Abstract
Experiments were conducted to study the kinetics of colour change of yellow sweet pepper during microwave-assisted convective drying at power levels of 280, 210, 140, and 70 W; temperatures of 60, 45, and 30°C; and a constant air velocity of 1.5 m/s. Colour change kinetics were determined using Hunter’s primary L, a, b values, chroma, hue, total colour change (ΔE), and browning index (BI) values. The results indicated that the degradation of a, ΔE, and hue followed zero order kinetics and L, b, chroma, and browning index followed first order kinetics. The rate constant was assumed to have Arrhenius-type dependence on temperature. Again, based on activation energy, b and hue parameters proved to be the most sensitive measures of color change, which could be taken into account during thermal processing of capsicum for getting a quality product.
INTRODUCTION
Sweet pepper is a non-pungent variety of pepper (Capsicum sp.) belonging to the solanaceous family. Health-promoting, nutritional, and sensory attributes make the pepper one of the most consumed vegetables. It is an important cash crop in India and the world’s second most important solanaceous vegetable after tomato, also known as bell pepper and locally as ‘shimla mirch’. Apart from being used as a vegetable, peppers are extensively utilized as a colouring agent by the food and cosmetics industries. It is rich in vitamins C, B, and E, flavanoids, capsaicin, and mineral content.[Citation1Citation−Citation3]
The visual appearance of food, i.e., colour, is an important attribute, which is the first impact made by a consumer at the point of sale.[Citation4] The colour of yellow sweet pepper is controlled by β-carotene and zeaxanthin as these are food pigments.[Citation5] However, excessive heating during thermal processing produces considerable losses in the quality and particularly in the organoleptic properties of foods.[Citation6] Several authors have studied the colour kinetics of food materials during thermal processing in terms of changes in Hunter tri-stimulus colour values L, a, and b.[Citation7−Citation10]
Hunter colour parameters (L, whiteness/darkness; a, redness/greenness; and b, yellowness/blueness) have proved valuable in describing visual colour deterioration and providing useful information for quality control in fruits and vegetables. There are other parameters derived from the Hunter L, a, and b scale: the total colour change, chroma, which indicates colour saturation and is proportional to its intensity; hue angle is frequently used to specify colour in food products. An angle of 0 or 360°C represents red hue, while angles of 90, 180, and 270°C indicate yellow, green, and blue hues, respectively. Hue angle has been used in the evaluation of colour parameters, especially in green vegetables, fruits, and meats. Browning index (BI) represents the purity of brown colour and is reported as an important parameter in drying processes where enzymatic and non-enzymatic browning takes place. The study of the colour change behavior of foods during drying has recently been a subject of interest for various investigators; for example, concentrated fruit pulp,[Citation11] spinach and mustard leaves,[Citation12] banana,[Citation13] garlic,[Citation14] rosehip,[Citation15] soybean,[Citation16] mango pulp,[Citation17] guava and papaya,[Citation18] okra,[Citation8] bamboo shoot slices,[Citation19] apple slices,[Citation20] and watermelon juice.[Citation21] Hence, if the kinetics of colour degradation are determined and the order of colour change is established, the total colour can be used to evaluate quality of food material during thermal processing. The literature review revealed that no information is available on thermal kinetics of colour degradation of yellow sweet pepper. This study was aimed at understanding the kinetics of colour degradation during microwave-assisted convective drying of yellow sweet pepper and also to calculate the activation energies for colour change kinetic parameters using the exponential expression based on the Arrhenius equation.
MATERIALS AND METHODS
Material
Fresh yellow sweet peppers were procured from the Center for Protected Cultivation Technology, Indian Agricultural Research Institute, New Delhi. The samples were washed and stored at 7 ± 0.5°C in refrigerated storage until analysis. Before the drying experiments, the samples were taken out of the refrigerator and sliced with a knife. At least 10 measurements of length, breadth, and thickness were made at different points of the sliced pepper with a vernier caliper (Mitutoyo, Japan) having an accuracy of 0.02 mm; only slices that fell within a 5% range of the average dimensions (length, breadth, and thickness) were used. The average value of length (60 ± 1.5 mm), breadth (6 ± 0.5 mm), and thickness (4 ± 0.41 mm) were calculated. Initial moisture content was measured by taking 30-g samples dried in an oven at 70°C for 24 h, which were calculated as 89.01 ± 0.45% (w.b.).
Pretreatment
A flow diagram of the used experimental protocol is shown in . The samples were osmotically dehydrated according to the Central Composite Rotatable Design (CCRD) using parameters, such as salt, sucrose, RPM (revolutions per minute), solution to sample ratio (SSR), and time, inside the incubator shaker shown in . These were optimized based on our objective requirements of weight loss, moisture loss, and solid gain, and the optimized osmotic dehydrated samples containing a moisture content of 71.29 ± 0.65% (w.b.) were taken for microwave drying.
Table 1 Design parameters for osmotic dehydration
Drying Equipment and Drying Method
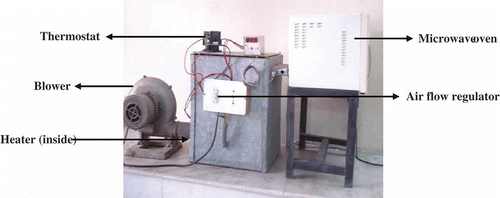
Drying experiments were performed in a laboratory scale microwave-convective dryer () consisting of four subsystems: air supply unit, heating unit, drying unit, and control unit, available in the Division of Post Harvest Technology, Indian Agricultural Research Institute, New Delhi. The blower (model: 0.24 HP, 50 HZ, continuous single phase) blows the air to the heating section to maintain the temperature up to a desired level, regulated by a thermostat from where it passes to the microwave oven. A thermostat (Multispan, MDC2901) was mounted over the blower to detect the change in the air temperature, which can be varied by manually using the regulator unit. The microwave oven (WP700L17.3 MW oven, LG make, 17 L capacity) with technical features of ˜230 V, 50 Hz, and 700 W with a frequency of 2450 MHz has the dimensions of 295, 458, and 370 mm and consisted of a turn table of 270 mm diameter at the base of the oven and it also operates in pulsed mode. The microwave oven has the capability of operating at ten different microwave output powers between 70 and 700 W measured using the IMPI-2 L test.[Citation22] The adjustment of microwave power level and processing time is done with the aid of an analogue control facility located on the microwave oven. The dryer was run without the sample placed in, for about 30 min to set the desired drying conditions before each drying experiment. Air velocity was measured with a hot wire anemometer (Model No.: AM-4204; Make: LT Lutron, Taipei, Taiwan) having a least count of 0.1 m/s. Preliminary experiments of microwave-hot air drying of yellow sweet pepper resulted in charring of the product towards the end of drying at a power level higher than 280 W. The combined microwave-hot air drying experiments were thus conducted with continuous microwave power of 280 W and step down intervals of 70 W, in conjunction with hot air at 30, 45, and 60°C temperatures at constant air velocity of 1.5 m/s.
Two hundred (200) g of osmotically dehydrated yellow sweet pepper was arranged in a single layer on the turn table and placed in the centre of the oven and the drying process was started for different combinations of microwave power and air temperature. Then, the samples were removed from the oven periodically and moisture loss was measured by weighing on the digital balance (Panacea GX 4000, Germany) with 0.01 g precision. Three replications of each experiment were performed according to preset conditions and the data was presented as the average of these results. The reproducibility of the experiments was within the range of ± 5%. Drying process continued until the moisture content of samples fell down to about 6% (w.b.). All weighing processes were completed in <10 s during the drying process.
Colour Measurement
Colour measurements of the yellow sweet pepper slices were carried out using a Hunter-Lab Colorimeter (Miniscan® XE Plus 4500 L). The instrument (45°/0° geometry, D 65 optical sensor, 10° observer) was calibrated with black and white reference tiles through the tri-stimulus values X, Y, Z, taking as standard values those of the white background (X = 79.01; Y = 83.96; Z = 86.76) tile. The colour values were expressed as L, a, and b at any time, respectively. A glass cell containing the microwave treated samples was placed above the light source and post-processing L, a, b values were recorded. Colour measurements were taken in triplicate and average values were taken for further calculation. In addition, the total colour change (ΔE) (Eq. 1), chroma (Eq. 2), hue angle (Eq. 3), and Browning index (Eq. 4) were calculated from the Hunter L, a, b scale and used to describe the colour change during drying:
Kinetic Consideration
To determine the reaction order of total colour change due to heat exposure, colour change was assumed to be a single entity. The following equations were used to represent the reaction rate:
Statistical Analysis
Experimental data for the different parameters were fitted to different kinetic models and processed using MATLAB version 7.1.6 (R2008a) software. Linear and non-linear regression were applied for zero order and first order kinetics, respectively, to calculate the corresponding reaction rate constant. Correlation coefficient and root mean square error (δ) values were used as the basis to select the best regression for the entire temperature range. In the second step, the Arrhenius equation was used to describe the temperature dependence of the reaction rate constant. In all cases, data fittings were considered significant to a probability level of 95%.
RESULTS AND DISCUSSION
Kinetics of Colour Change during Microwave Convective Drying
To investigate the combined effect of microwave output power and convective air temperature on colour change kinetics of yellow sweet pepper, four microwave output powers (70, 140, 210, and 280 W) and convective air temperatures (30, 45, and 60°C) at constant air velocity of 1.5 m/s were used for drying a constant amount of 200 g. In practice, any changes in a and b values have been associated with simultaneous changes in L value. Representation of quality in terms of total colour may, therefore, be more relevant from the processing point of view.[Citation9,Citation24] The estimated parameters of the kinetic models, the corresponding values of coefficients of determination (R2) and the root mean square error (δ) are represented in and .
Colour Parameter ‘L’
Lightness (L) decreased with drying time for all levels of power and temperature of yellow sweet pepper as shown in . Since L is a measure of the colour in the light-dark axis, this falling value indicates that the samples were turning darker. It has been observed that the variation in the brightness of heat-treated samples can be taken as a measurement of browning.[Citation25] A similar behaviour was found by Barreiro et al.,[Citation26] which was possibly due to the presence of heat sensitive reactions in the first phase of the curve involving the degradation of thermolabile pigments resulting in formation of dark compounds that reduced luminosity, while in the second phase more thermostable pigments were involved. As a whole, the development of discoloration of samples during drying by any technique may be related to pigment destruction, ascorbic acid browning, and non-enzymatic Maillard browning.[Citation27,Citation28]
Table 2 The estimated kinetic parameters and the statistical values of zero-order and first-order models for L, a, b, and total color change (ΔE) for various microwave output powers
Table 3 The estimated kinetic parameters and the statistical values of zero-order and first-order models for chroma, hue angle, and Browning index for various microwave output powers
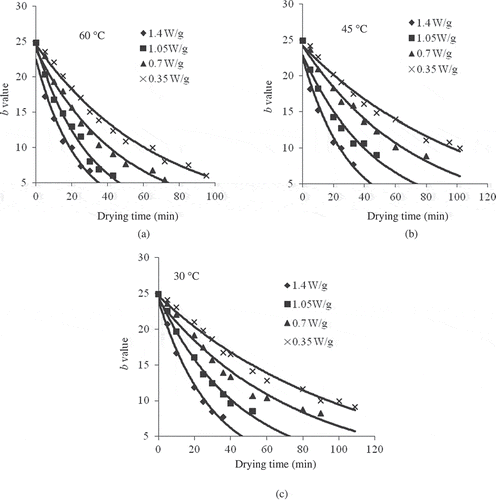
Figure 3 Kinetics of change of L value as a function of drying time at 1.4, 1.05, 0.70, and 0.35 W/g of microwave output powers and at temperatures of 60, 45, and 30°C.
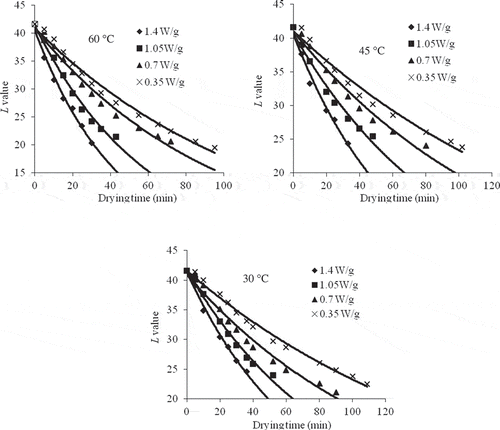
The L value decreased from the initial value of 41.61 to 20.98 with an increase in the power level from 0.35 to 1.4 W/g, which shows more deviation from lighter colour to darker. The time to reach an L value of about half of the initial was 30 min for 1.4 W/g and 60°C, which is about 110 min for 0.35 W/g and 30°C. Therefore, high power and temperature would give a destruction rate nearly 3.5 times more than the lower power levels. Indeed, final lightness relative to initial lightness declined exponentially and significantly (P < 0.05) with the corresponding increase in the drying temperature. Again, the kinetic rate constants (k) for the first order model increased from 0.0059 to 0.0226 min−1 with the increase in microwave power and convective air temperature. Thus, it may be concluded that with the increase in microwave power and air temperature, the rate of colour deterioration was faster as a result of high energy transferred to the inside of food material, which causes an increase in the temperature of the product.[Citation8] The coefficient of determination values (R2) for the first order model ranged from 0.9986 to 0.9707 with the standard error values ranging from 0.297 to 0.970, which were less than zero order model as shown in . As the values of R2 and standard error were taken as the best criteria for selecting a best fitted model, it could be observed that this relationship followed the first-order kinetic reaction and was consistent with previous works for grape juice;[Citation29,Citation30] double concentrated tomato paste;[Citation26] apple pulp, peach pulp, and plum pulp;[Citation11] peach puree[Citation31] and pear puree[Citation27].
Colour Parameter ‘b’
The relative visual b values decreased from 24.84 to 5.43 () during microwave treatment under various conditions of microwave power. This could be attributed to accelerate the carotenoid isomerization due to exposure to high temperature leading to loss of yellowness.[Citation32,Citation33] It was observed that the first-order kinetic model fitted well to parameter b, where a significant (P < 0.05) exponential regression with R2 varied between 0.9358 and 0.9848, with the standard errors (δ) for first order model varying from 0.368 to 0.971, were less than zero order model (). Hence, it could be concluded that the degradation of colour parameter b followed first order kinetic reaction. Similar results for the order of reaction were found by other authors in double concentrated tomato paste,[Citation26] peach puree,[Citation31] mango puree,[Citation34] and pineapple juice.[Citation35]
Colour Parameter ‘a’
, , nd 5c show an increase in the a values from 5.64 to 11.37 undergoing different processing steps, which signifies that the yellow sweet pepper slices lost their colour and became more red. This may be due to decomposition of pigments[Citation10] and formation of brown pigments.[Citation29,Citation36,Citation37] A similar behaviour for this parameter was found in black currant syrups,[Citation38], grape juice,[Citation30], blood orange juice,[Citation39] and purple carrots.[Citation40] In this case, zero order kinetic model adequately described the degradation of a value of yellow sweet pepper over the entire temperature range. The coefficient of determination values (R2) ranged between 0.9907 and 0.9997 with the kinetic rate constant varying between 0.0434 and 0.152 min−1.
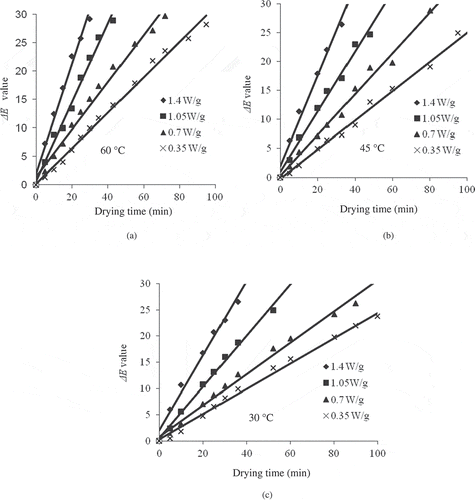
Colour Parameter ‘ΔE’
The total colour difference (ΔE) increased significantly during microwave drying with drying time as well as temperature. The values ranged from 5.05 to 29.87 as the microwave output power increased from 0.35 to 1.40 W/g (). In the current study, it was observed that the zero-order kinetic model fitted well in which a significant (P < 0.05) linear regression with the coefficient of determination values (R2) ranged between 0.9923 and 0.9978 (). The same order of reaction was found by Flora[Citation41] and Rhim et al.[Citation30] in grape juice, by Pagliarini et al.[Citation42] in milk and by Barreiro et al.[Citation26] in double concentrated tomato paste.
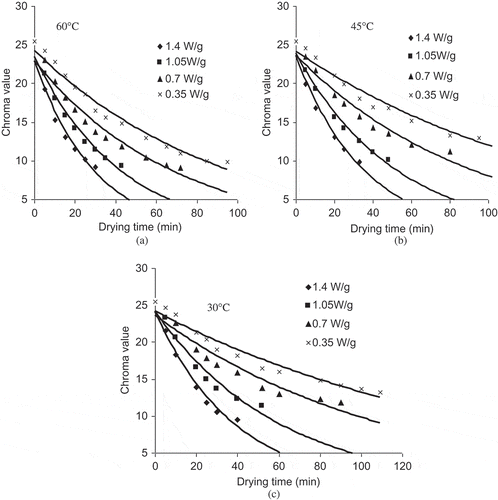
Colour Parameter ‘Chroma’
Chroma indicates the degree of saturation of colour and is proportional to the strength of the colour. It decreased from 25.472 to 9.067 (). Little change was found in chroma and they were not significantly different (P < 0.05), indicating stability of colour of yellow sweet pepper. The first order reactions associated with the degradation chroma was also calculated with the kinetic rate constant ranging from 0.0065 to 0.0371 min−1 (). Several investigators have reported similar observations.[Citation26,Citation43,Citation44]
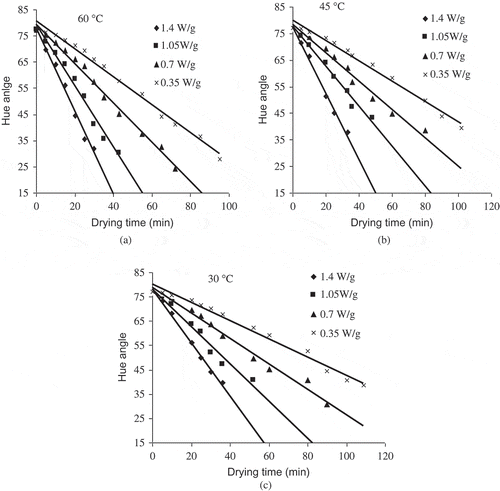
Colour Parameter ‘Hue’
Hue angle has been extensively used to characterise and evaluate colour parameters in food products (green vegetables, fruits, and meats). Hue angle decreased from 77.226 to 25.379 () following zero order reaction (). The decreasing trend of hue angle may be attributed to degradation of yellow carotenoid pigments (Zeaxanthin) due to microwave heating leading to orange colour product. It is in agreement with other literature like kiwi fruit[Citation37] and okra.[Citation8] The kinetic rate constant of zero order ranged from 0.3491 to 1.554 min−1, which was also proportional to the increase in the microwave output power and convective air temperature.
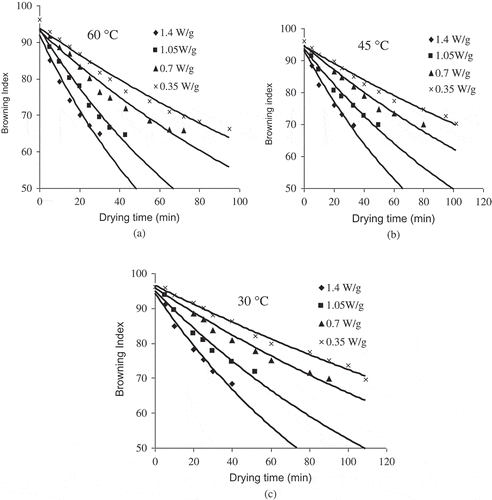
Colour Parameter ‘Browning Index (BI)’
Browning index signifies the purity of brown colour and an important parameter in processes where enzymatic and non-enzymatic browning takes place.[Citation44] It decreased from 96.153 to 64.612 and it was predicted that brown pigment formation adequately followed the first-order kinetic model (). In all cases, a significant (P < 0.05) exponential regression with R2 varied from 0.973 to 0.988 with the standard error values shown in . Similar results for the order of reaction were found by other authors in pear juice concentrate,[Citation45] apple juice,[Citation46] and pear puree.[Citation27] The kinetic constants’ values of first order reaction ranged between 0.0024 and 0.0131 min−1 for yellow sweet pepper (). The evident increase of the kinetic constants with the treatment temperature confirmed that non-enzymatic browning is favoured by the increase in treatment temperature.
Effects of Temperature on the Rate Constant
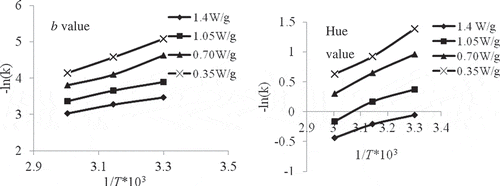
The dependence of rate constant on absolute temperature obeys the Arrhenius relationship (Eq. 8). A higher activation energy (Ea) value indicates greater heat sensitivity for colour degradation during a thermal process.[Citation47] Ea values of yellow sweet pepper ranged from 6.250 to 24.665 kJ/mole. Again, Ea values () of b were found to be higher than other colour parameters. Hence, b could be considered to be the most heat sensitive colour variable for yellow sweet pepper. Further, a higher Ea value over a lower temperature range reflects differences in their sensitivity to temperature. This is in agreement with pear puree.[Citation27]
Table 4 Activation energies calculated for the color degradation for quality parameters
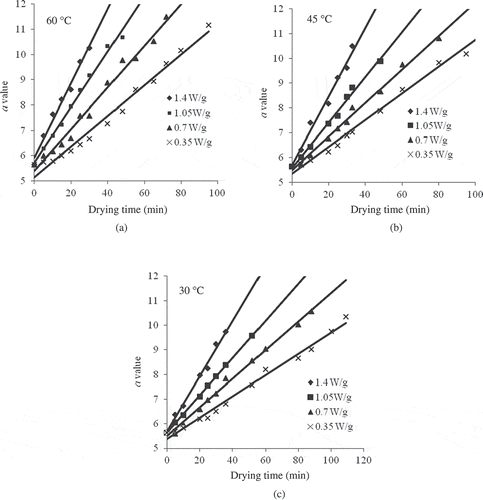
Considering for derived Hunter’s parameters, hue angle was most sensitive having Ea values ranging between 11.109 ± 1.543 to 20.438 ± 2.212 kJ/mole with the coefficient of determination varying from 0.982 to 0.991. The Arrhenius plot for the best color parameters (b and hue value) at different power levels is shown in . The slope values, Ea/R, were used to calculate the activation energies and the intercepts gave the frequency factors (k0). It may be concluded that fruit exhibits high activation energy for one parameter but less for another, reflecting differences in their sensitivity to temperature. Yellow sweet pepper had a higher Ea value over the lower temperature range for yellowness (b value), but lower value for brightness (L). A similar trend was also observed for pear puree[Citation27] that had a higher Ea value over the lower temperature range for yellowness (b value), but lower value for ΔE and the browning index compared to pineapple puree. This could be due to the differences in composition, such as sugar and amino acid content, pH, acidity, and also the temperature range of the study.[Citation12,Citation45] The results from this study indicated that the most sensitive parameter for the measurement of colour degradation in response to temperature treatment during processing was b value and hue for yellow sweet pepper, based on their Ea estimates.
CONCLUSION
The objective measurement of colour using the L, a, and b system can totally translate the real behaviour of dried yellow sweet pepper when subjected to microwave-assisted hot air drying. The b and hue parameters following first order and zero order, respectively, proved to be good indicators of the total colour change based on activation energy (Ea) of heat-treated yellow sweet pepper. The Arrhenius model described well the temperature dependence of the reaction rate constant for all the colour parameters considered. The activation energy for yellow sweet pepper varied from 12.603 ± 1.022 to 24.665 ± 1.403 kJ/mol for a and 11.109 ± 1.543 to 20.438 ± 2.212 kJ/mol for hue angle. Therefore, the above two parameters may be recommended as an on-line quality control parameter to monitor processing effects on colour change during microwave-assisted hot air drying of yellow sweet pepper.
REFERENCES
- Andrews, J. Peppers, the classification of capsicums. University of Texas Press: Austin, Texas, 1984.
- Choudhary, B. Vegetables, 1st Ed.; National Book Trust: New Delhi, India, 1967; 70.
- Choudhury, A.P.; Kumbhar, B.K.; Singh, B.P.N.; Narain, M. Osmotic dehydration of fruits and vegetables-a review. Indian Food Industry 1993, 12, 20–27.
- Sáenz, C.; Sepúlveda, A.E.; Calvo, C. Color changes in concentrated juices of prickly pear (Opuntiacus indica) during storage at different temperature. LWT–Food Science and Technology 1993, 26, 417–421.
- Ittah, Y.; Kanner, J.; Granit, R. Hydrolysis study of carotenoid pigments of paprika (Capsicum annuum L. variety Lahava) by HPLC/photodiode array detection. Journal of Agricultural and Food Chemistry 1993, 41 (6), 899–901.
- Hayakawa, K.; Timbers, G.E. Influence of heat treatment on the quality of vegetables: Changes in visual green color. Journal of Food Science 1977, 42 (3), 778–781.
- Ahmed, J.; Shivhare, U.S. Thermal kinetics of color change, rheology and storage characteristics of garlic puree/paste. Journal of Food Science 2001, 66, 754–757.
- Dadali, G.; Apar, D.K.; Ozbek, B. Colour change kinetics of okra undergoing microwave drying. Drying Technology 2007, 25, 925–936.
- Shin, S.; Bhowmik, S.R. Thermal kinetics of color changes in pea puree. Journal of Food Engineering 1994, 27, 77–86.
- Weemaes, C.A.; Ooms, V.; Loey, A.M.; Hendrickx, M.E. Kinetics of chlorophyll degradation and color loss in heated broccoli juice. Journal of Agricultural and Food Chemistry 1999, 47, 2404–2409.
- Lozano, J.E.; Ibarz, A. Color changes in concentrated fruit pulp during heating at high temperatures. Journal of Food Engineering 1997, 31, 365–373.
- Ahmed, J.; Kaur, A.; Shivhare, U. Colour degradation kinetics of spinach, mustard leaves and mixed puree. Journal of Food Science 2002, 67 (3), 1088–1091.
- Waliszewski, K.N.; Pardio, V.T.; Ramirez, M. Effect of EDTA on colour during osmotic dehydration of banana slices. Drying Technology 2002, 20 (6), 1291–1298.
- Cui, Z.; Xu, S.; Sun, D. Dehydration of garlic slices by combined microwave-vacuum and air drying. Drying Technology 2003, 21 (7), 1173–1184.
- Koyuncu, T.; Tosun, I.; Ustun, N.S. Drying kinetics and colour retention of dehydrated rosehips. Drying Technology 2003, 21 (7), 1369–1381.
- Prachayawarakom, S.; Prachayawasin, P.; Soponronnarit, S. Effective diffusivity and kinetics of urease inactivation and colour change during processing of soybeans with super-heated-steam fluidized bed. Drying Technology 2004, 22 (9), 2095–2118.
- Cunha, R.L.; Cruz, A.G.; Menegalli, F.C. Effects of operating conditions on the quality of mango pulp dried in a spout fluidized bed. Drying Technology 2006, 24, 423–432.
- Hawlader, M.N.A.; Perera, C.O.; Tian, M.; Yeo, K.L. Drying of guava and papaya: Impact of different drying methods. Drying Technology 2006, 24, 77–87.
- Bal, L.M.; Kar, A.; Satya, S.; Naik, S.N. Kinetics of color change of bamboo shoot during microwave drying. International Journal of Food Science and Technology 2011, 46 (4), 827–833.
- Chauhan, O.P.; Singh, A.; Singh, A.; Raju, P.S.; Bawa, A.S. Effects of osmotic agents on colour, textural, structural, thermal, and sensory properties of apple slices. International Journal of Food Properties 2011, 14, 1037–1048.
- Sharma, R.; Kaur, D.; Oberoi, D.P.S.; Sogi, D.S. Thermal degradation kinetics of pigments and visual color in watermelon juice. International Journal of Food Properties 2008, 11, 439–449.
- Buffler, C.R. Microwave Cooking and Processing: Engineering Fundamentals for the Food Scientist; Van Nostrand Reinhold: New York, 1993; 157–159.
- Laidler, K.J. Chemical Kinetics; McGraw-Hill: New York, 1965.
- Ahmed, J.; Shivhare, U.S.; Debnath, S. Color degradation and rheology of green chilli puree during thermal processing. International Journal of Food Science and Technology 2002, 37, 57–64.
- Labuza, T.P.; Lillemo, J.H.; Taoukis, P.S. Inhibition of polyphenol oxidase by proteolytic enzymes. Fruit Processing 1992, 2, 9–13.
- Barreiro, J.A.; Milano, M.; Sandoval, A.J. Kinetics of color change of double concentrated tomato paste during thermal treatment. Journal of Food Engineering 1997, 33, 359–371.
- Ibarz, A.; Pagan, J.; Garza, S. Kinetic models for color changes in pear puree during heating at relatively high temperatures. Journal of Food Engineering 1999, 39, 415–422.
- Salunkhe, D.K.; Bolin, H.R.; Reddy, N.R. Storage, Processing, and Nutritional Quality of Fruits and Vegetables, 2; CRC Press: Boca Raton, FL, 1991.
- Rhim, J.W.; Nunes, R.V.; Jones, V.A.; Swartzel, K.R. Determination of kinetic parameters using linearly increasing temperature. Journal of Food Science 1989, 54, 446–450.
- Rhim, J.W.; Nunes, R.V.; Jones, V.A.; Swartzel, K.R. Kinetics of color change of grape juice generated using linearly increasing temperature. Journal of Food Science 1989, 54, 776–779.
- Garza, S.; Ibarz, A.; Pagan, J.; Giner, J. Non-enzymatic browning in peach puree during heating. Food Research International 1999, 32, 335–343.
- Chen, H.E.; Peng, H.Y.; Chen, B.H. Changes of carotenoids, color and vitamin A contents during processing of carrot juice. Journal of Agricultural and Food Chemistry 1995, 43 (7), 1912–1918.
- Singleton, V.L.; Gortner, W.A.; Young, H.Y. Carotenoid pigments of pineapple fruit. I. Acid-catalyzed isomerization of the pigments. Journal of Food Science 1961, 26 (1), 49–52.
- Ahmed, J.; Shivhare, U.S.; Kaur, M. Thermal color degradation kinetics of mango puree. International Journal of Food Properties 2002, 5 (2), 359–366.
- Rattanathanalerk, M.; Chiewchan, N.; Srichumpoung, W. Effect of thermal processing on the quality loss of pineapple juice. Journal of Food Engineering 2005, 66, 259–265.
- Lopez, A.; Pique, M.T. Influence of the drying conditions on the hazelnut quality: III. Browning. Drying Technology 1997, 15 (3–4), 989–1002.
- Maskan, M. Kinetics of colour change of kiwi fruits during hot air and microwave drying. Journal of Food Engineering 2000, 48 (2), 169–175.
- Skrede, G. Color quality of blackcurrant syrups during storage evaluated by Hunter L, a, b values. Journal of Food Science 1985, 50, 514–517.
- Arena, E.; Fallico, B.; Maccarone, E. Influence of carotenoids and pulps on the color modification of blood orange juice. Journal of Food Science 2000, 65, 458–460.
- Uyan, S.E.; Baysal, T.; Yurdagel, U.; El, S.N. Effects of drying on antioxidant activity of purple carrots. Nahrung 2004, 48, 57–60.
- Flora, L.F. Time-temperature influence on muscadine grape quality. Journal of Food Science 1976, 41, 1312–1315.
- Pagliarini, E.; Vernile, M.; Peri, C. Kinetics study on color changes in milk due to heat. Journal of Food Science 1990, 55 (6), 1766–1967.
- Lee, H.S.; Coates, G.A. Thermal pasteurization effects on colour of red grape fruit juices. Journal of Food Science 1999, 64, 663–666.
- Palou, E.; Lopez-Malo, A.; Barbosa-Canovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. Journal of Food Science 1999, 64, 42–45.
- Beveridge, T.; Harrison, J.E. Non-enzymatic browning in pear juice concentrate at elevated temperatures. Journal of Food Science 1984, 49 (5), 1335–1340.
- Cohen, E.; Birk, Y.; Mannheim, C.H.; Saguy, I.S. A rapid method to monitor quality of apple juice during thermal processing. LWT–Food Science and Technology 1998, 31 (7–8), 612–616.
- Chutintrasri, B.; Noomhorm, A. Colour degradation kinetics of pineapple puree during thermal processing. LWT–Food Science and Technology 2007, 40 (2), 300–306.