Abstract
A casein hydrolysate generated by Alcalase had in vitro ACE-inhibitory activity of 44.4%, and was treated by Alcalase-catalyzed plastein reaction in ethanol-water medium. Alcalase addition, ethanol, substrate concentration, and reaction temperature optimized from experimental design were 8.36 kU/g peptides, 56.8 (v/v), 56.8% (w/v), and 37.5°C, respectively, when reaction time was fixed at 6 h. Two treated casein hydrolysates, namely TCH4 and TCH8, were obtained with reaction time of 4 and 8 h, and exhibited the highest ACE-inhibitory activity of 62.5% or the greatest reaction extent but an activity of 35.6%, respectively. Fractionation of TCH4 and TCH8 by applying ethanol-water of 7:3 (v/v) conferred the obtained supernatant (precipitate) fractionates higher (lower) activity than the parent substrate, while applying ethanol-water of 3:7 (v/v) or water led to an opposite result in activity for the fractionates. In vitro digestion of TCH4, TCH8, and their fractionates revealed that they had resistance in activity towards the investigated four proteases, as the resulted 47 out of 48 digests had higher activities than casein hydrolysate. TCH8 exhibited better protease resistance than TCH4. It is concluded that the applied plastein reaction can enhance ACE inhibition and protease resistance of casein hydrolysate.
INTRODUCTION
Hypertension is a major controllable risk factor in cardiovascular diseases, which is ranged as the world’s largest killers and claim 17.1 million lives per year according to the World Health Organization.[Citation1] Angiotensin I-converting enzyme (ACE, EC 3.4.15.1), one enzyme of the rennin-angiotensin system, can regulate peripheral blood pressure.[Citation2] ACE catalyzes the conversion of angiotensin I into the potent vasoconstrictor angiotensin II, and simultaneously, the degradation of bradykinin, a blood pressure lowering nonapeptide in kallikrein-kinin system.[Citation3] These two reactions cause a contraction of blood vessels and a consequent increase in blood pressure.
ACE-inhibitory peptides derived from food proteins are considered to be milder and safer in anti-hypertension than synthetic drugs. In vivo studies in spontaneously hypertensive rats or human volunteers indicate that these peptides can significantly reduce blood pressure, either after intravenous or oral administration, but have no effect on normotensive subjects.[Citation4] Furthermore, these peptides usually have multifunctional properties apart from better absorption.[Citation5] Oshima et al.[Citation6] generated ACE-inhibitory peptides from food protein by digestive proteases. Many other ACE-inhibitory peptides have been discovered from the fermented milk products[Citation7,Citation8] or protein hydrolysates,[Citation9−Citation11] as they could be generated from the inactive protein precursor by the digestion of proteases.[Citation12] The widely studied food protein sources of ACE-inhibitory peptides include milk proteins,[Citation13,Citation14] fish proteins,[Citation9] animal muscle proteins,[Citation10] egg proteins,[Citation15−Citation17] and soybean proteins.[Citation18,Citation19]
Plastein reaction involves three mechanisms as condensation,[Citation20] physical forces,[Citation21] and transpeptidation,[Citation22] and has been well-studied by food chemists owing to its application in debittering treatment for protein hydrolysates,[Citation23] amino acid fortification,[Citation24] and property modification for protein ingredients.[Citation21] A recent application of plastein reaction is to enhance bioactivity of protein hydrolysates. Casein hydrolysate modified by plastein reaction has an enhanced ACE-inhibitory[Citation25−Citation27] or antioxidant activity,[Citation28,Citation29] and extrinsic proline addition in the reaction system can result in casein hydrolysate higher ACE inhibition.[Citation30] Plastein reaction is also able to confer casein hydrolysate stronger resistance in ACE-inhibitory activity towards in vitro digestion of some proteases.[Citation31]
Plastein reaction in these mentioned studies is carried out in a water medium. Whether a plastein reaction in other mediums (e.g., a mixed solvent system consisting of water and one of miscible organic solvents) has potential impact on ACE inhibition and protease resistance of protein hydrolysates is unknown yet. Theoretically, the added miscible organic solvents can impact water content (i.e., water activity), practical activity of proteases, consequentially, the reaction extent and activity of the modified products. A detailed investigation is thus strongly suggested. In the present study, an Alcalase-catalyzed plastein reaction was employed to treat a casein hydrolysate in ethanol-water medium. Suitable reaction conditions, including Alcalase addition, ethanol and substrate concentration, and reaction temperature were studied and selected from an experimental design by response surface methodology. Two treated hydrolysates of different activities and reaction extent were subjected to solvent fractionation and protease digestion in vitro. The impacts of the applied plastein reaction on ACE inhibition or protease resistance of the treated hydrolysates were thus evaluated. The aim of the present study was to reveal potential application of plastein reaction in ethanol-water medium to enhance ACE inhibition and activity stability of casein hydrolysate.
MATERIALS AND METHODS
Materials
Caseinate, hippuryl-histidyl-leucine (HHL), rabbit lung acetone powder (as ACE source), and trypsin (89 kU/g) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Alcalase (118 kU/mL), pepsin (18 kU/g), and papain (29 kU/g) were purchased from Novozyme China (Tianjin, China), Hui Shi Biochem Reagent Co. (Shanghai, China) and Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China), respectively. All other chemicals and reagents used were of analytical grade. Highly purified water by Milli-Q PLUS (Millipore Corporation, New York, NY, USA) was used in buffer and solution preparation.
Preparation of Casein Hydrolysate
Caseinate solution (10%, w/v) was adjusted into pH 8.0 by adding 0.2 mol/L NaOH, and pre-incubated in water bath of 55°C for 10 min. Alcalase solution was prepared immediately prior to use. After withdrawal of a 20 mL sample (zero-time sample), the proteolysis was started by adding Alcalase solution to the remaining casein solution at 1 kU/g proteins, and carried out at 55°C with continuous gentile stirring. Hydrolyzed samples of 20 mL were withdrawn from the reaction system after 1, 2, 3, 4, 5, 6, 7, and 8 h of hydrolysis, respectively, heated at 95°C for 15 min, cooled to room temperature, and centrifuged at 11,000 × g for 20 min. The collected supernatants (casein hydrolysates) were evaluated for degree of hydrolysis (DH) and in vitro ACE-inhibitory activities. The hydrolysate of the highest activity was bulk prepared, lyophilized and used as the substrate of plastein reaction.
Plastein Reaction of Casein Hydrolysate in Ethanol-Water Medium
Some conditions were studied by using response surface methodology with a central composite design (CCD). In the CCD, ethanol concentration (%, v/v), substrate concentration (%, w/v), Alcalase addition (kU/g peptides) and reaction temperature (°C) were studied at five different levels shown in , with a fixed reaction time of 6 h. Decrease of free amino groups of the treated hydrolysates was used as the response. After the reaction, the treated hydrolysates were heated at 95°C for 15 min and assayed for content of free amino groups. Eight treated hydrolysates were prepared with the optimized conditions and reaction time of 1−8 h, respectively, and evaluated for their decrease of free amino groups and ACE-inhibitory activities. One treated hydrolysate of the highest activity and another treated hydrolysate of the greatest reaction extent (i.e., the largest decrease of free amino groups) were bulk prepared, lyophilized, and subjected to solvent fractionation and protease digestion.
Table 1 Reaction conditions investigated for the plastein reaction of casein hydrolysate by response surface methodology
Solvent Fractionation of the Treated Casein Hydrolysates
The treated hydrolysates or original casein hydrolysate were dispersed in ethanol-water solvents in ratios of 3:7 and 7:3 (v/v), respectively, at a fixed peptide content of 50% (w/v). The mixture was centrifuged at 9000 × g for 30 min to separate supernatant and precipitate fractionates. The two fractionates were evaporated at 80°C to remove ethanol, lyophilized, dissolved in water, and evaluated for peptide recoveries and ACE-inhibitory activities. At the same time, pure water was used to fractionate the two treated hydrolysates by the same procedure and conditions. The resulted fractionates were lyophilized, and subjected to same treatment and evaluation.
In Vitro Digestion of the Treated Casein Hydrolysates
The two treated hydrolysates and their four fractionates by ethanol-water in ratio of 7:3 (v/v) were subjected to in vitro digestion to examine activity resistance towards four proteases. Pepsin and trypsin were used as two digestive proteases while Alcalase and papain were selected as bacterial and plant protease. The evaluated samples were dissolved in water to give a fixed peptide content of 10% (w/v). Final pH of the solutions was adjusted to the optimal value of the used protease by 2 mol/L NaOH or HCl. The pH-adjusted solutions were incubated at water bath for 5 min, and digested by adding Alcalase (1 kU/g peptides, pH 8.0, 55°C), papain (2 kU/g peptides, pH 6.5, 45°C), trypsin (2 kU/g peptides, pH 8.0, 37°C), and pepsin (4 kU/g peptides, pH 2.0, 37°C) for 10, 30, or 60 min, respectively. A blank sample (zero time) was also prepared by adding protease solution preheated at 95°C for 15 min. After the digestion, all solutions were heated at 95°C for 15 min to inactive the proteases. The digests were lyophilized and analyzed for the content of free amino groups and residual ACE-inhibitory activities.
Protease Activity, Protein Content, DH, and In Vitro ACE-Inhibitory Activity
Protease activity was assayed as per the method of Sarath et al.[Citation32] with caseinate as substrate. Peptide content was measured by the Kjeldahl method with a conversion factor of 6.38.[Citation33] Content of free amino groups was measured by o-pthaldialdehyde assay,[Citation34] and used to calculate DH described by Adler-Nissen.[Citation35] L-Leucine (0−36 μg/mL) was used to prepare standard solution.
In vitro ACE-inhibitory activity was measured according to the method of Cushman et al.[Citation36] with some modifications. The analysis samples were dissolved in water in a fixed peptide content of 0.3 mg/mL. Sample solution (or deionized water) of 100 μL and HHL solution (5 mmol/L in 0.1 mol/L borate buffer, pH 8.3, containing 0.3 mmol/L NaCl) of 250 μL were mixed and incubated at 37°C for 5 min. Then, 150 μL of ACE extract from rabbit lung acetone powder was added. The mixture was incubated for 90 min, and the reaction was stopped by adding 0.5 mol/L HCl of 250 μL. Hippuric acid formed in the reaction system was extracted by ethyl acetate of 3 mL with vigorously shaking of 5 min. After standing for 5 min, 2 mL of the upper layer was transferred to clean tubes and evaporated by heating at 85°C for 35 min in a water bath. Hippuric acid left in the tubes was dissolved in 1 mol/L NaCl of 3 mL, and the absorbance was measured at 228 nm using a UV-spectrophotometer (UV-2401PC, Shimadzu, Kyoto, Japan). HCl solution was added immediately before ACE extract in zero-time control assaying, and ACE extract was heated at 75°C for 5 min to ensure absolutely inactivation. ACE inhibitory activity (%) was calculated as follows.
Statistical Analysis
All experiments or analyses in the present study were performed three times. The results were expressed as means ± standard deviations (SD). The significant differences among the means were evaluated by one-way analysis of variance (ANOVA) at P < 0.05 with Duncan’s multiple comparison test by the SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Design Expert 7.0 software (Stat-Ease Inc., Minneapolis, MN, USA) was used in CCD analysis.
RESULTS AND DISCUSSION
Suitable Plastein Reaction Conditions for Casein Hydrolysate
The analysis results show that DHs of the eight casein hydrolysates prepared by hydrolysis time of 1−8 h range from 6.9–11.8%. One casein hydrolysate prepared by a hydrolysis time of 6 h has a DH of 11.6% and is selected as the substrate of plastein reaction, as it shows the highest activity (44.4%) against ACE (IC50 = 42.8 μg/mL). With the applied CCD as in , a detailed experiment containing of 30 runs is completed. Final experimental results are given in , and the analysis results about the linear, quadratic, and cross-product effects of the investigated conditions on the response are listed in . Ethanol (X1), substrate concentration (X2), Alcalase addition (X3), reaction temperature (X4), quadratic term coefficient (X42), and the interaction coefficient (X1X2, X1X3, X2X3) all have significant impact on the plastein reaction of casein hydrolysates (P < 0.05). Other quadratic term coefficient (X12, X22, and X32) and interaction coefficient (X1X4, X2X4, and X3X4) give insignificant impact on the plastein reaction (P > 0.05) (not shown in ). Suitable ethanol and substrate concentration, Alcalase addition and temperature given by the software are 56.8% (v/v), 56.8% (w/v), 8.36 kU/g peptides and 37.5°C, respectively. When these optimized conditions are used for casein hydrolysate, actual decrease of free amino groups of the treated hydrolysate is (300.9 ± 2.1) μmol/g peptides, slight higher than the predicted value 296.8 μmol/g peptides.
Table 2 ANOVA response for linear, quadratic, and interactive effect of the investigated variables in statistical significancea
Figure 1 Response surface graphs for the impacts of the selected reaction conditions on the decrease of free amino groups of the treated casein hydrolysates.
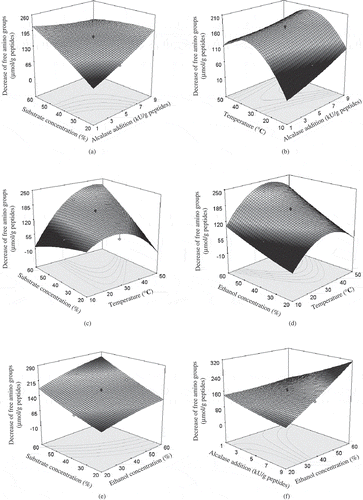
Plastein reaction of casein hydrolysate by Alcalase in water medium has a decrease of free amino groups about 110 or 180 μmol/g peptides.[Citation25,Citation31] The two values are lower than that of the present one (300.9 μmol/g peptides). This is supported by a previous research, in which addition of organic solvent such as glycerol into plastein reaction system can enhance plastein yield.[Citation21] Addition of organic solvent decreases water activity (aw) of the reaction system,[Citation37] and lower aw can shift reaction equilibrium towards synthesis reaction.[Citation22] Thus, ethanol concentration in reaction system is selected as 56.8% (v/v), which leads to much condensation reaction (i.e., the treated hydrolysate higher value in decrease of free amino groups). Plastein reaction usually carries out at substrate concentration about 30−50% (w/w), and is found to be an exothermic reaction.[Citation38] A higher substrate content and lower reaction temperature should be beneficial to condensation reaction. It is reasonable that the selected substrate concentration and reaction temperature are 56.8% (w/v) and 37.5°C, respectively. Owing to a lower aw in the present reaction system, practical activity of Alcalase is inhibited; consequentially, higher Alcalase addition of 8.36 kU/g peptides is needed. Unlikely, lower Alcalase addition about 3.1 or 7.7 kU/g peptides are applied in water medium.[Citation25,Citation31]
Reaction Extent and ACE Inhibition of the Treated Casein Hydrolysate
Eight treated hydrolysates are prepared by the selected conditions and reaction time of 1−8 h, respectively. Evaluation results for their decrease of free amino groups and activities in vitro are given in . The data show that decrease of free amino groups of the treated hydrolysates increases as reaction time is prolonged, indicating longer reaction time leads to much condensation. On the contrary, the activity of the treated hydrolysates behaves an increasing trend first (reaction time of 1−4 h) but then a decreasing trend (reaction time of 5−8 h). A treated hydrolysate by a reaction time of 4 h (TCH4) has the highest activity (62.5%) or lowest value of IC50 (27.7 μg/mL), while another one by a reaction time of 8 h (TCH8) shows the largest value in decrease of free amino groups (i.e., greatest reaction extent) but the lowest activity (35.6%). It indicates that only suitable reaction extent can enhance ACE inhibition of the treated hydrolysate. IC50 value of a synthetic ACE inhibitor captopril is 0.0043 μmol/L (i.e., 0.9 μg/mL).[Citation39] It means that these treated hydrolysates are weaker ACE inhibitors than captopril. TCH4 and TCH8 are thus selected to investigate their solvent fractionation or protease resistance.
Table 3 Decrease of free amino groups, ACE-inhibitory activities, and IC50 values of casein hydrolysate and treated casein hydrolysates by the plastein reaction at different reaction times
Plastein reaction is a kinetically-driven reversal of the usual protein hydrolysis, in which protease catalyses a condensation reaction to form new peptides of higher molecular weights.[Citation20] Owing to the occurred condensation and transpeptidation, some new peptides with higher activities might be generated, leading to the treated hydrolysates enhanced activities.[Citation30] The treated hydrolysates (TCH1−TCH6) exhibit higher activities than casein hydrolysate (46.6−62.5 vs. 44.4%). Unfortunately, too much plastein reaction will give an adverse impact on the activity of the treated hydrolysate, e.g., a reaction time longer than 4 h. This profile results in TCH4 the highest activity than others. ACE-inhibitory peptides are usually in 2−12 amino acid residues.[Citation3] Too much plastein reaction (or too longer reaction time) for casein hydrolysate means that those newly generated peptides of higher activities have opportunity to be further modified, resulting in the treated hydrolysates too longer peptide lengths and more important, the active sites of the generated peptides blocked or destroyed. The treated hydrolysates thus prepared show relative lower activity. A support might be provided by TCH8, who has the greatest reaction extent but the lowest activity among the treated hydrolysates ().
Table 4 Effect of solvent fractionation of casein hydrolysate and two treated casein hydrolysates by plastein reaction on their peptide recoveries and in vitro ACE-inhibitory activities
Impact of Solvent Fractionation on ACE-Inhibitory Activity of the Treated Casein Hydrolysate
The results given in indicate that when water and two ethanol-water (E-W) solvents in ratios of 3:7 and 7:3 (v/v) are used to fractionate casein hydrolysate, TCH4 and TCH8, the obtained fractionates have different activity changes. The obtained precipitate (supernatant) fractionates of TCH4 and TCH8 by ethanol-water in ratio of 3:7 (v/v) or water have higher (lower) activity than the parent substrate. Contrary to these results, fractionation of casein hydrolysate, TCH4 and TCH8 by ethanol-water in ratio of 7:3 (v/v) confers the supernatant (precipitate) fractionates higher (lower) activity than the parent substrate.
Plastein reaction yields some plasteins insoluble in water,[Citation21] which leads to the treated hydrolysates less soluble in three fractionation solvents than casein hydrolysate. More ethanol in the fractionation solvent results in lower polarity, and finally more peptide inextractable (i.e., lower peptide recovery in soluble part). ACE-inhibitory peptides are rich in hydrophobic amino acids.[Citation2,Citation10] When fractionation solvent is in lower polarity (e.g., ethanol-water in ratio of 7:3, v/v), the soluble (precipitate) fractionate would be rich (poor) in these peptides containing more hydrophobic amino acids. The soluble fractionate thus has higher activity but the precipitate fractionate has lower one. Plastein products are rich in hydrophobic amino acids,[Citation21] fractionation by solvents of higher polarity (e.g., ethanol-water of 3:7, v/v; or water) would ensure plastein products mainly in precipitate fractionate. As one might expect, the obtained precipitate fractionate gives higher activity. These results are consistent to the results of Sun and Zhao,[Citation31] in which casein hydrolysate treated in water medium is fractionated by several solvents of different polarity. Another support is the result from Hang and Zhao,[Citation40] in which ethanol-water solvent in ratio of 6:4 other than 2:8 or 6:4 (v/v) is more efficient to separate most active peptide fractions from a fermented soybean product.
Protease Resistance of the Treated Casein Hydrolysate
Protease resistance of TCH4, TCH8, and their four fractionates (by ethanol-water in ratio of 7:3, v/v) are examined by the residual activities of the resulted digests (). Four selected proteases all induce proteolysis on these substrates, among which Alcalase shows the strongest ability as it results in much increase in free amino groups of the digests. The data in show that TCH4, TCH8, and the four fractionates have different resistance in activity towards the carried out proteolysis, depending on the protease used. Totally, Alcalase induces much activity loss in the most cases whereas papain might enhance activity of the resulted digests in the some cases, if hydrolysis time is fixed in the same level.
Among the generated digests of TCH4, only those by papain, trypsin, and pepsin with a hydrolysis time of 10 min have residual activities higher than TCH4 (64.0−72.8 vs. 62.5%). For the supernatant fractionate of TCH4 (FTCH4-SF), only three digests by papain, trypsin, and pepsin with a hydrolysis time of 10 min have higher (73.7%) or same (61.5 and 62.7%) activities than TCH4, and seven out of eight digests show lower activities (50.0−62.7%) than FTCH4-SF (69.5%). For the precipitate fractionate of TCH4 (FTCH4-PF), three resulted digests by papain, trypsin, and pepsin with a hydrolysis time of 10 min exhibit similar activity as FTCH4-PF (58.3−59.1 vs. 58.6%), while other digests have residual activities of 45.1−52.3%. TCH8 behaves different behavior in protease resistance than TCH4. All digests of TCH8 have residual activities higher than TCH8 (42.9−74.8 vs. 35.6%). Digestion of the supernatant fractionate of TCH8 (FTCH8-SF) results in the residual activities in 46.2−71.4%, totally higher than the activity of FTCH8-SF (46.0%). Similar result is also found for the digests of the precipitate fractionate of TCH8 (FTCH8-PF) (residual activities 40.7−65.1 vs. 33.7%). These facts reveal that: (1) TCH4 and TCH8 have some resistance in activity to the selected proteases; (2) TCH8 of the greatest reaction extent has better protease resistance; (3) totally, the supernatant fractionates of TCH4 and TCH8 have higher activities, resulting in the digests higher residual activities; (4) among all 48 digests, only one digest from FTCH8-PF by Alcalase with a hydrolysis time of 30 min has an activity of 40.7%, lower than casein hydrolysate (44.4%). It is thus concluded that the applied plastein reaction can confer casein hydrolysate higher activity and better protease resistance.
Table 5 Impacts of protease digestion of TCH4, TCH8, and their fractionates on in vitro ACE-inhibitory activities
Alcalase has specificity mainly for hydrophobic amino acids.[Citation41] It can release these amino acids from TCH4, TCH8, and the corresponding fractionates to decrease ACE inhibition of the digests. Longer digestion time destroys more active peptides generated during plastein reaction, and leads to greater activity loss. This shares similarity to a previous research.[Citation31] Also, limited digestion of these samples by some proteases (e.g., digestive time of 10 min) might release some peptide fractions or amino acids previously blocked the active sites of the most active peptides, resulting in the digests higher activity than the original substrate (e.g., TCH8 and TCH8-SF). This phenomenon is not found in the digestion of the treated casein hydrolysates in water medium.[Citation31] A possible reason might be lower reaction extent for these hydrolysates treated in water medium. At the same time, digestive time of 30 or 60 min will induce too much damage to the most active peptides. The digests obtained thereof show decreased activities with increasing digestive time.
Protease resistance of protein hydrolysates in ACE inhibition might be an important scientific issue, as it means activity stability (and potential in vivo activity) when being severed as functional ingredients for oral administration. In the present study, casein hydrolysate treated by plastein reaction in ethanol-water medium shows an enhanced activity and protease resistance, indicating plastein reaction might be an approach to obtain protein hydrolysates with better activities and digestive stability.
CONCLUSION
Alcalase-catalyzed plastein reaction of casein hydrolysate in ethanol-water medium can lead to larger reaction extent than in water medium. With the optimized conditions from response surface methodology, a reaction time of 4 h confers the treated hydrolysate the highest ACE-inhibitory activity, while a longer reaction time of 8 h gives the treated hydrolysate the greatest reaction extent but lowest activity. When the treated hydrolysates is fractionated by different solvents, applying the solvent of higher polarity (water or ethanol-water in ratio of 3:7, v/v) leads to the obtained soluble or precipitate fractionate lower or higher activity than the parent substrate, whereas applying the solvent of lower polarity (ethanol-water in ratio of 7:3, v/v) results in opposite activity change. The carried out plastein reaction also confers the treated hydrolysate resistance in activity towards to four proteases, especially the treated hydrolysate of the greatest reaction extent. Plastein reaction in ethanol-water medium can be used to enhance ACE inhibition and protease resistance of protein hydrolysates.
ACKNOWLEDGMENT
The authors thank the anonymous reviewers and the editors for their valuable advice to this article.
FUNDING
This work was funded by the Innovative Research Team of Higher Education of Heilongjiang Province (Project No. 2010td11) and the National Natural Science Foundation of China (Project No. 30972132).
REFERENCES
- Papadogiannis, D.E.; Protogerou, A.D. Blood pressure variability: A confounder and a cardiovascular risk factor. Hypertension Research 2011, 34, 162–163.
- Sieber, R.; Bütikofer, U.; Egger, C.; Portmann, R.; Walther, B.; Wechsler, D. ACE-inhibitory activity and ACE-inhibiting peptides in different cheese varieties. Dairy Science and Technology 2010, 90 (1), 47–73.
- Li, G.H.; Lea, G.W.; Shi, Y.H.; Sundar, S. Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutrition Research 2004, 24 (7), 469–486.
- Erdmann, K.; Cheung, B.W.Y.; Schröder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. The Journal of Nutritional Biochemistry 2008, 19 (10), 643–654.
- Yu, Y.K.; Hu, J.E.; Miyaguchi, Y.; Bai, X.F.; Du, Y.G.; Lin, B.C. Isolation and characterization of angiotensin I-converting enzyme inhibitory peptides derived from porcine hemoglobin. Peptides 2006, 27 (11), 2950–2956.
- Oshima, G.; Shimabukuro, H.; Nagasawa, K. Peptide inhibitors of angiotensin I-converting enzyme in digests of gelatin by bacterial collagenase. Biochimica et Biophysica Acta-Enzymology 1979, 566 (1), 128–137.
- Gobbetti, M.; Ferranti, P.; Smacchi, E.; Goffredi, F.; Addeo, F. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milks started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4. Applied and Environmental Microbiology 2000, 66 (9), 3898–3904.
- Pihlanto, A.; Virtanen, T.; Korhonen, H. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effect of fermented milk. International Dairy Journal 2010, 20 (1), 3–10.
- Raghavan, S.; Kristinsson, H.G. ACE-inhibitory activity of tilapia protein hydrolysates. Food Chemistry 2009, 117 (4), 582–588.
- Jang, A.; Lee M. Purification and identification of angiotensin converting enzyme inhibitory peptides from beef hydrolysates. Meat Science 2005, 69 (4), 653–661.
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Identification of a novel angiotensin I-converting enzyme inhibitory peptide corresponding to a tryptic fragment of bovine β-lactoglobulin. FEBS Letters 1997, 27 (2–3), 99–101.
- Meisel, H. Biochemical properties of regulatory peptides derived from milk proteins. Biopolymers 1997, 43 (2), 119–128.
- Miguel, M.; Contreras, M.M.; Recio, I.; Aleixandre, A. ACE-inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chemistry 2009, 112 (1), 211–214.
- Contreras, M.M.; Carrón, R.; Montero, M. J.; Ramos, M.; Recio, I. Novel casein-derived peptides with antihypertensive activity. International Dairy Journal 2009, 19 (10), 566–573.
- Miguel, M.; Alonso, M.J.; Salaices, M.; Aleixandre, A.; López-Fandiño, R. Antihypertensive, ACE-inhibitory, and vasodilator properties of an egg white hydrolysate: Effect of a simulated intestinal digestion. Food Chemistry 2007, 104 (1), 163–168.
- Fujita, H.; Usui, H.; Kurahashi, K.; Yoshikawa, M. Isolation and characterization of Ovokinin, a bradykinin B1 agonist peptide derived from ovalbumin. Peptides 1995, 16 (5), 785–790.
- Rao, S.Q.; Sun, J.; Liu, Y.T.; Zeng, H.W.; Su, Y.J.; Yang, Y.J. ACE inhibitory peptides and antioxidant peptides derived from in vitro digestion hydrolysate of hen egg white lysozyme. Food Chemistry 2012, 135 (3), 1242–1252.
- Tomatsu, M.; Shimakage, A.; Shinbo, M.; Yamada, S.; Takahashi, S. Novel angiotensin I-converting enzyme inhibitory peptides derived from soya milk. Food Chemistry 2013, 136 (2), 612–616.
- Wu, J.; Ding, X. Hypotensive and physiological effect of angiotensin I-converting enzyme inhibitory peptides derived from soy protein on spontaneously hypertensive rats. Journal of Agricultural and Food Chemistry 2001, 49 (1), 501–506.
- Yamashita, M.; Arai, S.; Fujimaki, M. Plastein reaction for food protein improvement. Journal of Agricultural and Food Chemistry 1976, 24 (6), 1100–1104.
- Andrews, A.T.; Alichanidis, E. The plastein reaction revisited: Evidence for a purely aggregation reaction mechanism. Food Chemistry 1990, 35 (4), 243–261.
- Combes, D.; Lozano, P. α-Chymotrypsin in plastein synthesis influence of water activity. Annals of the New York Academy of Science (Enzyme Engineering XI) 1992, 672, 409–414.
- Eriksen, S.; Fagerson, I.S. The plastein reaction and its application: A review. Journal of Food Science 1976, 41 (3), 490–493.
- Yamashita, M.; Arai, S.; Imaizumi, Y.; Amano, Y.; Fujimaki, M. A one-step process for incorporation of L-methionine into soy protein by treatment with papain. Journal of Agricultural and Food Chemistry 1979, 27 (1), 52–56.
- Zhao, X.H.; Li, Y.Y. An approach to improve ACE inhibitory activity of casein hydrolysates with plastein reaction catalyzed by Alcalase. European Food Research and Technology 2009, 229 (5), 795–805.
- Gao, B.; Zhao, X.H. Modification of soybean protein hydrolysates by alcalase-catalyzed plastein reaction and the ACE-inhibitory activity of the modified product in vitro. International Journal of Food Properties 2012, 15 (5), 982–996.
- Xu, W.; Li, T.J.; Zhao, X.H. Coupled Neutrase-catalyzed plastein reaction mediated the ACE-inhibitory activity in vitro of casein hydrolysates prepared by Alcalase. International Journal of Food Properties 2013, 16 (2), 429–443.
- Zhao, X.H.; Wu, D.; Li, T.J. Preparation and the radical scavenging activity of papain-catalyzed casein plasteins. Dairy Science and Technology 2010, 90 (5), 521–535.
- Zhao, X.H.; Song J.T. Evaluation of antioxidant properties in vitro of plastein-reaction-stressed soybean protein hydrolysate. International Journal of Food Properties 2014, 17 (1), 152–162.
- Zhao, X.H.; Li, Y.Y. Preparation of Alcalase-catalyzed casein plasteins in the presence of proline addition and the ACE-inhibitory activity of the plasteins in vitro. European Food Research and Technology 2010, 231 (2), 197–201.
- Sun, H.; Zhao, X.H. Angiotensin I-converting enzyme inhibition and enzymatic resistance in vitro of casein hydrolysate treated by plastein reaction and fractionated with ethanol/water or methanol/water. International Dairy Journal 2012, 24 (1), 27–32.
- Sarath, G.; De La Motte, R.S.; Wagner, F.W. Protease assay methods. In: Proteolytic Enzymes, a Practical Approach; Beynon, R.J.; Bond, J.S.; Eds.; IRL Press: Oxford, UK, 1989, 25–55.
- International Dairy Federation (IDF). Determination of the Nitrogen (Kjeldahl method) and Calculation of the Crude Protein Content. IDF Standard 20B. International Dairy Federation: Brussels, Belgium, 1993.
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. Journal of Dairy Science 1983, 66 (6), 1219–1227.
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. Journal of Agricultural and Food Chemistry 1979, 27 (6), 1256–1261.
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin -converting enzyme of rabbit lung. Biochemical Pharmacology 1971, 20 (7), 1637–1648.
- George, B.; Janssen, A.E.M.; Halling, P.J. Water activity fails to predict critical hydration level for enzyme activity in polar organic solvents: Interconversion of water concentrations and activities. Enzyme and Microbial Technology 1997, 20 (6), 471–477.
- Williams, R.J.H.; Brownsell, V.L.; Andrews, A.T. Application of the plastein reaction to mycoprotein: I. Plastein synthesis. Food Chemistry 2001, 72 (3), 329–335.
- Qian, Z.J.; Je, J.Y.; Kim, S.K. Antihypertensive effect of angiotensin I converting enzyme-inhibitory peptide from hydrolysates of bigeye tuna dark muscle, Thunnus obesus. Journal of Agricultural and Food Chemistry 2007, 55 (21), 8398–8403.
- Hang, M.; Zhao, X.H. Fermentation time and ethanol/water-based solvent system impacted in vitro ACE-inhibitory activity of the extract of Mao-tofu fermented by Mucor spp. CyTA-Journal of Food 2012, 10 (2), 137–143.
- Markland, F.S.; Smith, E.L. Substilisins: Primary Structure, Chemical, and Physical Properties, Boyer, P.D.; Ed.; The Enzyme, 1st Ed., Academic Press: New York. 561–608, 1971.
