percentage of eugenol. In addition, the phenolic content was observed to be dependent on type of extraction method and solubility of oil components.[Citation33]
Eugenol Content Estimated by RP-HPLC Method
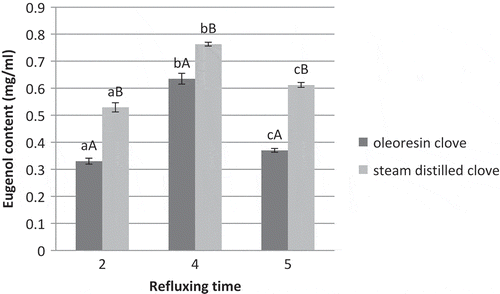
Standard eugenol of known concentrations (ranging from 0.25–1.5 ppm) demonstrated a retention time of 14.22 ± 0.5 min in RP-HPLC. Eugenol content of oleoresin and steam distilled clove extract were calculated using a standard curve. It was observed from that eugenol content increased proportionately with the increase in refluxing time from 2–4 h, however it decreased with further increase in refluxing time to 5 h. There by, the optimal time for complete extraction of eugenol from samples was elucidated to 4 h (through water refluxing condenser).
Antioxidant Activity by β-Carotene–Linoleic Acid Model System
Antioxidant activity of clove extracts and BHA were evaluated at a concentration of 200 ppm using β-carotene–linoleic acid coupled oxidation model system () and results indicated that BHA showed significantly (P < 0.05) higher antioxidant activity compared to clove extracts (steam distilled and oleoresin). However, steam distilled clove extract showed higher resistance against bleaching effect of β-carotene than that exhibited by clove oleoresin. The variation in antioxidant activity of steam distilled clove extract and clove oleoresin can be due to the difference in total phenolic content which may further varied by extraction methods and solubility of oil components. It has been reported that the eugenol content was higher in clove oil obtained by steam distillation compared to other extraction methods.[Citation32] Cai et al.[Citation34] ascertained a linear correlation between total phenolic content and antioxidant activity for aqueous and ethanolic extracts of Chinese medicinal plants.
Table 1 Antioxidant activity (%) and radical scavenging activity of clove extract (steam distilled and oleoresin) and synthetic antioxidant (BHA) at 200 ppm concentration
Radical-Scavenging Activity by DPPH Assay
The radical-scavenging activity of clove extracts (steam distilled and oleoresin) and BHA were evaluated at a concentration of 200 ppm using DPPH assay. It can be inferred from that the radical-scavenging potential of BHA was higher than steam distilled clove extract and its oleoresin counterpart. The difference in radical scavenging activity of steam distilled clove and clove oleoresin may be due to high phenolic content of steam distilled clove extract than its oleoresin counterpart. It can be noted that results obtained using DPPH radical scavenging assay were consistent with the results obtained from β-carotene–linoleic acid model system.
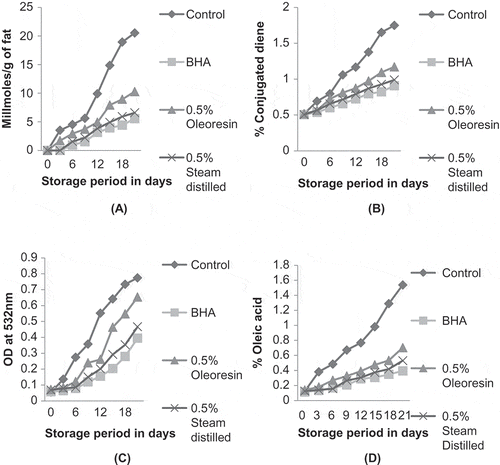
Peroxide Value
Peroxide value represents primary reaction products of lipid oxidation, which can be measured by their ability to liberate iodine from potassium iodide. From , it can be inferred that the peroxide value of control ghee increased significantly (P < 0.05) throughout the 21 days of the storage period. Ghee added with synthetic antioxidant (BHA) and steam distilled clove extract were found to have no peroxides even after being exposed to high temperature at the 3rd day of the storage period. At the end of the storage period, ghee containing BHA (0.02%) showed significantly (P < 0.05) lower values of peroxides compared to clove extracts (steam distilled and oleoresin) containing samples. Whereas, ghee incorporated with clove oleoresin showed a significant (P < 0.05) rise in peroxide value as compared to its steam distilled counterpart. This indicated that ghee containing steam distilled clove extract was more effective in retarding peroxides development than its oleoresin counterpart. Lower antioxidant property of clove oleoresin might be due to some chemical changes such as oxidation, occurring during its manufacture and storage which caused destruction of several bioactive compounds.[Citation35] The results obtained were in agreement with the results of Siddiq[Citation36] who reported that the addition of methanolic and acetone extracts of Moringa oleifera to sunflower oil significantly inhibited the development of peroxides under accelerated conditions.
Conjugated Diene
On the completion of 21 days of storage at 80 ± 1°C, it was inferred () that development of conjugated dienes were less in ghee containing the steam distilled clove extract as compared to ghee with clove oleoresin. This difference between the results might be due to higher amount of principal phenolic compound, i.e., eugenol percentage in steam distilled clove extract. Research findings revealed that clove oil obtained by steam distillation contains higher percentage of eugenol.[Citation32] However, ghee containing BHA showed the lowest amount of conjugated dienes as compared to other clove extracts (steam distilled and oleoresin) containing ghee samples. In research carried out by Frankel,[Citation37] the antioxidant activity of rosemary in several oil types stored at 60°C for 20 days was assessed after the addition of rosemary extract containing 44 mg/kg carnosic acid and 6 mg/kg carnosol. In soybean oil, rosemary extract at a concentration of 1000 mg/kg inhibited diene formation when compared to control.
Thiobarbituric Acid
It was evident from that the steam distilled clove extract, oleoresin as well as BHA, were significantly effective in lowering of TBA values of ghee as compared to control. Clove oleoresin showed significantly higher TBA values than its steam distilled counterpart. Clove oleoresin might have lower antioxidant potential due to some chemical changes, such as oxidation, occuring in oleoresin during its manufacture and storage which caused destruction of several bioactive compounds.[Citation35] However, BHA containing ghee showed higher antioxidant activity than clove extract added sample throughout the 21 days of the storage period.
FFA
FFAs are formed by hydrolysis as well as by cleavage and oxidation of double bonds,[Citation38] and its measure is used as an indicator of hydrolytic rancidity of fat and oils. It can be inferred from , that ghee samples containing BHA showed higher resistance against the development of FFA compared to those containing clove extracts (steam distilled and oleoresin). However, ghee incorporated with steam distilled clove extract displayed lower FFA values than ghee containing clove oleoresin. Steam distilled extract acts as stronger antioxidant as compared to oleoresin, which may be due to difference in level of antioxidant compounds. This difference may also be due to the higher amount of principal phenolic compound, i.e., eugenol in steam distilled clove extract. The results are in accordance with the findings of Guan et al.[Citation32] which indicated that clove oil obtained by steam distillation contained highest percentage of eugenol.
Stability of Ghee Incorporated with Antioxidants Estimated by Rancimat
The induction time of ghee samples incorporated with clove extracts (steam distilled and oleoresin) and BHA are presented in . It can be concluded that clove extracts (steam distilled and oleoresin) and BHA acts as effective antioxidants in ghee by limiting the formation of volatile oxidative products. Ghee containing clove extracts and BHA displayed significantly higher (P < 0.05) IP compared to control ghee sample. It was also observed that steam distilled clove extract was more effective in stabilizing ghee against oxidative deterioration as compared to BHA followed by clove oleoresin. Steam distilled clove extract exhibited higher antioxidant activity, which may be attributed to presence of principle active compound, eugenol and also some other compounds such as eugenol acetyl, β-caryophyllene, etc., which are stable at high temperatures.[Citation39] The results were in accordance with those obtained by Griffiths et al.,[Citation40] which showed that the steam distilled propolis extract exhibited significantly higher antioxidant activity than rosemary oleoresin.
Table 2 Stability of ghee with antioxidants estimated by Rancimat test (induction period in h)
Fat Frying at 180°C
It was observed from that adding clove extracts (steam distilled and oleoresin) and BHA to ghee significantly lowered the peroxide formation as compared to control ghee. Ghee containing steam distilled clove extract showed zero peroxide value at the end of the first frying. However, peroxide value increased for all the ghee samples. Steam distilled clove extract offered more resistance in formation of peroxides than BHA and oleoresin throughout the frying process. Lower antioxidant potential of oleoresin may be due to the fact that it was highly prone to oxidation during deep fat frying/processing and storage which might have caused a decrease in its antioxidant properties.[Citation30] The potential decrease in antioxidant activity of BHA at high temperatures have been reported by many researchers,[Citation41−Citation43] and showed that BHA volatilized during frying and was ineffective in enhancing oxidative stability of frying oil.[Citation44,Citation45]
Figure 3 Ghee samples added with clove extracts (steam distilled and oleoresin) and BHA during simulated deep frying. (a) Peroxide value; (b) conjugated diene; (c) thiobarbituric acid; (d) free fatty acid.
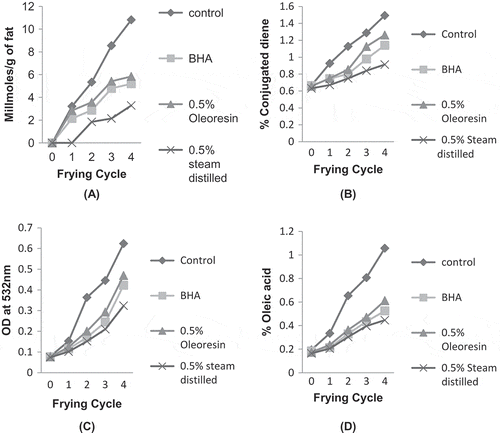
It was observed from that there was a significant increase in conjugated dienes in all ghee samples throughout the frying process. However, clove oleoresin showed higher conjugated diene content than its steam distilled counterpart and BHA. Control ghee was least stable against oxidative deterioration during deep fat frying. In addition, steam distilled clove extract exhibited higher antioxidant activity in preventing the development of conjugated diene in ghee. This might be due to the fact that clove extract obtained by steam distillation contains higher percentage of eugenol (58.2%), than that obtained by super critical fluid extraction (SFE) (53.8–55.9%).[Citation46] Besides eugenol, eugenol acetate, and β-caryophyllene in the clove extracted by steam distillation was as high as 20.59%, however, at frying temperature, thermal degradation of eugenol acetate and other components may take place whereas, eugenol is thermally stable.[Citation39]
TBA value of ghee containing clove extracts and BHA was significantly lower than that of control ghee as depicted in . Ghee containing steam distilled clove extracts showed higher resistance in development of TBA value than ghee incorporated with BHA and clove oleoresin. Among all the antioxidants, clove oleoresin displayed least antioxidant property compared to steam distilled clove extract and BHA. The results showed that steam distilled clove extract was more effective in hindering the formation of TBA which may be due to the high principle phenolic compound, eugenol. The research by Guan et al.[Citation32] indicates that the eugenol percentage was higher in clove oil obtained by steam distillation method. In case of oleoresin, some chemical changes such as oxidation occurs during its manufacturing and storage which may cause destruction of several bioactive compounds.[Citation35] The results were in accordance with Perez Galvez et al.[Citation47] who reported that lower antioxidant activity was observed for paprika oleoresin which might be due to high thermal treatments used for its extraction.
Frying at higher temperature in presence of water, steam and oxygen initiates the oxidation and hydrolysis degradation reaction in the frying oil. Water, being a weak neucleophilic compound, attacked the ester linkage of triacylglycerol and produced di- and monoacylglycerol, glycerol, and FFAs.[Citation48] Ghee containing steam distilled clove extract showed higher antioxidant activity () and resisted the formation of FFA compared to other ghee samples (BHA and oleoresin). However, oxidation increased with increase in number of frying cycles. Chung et al.[Citation49] reported that FFA content in frying oil increased along with the increasing numbers of frying. In addition, steam distilled extract of propalis showed higher antioxidant activity compared to rosemary oleoresin by limiting the peroxidation and hydrolysis in frying oil.[Citation40]
In the course of deep fat frying, the oxidative stability of ghee samples incorporated with clove extracts (steam distilled and oleoresin) and BHA were evaluated using Rancimat and the results are presented in . The IP of control ghee was found to be lower than ghee added with antioxidants. In addition, ghee containing steam distilled clove extract was found to be more stable and had higher IP than ghee with BHA, followed by clove oleoresin incorporated ghee. It was also observed that, decrease in the oxidative stability of ghee samples during the frying process can be concluded by observing the significant decrease in IP through successive frying cycles. Ghee incorporated with synthetic antioxidant (BHA) showed lower IP as it had greater volatility at higher temperatures and eventually loses its antioxidant potency. Many researchers have reported that BHA volatilized during frying, and was ineffective in enhancing oxidative stability of frying oil.[Citation44] BHA acts as potent antioxidant at room or moderate temperatures but is less effective under frying conditions.[Citation50] At high temperatures, BHA evaporates with steam, particularly when a large quantity of water was expelled from foods during frying or baking.[Citation41,Citation42] The lower antioxidant activity of clove oleoresin might be attributed to the higher temperature used in its preparation which may cause degradation of some antioxidant compounds.[Citation35]
Table 3 Oxidative stability of fried ghee samples incorporated with clove extracts (steam distilled and oleoresin) and BHA expressed its induction period in h
CONCLUSION
The present work proves that clove extracts were active in resisting the oxidative degradation of ghee during accelerated and deep fat frying condition. Among the clove extracts, steam distilled clove extract was demonstrated to be more potent antioxidant in ghee. A positive correlation between total phenolic content, antioxidant potential, and free radical scavenging activity was observed for clove extracts. Higher phenolic content, i.e., eugenol in steam distilled clove extract was observed using RP-HPLC. Synthetic antioxidant, BHA showed higher antioxidant activity under accelerated storage conditions whereas it had lower antioxidant potential during deep frying studies as compared to clove extracts (steam distilled and oleoresin).
ACKNOWLEDGMENT
The authors would like to express appreciation to Katayni Export, New Delhi, for providing steam distilled clove extracts for this study.
REFERENCES
- Pawar, N.; Arora, S.; Bijoy, R.R.; Wadhwa, B.K. The effect of Asparagus racemosus (Shatavari) extract on oxidative stability of ghee, in relation to added natural and synthetic antioxidant. International Journal of Dairy Technology 2012, 65 (2), 293–299.
- Che Man, Y.B.; C.P. Tan. Effects of natural and synthetic antioxidants on changes in refined, bleached, and deodorized palm olein during deep-fat frying of potato chips. Journal of American Oil and Chemical Society 1999, 76, 331–340.
- Stevenson, S.G.; Mand Esjin, N.M. Quality control in the use of deep frying oils. Journal of American Oil chemists Society 1984, 61, 1102–1108.
- Nerin, C.; Tover, L.; Salafranca, J. Behaviour of a new antioxidant active film versus oxidizable model compounds. Journal of Food Engineering 2008, 84, 313–320.
- Pukalskas, A.; Van Beek, A.T.; De Waard, P. Development of a triple hyphenated HPLC radical scavenging detection-DAD-SPE-NMR system for the rapid identification of antioxidants in complex plant extracts. Journal of Chromatography A 2005, 1074, 81–88.
- Sherwin, E.R. Antioxidants. In: Food Additives; Branen, A.L.; Davidson, P.M.; Salminen, S.; Eds.; Marcel Dekker: New York, 1990, 139–193.
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Zeitschrift fur Lebensmittel-Untersuchung und -Forschung 1993, 196 (4), 329–338.
- Grice, H.C. Safety evaluation of butylated hydroxyl toluene (BHT) in the liver, lung, and gastrointestinal tract. Food and Chemical Toxicology 1986, 24, 1127–1130.
- Wichi, H.P. Enhanced tumor development by butylated hydroxyl anisole (BHA) from the perspective of effect on the fore stomach and oesophageal squamous epithelium. Food and Chemical Toxicology 1988, 26, 717–723.
- Gülcin, I.; Küfrevioğlu, O. I.; Oktay, M.; Büyükokuroğlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). Journal of Ethnopharm 2004, 90, 205–215.
- Beddows, C.G.; Jagait, C.; Kelly, M.J. Effect of ascorbyl palmitate on the preservation of α-tocopherol in sunflower oil, alone and with herbs and spices. Food Chemistry 2001, 73, 255–261.
- Pokorny, J.; Korczak, J. Preparation of Natural Antioxidants. Antioxidants in Food–Practical Application, Woodhead Publishing Ltd.: Cambridge, 2001, 311–330.
- Rowan, C. Extracting the best from herbs. Food Engineering International 2000, 25, 31–34.
- Bhuiyan, I.M.; Begum, J.; Akter, F. Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum (L.) Alston). African Journal of Plant Science 2010, 4 (11), 451–454.
- Kildeaa, M.A.; Allanb, G.L.; Kearney, R.E. Accumulation and clearance of the anaesthetics clove oil and AQUI-S_ from the edible tissue of silver perch (Bidyanus bidyanus). Aquaculture 2004, 232, 265–277.
- Boulos, L. Medicinal Plants of North Africa, reference publication; Algonac, MI, 1993, pp. 287.
- Keene, J.L.; Noakes, D.L.G.; Moccia, R.D.; Soto, C.G. The efficacy of clove oil as an anaesthetic for rainbow trout, on corhynchus mykiss (Walbaum). Aquaculture Research 1998, 29, 89–101.
- Rajakumar, D.V.; Rao, A. Dehydrozingerone and isoeugenol as inhibitors of lipid peroxidation and as free radical scavengers. Biochemistry Pharmacology 1993, 46, 2067–2072.
- Krishnamurthy, R.G.; Kawda, T.; Chang, S.S. Chemical reactions involved in the deep fat frying of food. I. Laboratory apparatus for frying under simulated restaurant conditions. Journal of American Oil Chemist and Society 1965, 42 (10), 878–882.
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.; Pihlaja, K.; Kujala, S.T.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. Journal of Agriculture and Food Chemistry 1999, 47, 3954–3962.
- Yun, J. Quantitative analysis of eugenol in clove extract by a validated HPLC method. Journal of AOAC International 2010, 93 (6), 1806–1810.
- Marco, G.J. A rapid method for evaluation of antioxidants. Journal of American Oil Chemists Society 1968, 45, 594–596.
- Miller, H.E. A simplified method for the evaluation of antioxidants. Journal of the American Oil Chemists Society 1971, 48, 91–94.
- Blois, M.S. Antioxidant determination by the use of stable free radical. Nature 1958, 181, 1199–1200.
- IS: 3508. Indian Standards, Methods for Sampling and Test for Ghee (butter fat), Bureau of Indian Standards, New Delhi, 1966.
- AOAC. Official Methods of Analysis, 16th edition, Association of Official Agricultural and Chemists: Washington, D.C., 1971, 877–988.
- Patton, S.; Kurtz, G.W. 2-Thiobarbituric acid as a reagent for detecting milk fat oxidation. Journal of Dairy Science 1951, 34, 669–674.
- Frankel, E.N. In search of better methods to evaluate natural antioxidants and oxidative stability in food lipids. Trends in Food Science and Technology 1993, 4, 220–225.
- Snedecor, G.W.; Cochran, W.G. Statistical Method, 8th Ed.; Affiliated East-West Press: New Delhi, 1994.
- Zhou, C.; Li, X.; Xu, C.; Sun, C.; Chen, K. Hydrophilic and lipophilic antioxidant activity of loquat fruits. Journal of Food Biochemistry 2011, 36 (5), 621–626.
- Shan, B.; Yizhong, Z.; Cai Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. Journal of Agriculture and Food Chemistry 2005, 53 (20), 7749–7759.
- Guan, W.; Shufen, L.; Ruixiang, Y.; Saokun, T.; Can, Q. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chemistry 2007, 101 (4), 1558–1564.
- Reverchon, E. Supercritical fluid extraction and fractionation of essential oils and related products. Journal of Supercritical Fluids 1997, 10, 1–37.
- Cai, L.; Wu, C.D. Compounds from syzygium aromaticum possessing growth inhibitory activity against oral pathogen. Journal of Natural Products 1996, 59 (10), 987–990.
- Gilbertson, G. Oleoresin as flavor ingredients. The Flavour Industry 1971, 43, 403–405.
- Siddiq, A.; Anwar, F.; Manzoor, M.; Fatima, A. Antioxidant activity of different solvent extract of Moringa Oleifera leaves under accelerated storage of sunflower oil. Asian Journal of Plant Science 2005, 4 (6), 630–635.
- Frankel, E.N.; Huang, S.W.; Prior, E.; Aeschbach, R. Evaluation of antioxidant activity of rosemary extracts, carnosol, and carnosic acid in bulk vegetable oils and fish oil and their emulsions. Journal of the Science of Food and Agriculture 1996, 72, 201–208.
- Paul, G.; Mittal, S. Regulating the use of degraded oil/fat in deep fat/oil food frying. Critical Review of Food Science Nutrition 1997, 37, 635–662.
- Yadollah, Y.; Fatemeh, S.; Naader, B. Comparison of essential oil of Carum copticum obtained by supercritical carbon dioxide extraction and hydro distillation methods. Food Chemistry 2004, 86, 587–591.
- Griffiths, A.; Makoto, M.; Elizabeth, A.; Yoshinori, S.; Koichi, S. (2007). Activity of gaseous phase steam distilled propolis extracts on peroxidation and hydrolysis of rice lipids. Journal of Food Engineering 2007, 80, 850–858.
- Lee, H.S.; Kim, D.H. Variation of antioxidant retention and some properties of soybean oil during simulated frying operation. Han’guk Sikp’um Kwahakhoe Chi 1979, 11 (2), 86–92.
- Kim, C.M.; Pratt, D.E. Degradation products of 2-tertylhydroquinone at frying temperature. Journal of Food Science 1990, 55 (3), 847–850.
- Warner, C.R.; Daniels, D.H.; Lin, F.S.D.; Joe, F.L.J.; Fazio, T. Fate of 30 antioxidants and antioxidant derived product in deep-fat frying and cookie baking. Journal of Agriculture and Food Chemistry 1986, 34 (11), 1–5.
- Hawrysh, Z.J.; Shand, P.J.; Lin, C.; Tokarska, B.; Hardin, R.T. Efficacy of tertiary butylhydroquinone on the storage and heat stability of liquid canola shortening. Journal of American Oil Chemists Society 1990, 67, 585–90.
- Warner, M.M.; Gehring, M.M. High temperature natural antioxidant improves soya oil for frying. Journal of Food Science 2009, 74 (6), 500–505.
- Lee, K.G.; Shibamoto, T. Antioxidant property of aroma extract isolated from clove buds. Food Chemistry 2001, 74 (4), 443–448.
- Perez-Galvez, A.; Jaren-Galan, M.; Minguez-Mosquera, M.I. Impact of the increased thermal processing on retinol equivalent values of paprika oleoresins. Journal of Food Engineering 2005, 71, 379–385.
- Gutierrez, R.; Quijino, G.; Dobarganes, M.C. Analytical procedures for the evaluation of used frying fats. In: ID Frying of Food: Principles, Changes, New Approaches; Varela, G.; Bender, A.E.; Morton.; Eds.; Eills horwood: Chichester, 1988, 141–154.
- Chung, J.; Lee, J.; Choe, E. Oxidative stability of soybean and sesame oil mixture during frying of flour dough. Journal of Food Science 2004, 69, 574–578.
- Kurechi, T.; Kato, T. Studies on the antioxidants. XI. Oxidation products of concomitantly butylated hydroxyanisole and butylated hydroxytoluene. Journal of American Oil Chemists Society 1980, 57, 220–223.