Abstract
The physico-chemical properties of starch from jackfruit seed and mung bean were investigated. Jackfruit seed starch had much higher resistant starch content (26.99%) than that of mung bean starch (4.04%). Furthermore, jackfruit seed starch had a higher gelatinization temperature (To) that required more gelatinization energy (ΔH) compared to mung bean starch. However, mung bean starch had higher amylose content and its granules were much larger than that of jackfruit seed starch. Mung bean starch had the highest peak viscosity, breakdown, and setback whereas jackfruit seed starch had the highest pasting temperature. Amylopectin chain length of mung bean starch contained higher proportion of short chains (degrees of polymerization 6–12) but lower proportion of very long chains (degrees of polymerization > 37 ) comparing with jackfruit seed starch. The X-ray diffraction patterns showed both starches to be Type-A crystallinity. In addition, both starch gels showed higher the storage modulus (G′) than the loss modulus (G˝) designating as rubber like material. However, mung bean starch gel exhibited higher G’ and less tan δ than that of jackfruit seed starch indicating much stronger of gel structure.
INTRODUCTION
Starch is important source of energy for humans. Moreover, it is a highly valued raw material in the food industry because it has good physico-chemical properties for manufacture of various food products. Starch contributes greatly to the textural properties of various foods and has many industrial applications as a thickener, colloidal, stabilizer, gelling agent, bulking agent, water retention agent, and adhesive.[1Citation] Jackfruit (Artocarpus heterophyllus Lam.) is one of the most popular tropical fruits grown in Asia including Thailand.[2Citation] The ripe fruit contains well flavored yellow sweet bulbs and seeds (embedded in the bulb). Seeds make-up around 10 to 15% of the total fruit weight and have high carbohydrate and protein contents.[3Citation,4Citation] Seeds are normally discarded and fresh seeds cannot keep for a long time. So jackfruit seed starch can be an alternative product, which be used in some food products. Starch from the mung bean (Vigna radiata) is widely used in Thailand, particularly in the manufacture of glass noodles and traditional Thai desserts. Known to be a rich source of protein (21–28%), the seeds also contain a high amount of starch which is mainly amylose (30–45%). Mung bean starch is mostly used for the preparation of glass noodles or mung bean vermicelli. When heated, the starch solution becomes a “transparent” gel, a unique characteristic of mung bean starch, while remaining resilient with strong gel-strength.[5Citation] Comparing to the other locally used starches such as rice or cassava, mung bean starch is relatively expensive. The investigation of this study was to compare physico-chemical properties of starches from jackfruit seed and the mung bean.
MATERIALS AND METHODS
Materials
The commercial mung bean starch (Ton Son brand, Sitthinan Co., Ltd. Thailand) was obtained from local market in Songkhla. Jackfruit seed (Thongprasert variety) was collected from ripe fruit grown in the southern part of Thailand.
Starch Preparation of Jackfruit Seed Starch
The jackfruit seed starch was isolated by using the slightly modified method of Rengsutthi and Charoenrein.[6Citation] The seeds were cleaned and washed with water. The brown spermoderm covering cotyledon was removed and cleaned with distilled water. The cotyledons were sliced (2 mm thickness) and tray dried at 45°C until the moisture content was less than 13 g/100 g sample. The dried jackfruit seed pieces were ground with milling. The flour was packed in an aluminum foil bag and kept at room temperature until use. The flour was dissolved in 0.05 sodium hydroxide solution and stirred for 4 h. The slurries were centrifuged at 3000 rpm for 15 min. The supernatant was drained and upper brown sediment was scraped and followed by a second extraction with 0.05 M sodium hydroxide solution. The sediment was mixed with distilled water and filtered by a sieve (200 mesh) to eliminate fibers. The filtrated was neutralized with 1.0 M hydrochloric acid to pH 7.0 and the slurries were centrifuged at 3000 rpm for 15 min. The supernatant was drained and the upper brown sediment was scraped and the remaining was washed with distilled water for three times and centrifuged at 3000 rpm for 15 min. The starch cake was dried at 45°C until the moisture content was less than 13 g/ 100 g. The starch was blended and passes through a sieve (60 mesh). The starches were packed in an aluminum foil bag until use.
Chemical Analysis
The proximate compositions (moisture, fat, protein, fiber, and ash) of the mung bean starch were determined following standard methods of analysis.[7Citation] The amylose contents the samples were analyzed using iodine binding procedures.[8Citation,9Citation]
Amylopectin Chain Length Analysis
The degrees of polymerization (DP) of amylopectin branch chain was determined by high performance anionic exchange chromatography (HPAEC) performed using a Dionex system and equipped with pulsed amperometric detector (PAD) as described by Bertoft.[10Citation] The starches were diluted (4 mg/ml) with dimethlysulfoxide (DMSO). To debranch amylopectin, 700 unit isoamylase enzymes were added. The samples were incubated at room temperature overnight and the enzyme was denatured by heating in a water bath at 95°C for 5 min. Then the samples were filtered and injected (20 μl) into HPAEC system. A gradient profile of the solvents made by mixing eluent A (150 mM NaOH) and eluent B (150 mM NaOH containing 500 mM NaAc) was summarized as follows: from 0 to 9 min a fraction of eluent B increased from 15 to 36%; from 9 to 18 min increased from 36 to 45%; and from 18 to 110 min increased from 45 to 100%.
X-ray Diffraction
X-ray diffractogram of starches were obtained with an X-ray diffractometer (X’ Pert. MPD, Phillips, Eindhoven, Netherland). The starches powers were tightly packed into the sample holder. The diffraction data were collected over an angular range from 4 to 30° (2θ). The X-ray patterns were evaluated according to Zobel et al.[11Citation] The crystallinity was calculated as the area ratio of the crystallinity sharp peak over on the methods of Nara et al.[12Citation]
Particle Size Analysis and Morphology
Particle size distribution of starches were measured by using particle size analyzer (PSA, COULTER LS 230, USA) and used water for disperse phases. The starches granule morphology was observed by scanning electron microscopy (SEM, model JSM-5800 LV, JEOL, Tokyo, Japan).[13Citation]
Gelatinization Properties
Gelatinization properties were determined using a differential scanning calorimeter (DSC 7, Perkin Elmer Inc., Norwalk, CT, USA). Starch-water slurry of 1:4 weight ratios was hermetically sealed in a pre-weight aluminum pan and reweighed on a microbalance. After sealing the pan and equilibration for about 12 h, the slurry was heated from 20–95°C at a rate of 10°C/min in a DSC and onset (To), peak (Tp) and conclusion (Tc) temperature of gelatinization were determined. The enthalpy of gelatinization (ΔH) was calculated in terms of joules per unit weight of dry starch (J/g).
Pasting Properties
The pasting characteristics of the different starches were determined in a rapid visco analyzer, RVA (RVA-4D, Newport Scientific, Australia) and 3 g starch samples (based on 14% moisture content) were used. The amount of added water was adjusted according to the moisture and the total weight of the sample plus water was held constant at 28 g. After pouring the starch and water into the sample holder, the paddle was inserted and jogged up and down through starch slurry to eliminate lumps. The slurry is initially stirred with a rotating paddle at 960 rpm for 10 sec to thoroughly disperse the sample in the solvent and then at constant speed of 160 rpm for the remainder of the test. The standard profile was the “13 min” test. The initial temperature was 50°C (1 min) and then increased to 95°C (ramp time 3 min 42 s) where the sample was held for 2 min 30 s before cooling to 50°C (ramp time 3 min 48 s) and held at this temperature (2 min) The measured characteristics recorded were: peak, breakdown and setback viscosity, and pasting temperature.
Viscoelastic Properties
Starch pastes (8% w/w) were prepared for viscoelastic analysis with rheometer (RheoStress, HAKKE., Germany). The rheometer equipped with cone and plate geometry (CP4/40). The measurements were performed in the linear viscoelastic range (2% strain) and over a frequency range of 0.1–10 Hz. The temperatures during measurement were controlled at 25°C. The viscoelastic properties such as storage modulus (G’), loss modulus (G”) and loss tangent (tan Δ) were determined.
Resistant Starch (RS)
RS content of all starches was measured enzymatically according to McCleary and Monaghan.[14Citation] Samples were incubated in shaking water bath with pancreatic α-amylase and amyloglucosidase (AMG) for 16 h at 37°C to hydrolyzed digestible starch to glucose. The reaction was terminated with 4 ml ethanol and the digested RS III was recovered by centrifugation (3000 rpm, 10 min).The supernatant was then decanted and wash with 50% ethanol for twice to remove the digested starch. The sediment was solubillized in 2 ml of KOH in an ice bath, neutralized with 8 ml sodium acetate (1.2 M) and the RS hydrolyzed to glucose with of AMG (0.1 ml, 3300 U/ml).The glucose oxidase/peroxidase reaction was used to measure glucose release from the digested and RS. Absorbance was read at 510 nm after 20 min incubation period 50°C. RS and digested starch were calculated as glucose × 0.9. The total starch was calculated as the sum of RS and digested starch.
Statistical Analysis
All samples were compared with a complete randomize design (CRD). The difference in means was determined by Duncan’s new multiple’s range test.
RESULTS AND DISCUSSION
Chemical Composition and RS
Chemical compositions of the investigated starches are present in . It was found that protein, fat, ash, and fiber contents of starches from both jackfruit seed and mung beans were less than 0.5 g/100 g of starch, indicating their high purity.[15Citation] Amylose content of starches was determined by interaction with iodine (). The amylose content of mung bean starch (24.5%) was higher than that of jackfruit seed starch (20.82%). The different of amylose content for both starches may be due to the various sources and botanical properties. In addition, amylose and lipid suppress swelling and maintain the integrity of the swollen starch granules.[16Citation] The RS content for both starches was shown in . Mung bean starch had a lower RS content (4.04%) than that of jackfruit seed starch (26.88%). This agreed with the result obtained by Tongdang.[17Citation]
Table 1 Chemical compositions and resistant starch content of starches obtained from jackfruit seed and mung beans
Starch Granule Morphology and Particle Size Distribution
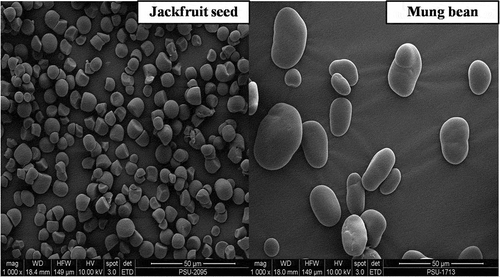
The scanning electron micrographs of both starches granules are shown in . The magnification used was 1000 × for both starches. The surface of both jackfruit seed and mung bean starches had smooth surface without other components. Jackfruit seed starch granules were round to bell shapes. On the other hand, the mung bean starch granules were oval shaped granules. The result is in agreement with those reported by Tongdang[17Citation] and Rengsutthi and Charoenrein.[6Citation]
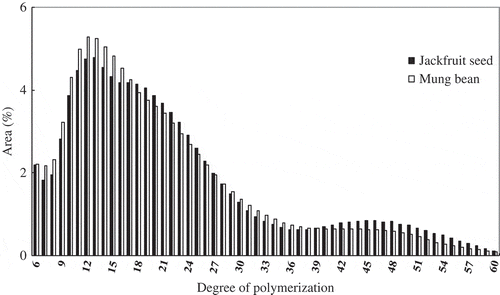
Figure 1 Distribution curve of amylopectin chain length for starches obtained from jackfruit seed and mung beans.
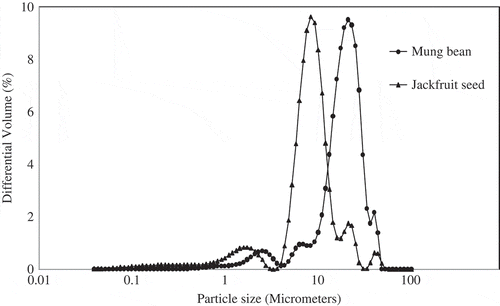
shows the granule sizes distributions of jackfruit seed and mung bean starches. The distribution profile shows that both starches exhibited the same pattern of multimodal distribution of size. The average granule size of mung bean starch (22.09 μm) was much larger than that of jackfruit seed starch (10.48 μm). This study agrees well with previous works.[17Citation,18Citation] The granule sizes of starches could influence the functional properties of starches.[17Citation]
Amylopectin Chain Length
The distribution profiles of amylopectin chain length of starches obtained from jackfruit seed and mung beans are shown in . The chain length of amylopectin for both starches were fractionated into four fractions; short chains with DP 6–12, medium chains with DP 13–24, long chain with DP 25–36, and very long chains with DP greater than 37. The distribution profiles for both starches displayed a shoulder at DP 18–21, which has been reported by Hanashiro et al.[19Citation] The peak area ratios of each chain fraction are shown in . Amylopectin of mung bean starch contained a higher proportion of short chains (DP 6–12) than jackfruit seed starch, while jackfruit seed starch contained more very long chains (DP > 37) than mung bean starch.
Table 2 Chain length distribution of amylopectin components for native starches obtained from jackfruit seed and mung beans
Starch Structure
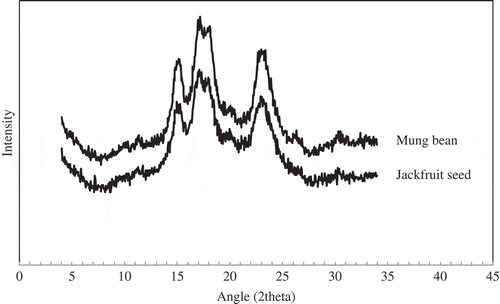
Both starches form jackfruit seed and the mung bean have the characteristic A-type crystalline pattern with unresolved peaks at 17.0 and 17.9 (2θ) and two individual peaks at 15.2 and 23°(2θ) (). Tulyathan,[20Citation] Rengsutthi, and Charoenrein[6Citation] reported that jackfruit seed starch showed an A-type crystallinity pattern. In addition, Kittipongpatana,[21Citation] Liu, and Shen[22Citation] reported that mung bean starch also showed an A-type crystallinity pattern. The A-type crystallinity pattern corresponds to a close packing of amylopectin double helices. The relative crystallainity (RC) of jackfruit seed starch (26.99%) was much greater than that of mung bean starch (20.80%) which indicates a strong crystallinity of granule. According to Zobel,[23Citation] crystallinity values for granular starches range from 15 to 45%. This observation supports the view that amylopectin is the principal component of native starches.
Thermal Properties
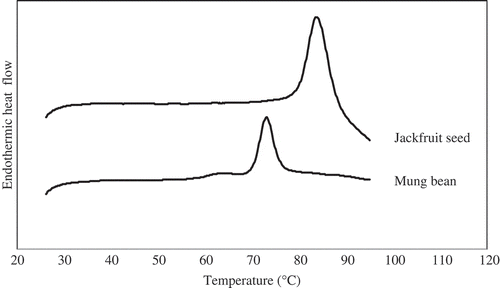
The gelatinization thermograms of jackfruit seed starch and mung bean starches measured by DSC were shown in . Both starches exhibited the typical endothermic enthalpy peak showing the melting transition of the crystalline regions of the starch granules. The gelatinization parameters: onset (To), peak (Tp), and conclusion (Tc) temperature of gelatinization and the enthalpy of gelatinization (ΔH) for both starches are shown in . Jackfruit seed starch had To, Tp, and Tc higher than that of mung bean starch. The difference in gelatinization temperature depends on the microstructure and degree of crystallinity within the granule and also on granule size and the amylose to amylopectin ratio.[24Citation] The higher ΔH value was also obtained for jackfruit seed starch comparing with mung bean starch. This indicated that the energy needed to melt the crystalline of jackfruit seed starch was much more than that of mung bean starch, revealing the strong bonding of molecules in the granules.[19Citation] The higher ΔH value of jackfruit seed starch has been attributed to its larger proportion of amylopectin (higher RC), which resulted in a higher number of double helices within the crystalline domains of the granules.[25Citation] The gelatinization temperature range (To–Tc) of jackfruit seed starch (10.20°C) was broader than that of mung bean starch (7.51°C). The result suggests that the degrees of crystallite heterogeneity within the granules for starches are different.[13Citation]
Table 3 Gelatinization parameters and viscosity parameters from RVA of starches obtained from jackfruit seed and mung beans
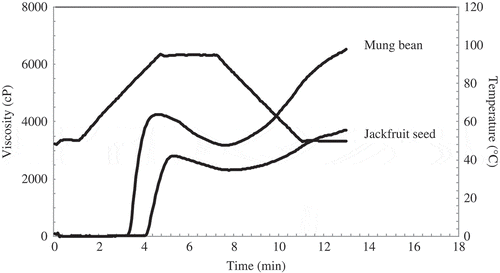
Pasting Properties
The pasting profiles of jackfruit seed and mung bean starches measured by RVA are shown in and . Mung bean starch had the highest peak viscosity (4261.67 mPa.s), breakdown (1072 mPa.s), and setback (3306.33 mPa.s) whereas jackfruit seed starch showed the highest pasting temperature (87.40oC). Peak viscosity and breakdown describe the fragility of swollen starch granules. During the swelling, amylose and some amylopectin leach out. Swollen granules and leached soluble components cause a rise in viscosity.[26Citation] With increasing heat, swollen granules become more fragile and may break resulting in a decrease in viscosity. The difference between the highest level of viscosity of swollen starch granules and their disintegration is recognized as paste breakdown.[27Citation] The different in peak viscosity, breakdown and pasting temperature for both starches were governed by amylopectin chain length distribution and not by amylose content. A higher proportion of short chains amylopectin (DP 6–12) for mung bean starch resulted in higher breakdown due to greater fragility of swollen granules.[28Citation] In addition, increasing the proportion of long chains amylopectin (DP > 12) for jackfruit seed starch increased pasting temperature and decreased breakdown. This can be explained by the amylopectin structures having more long chains, which are involved in more than one cluster and may have less tendency to be dispersed because of entanglement with other amylopectin molecules. Setback is defined as the degree of re-association between the starch molecules involving amylose.[29Citation] The setback of mung bean starch was higher than that of jackfruit seed starch. The probable reason is that higher amylose content of mung bean starch facilitates to develop more three-dimensional network structure. Accordingly, amylose content and amylopectin chain length distribution predominantly affected the pasting properties of starches.[30Citation]
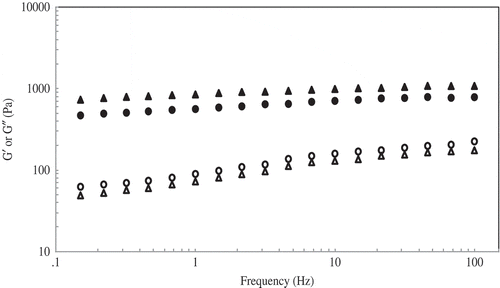
Viscoelastic Properties
The viscoelastic properties of starches were determined in the linear region at frequency range of 0.1–10 Hz and 2% strain (). For both starches, storage modulus (G′) was higher than loss modulus (G″) as shown in , indicated the strong interactions between components or between phases. In addition, the G′ for both starches was frequency independence behaving as rubber like material. The G′ (frequency at 1.0 Hz) of mung bean starch (759.25 Pa) was higher than jackfruit seed starch (556.33 Pa), while tan δ showed the opposite trend. This indicated that gel from mung bean starch was stronger than that of from jackfruit seed starch. For higher amylose content of mung bean starch, greater amounts of exuded linear starch molecules (amylose and long B-chain amylopectin) are expected. This resulted in an increase the extent of interactions between components or between phases, and accordingly three-dimensional network structures were more developed.[31Citation,32Citation]
Table 4 Viscoelastic parameters (at concentration 8% (w/w) and 1 Hz.) of starches obtained from jackfruit seed and mung beans
CONCLUSION
The physico-chemical properties of starches obtained from jackfruit seed and mung bean starch were different. The amylose content and amylopectin played the important roles in physico-chemical properties of both starches. Mung bean starch has higher amylose content resulting in higher setback and higher storage modulus comparing with jackfruit seed starch. Because of higher amount of crystallinity and higher proportion of long chains amylopectin, jackfruit seed starch has higher gelatinization temperature, enthalpy of gelatinization and pasting temperature but lower peak viscosity and breakdown.
FUNDING
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission.
REFERENCES
- Sirivongpaisal, P. Structure and functional properties of starch and flour from bambarra groundnut. Songklanakarin Journal of Science and Technology 2008, 30, 51–56.
- Mukprasirt, A.; Sajjaanantakul, K. Physico-chemical properties of flour and starch from jackfruit seeds (Artocarpus heterophyllus Lam.) compared with modified starches. International Journal of Food Science and Technology 2004, 39, 271–276.
- Bobbio, A.A.; Bobbio, P.A.; Rodrrigues, L.R. Isolation and characterization of the physicochemical properties of the starch of jackfruit seeds (Artocarpus heterophyllus). Cereal Chemistry 1987, 55 (4), 505–511.
- Kumar, S.; Singh, A.B.; Abidi, A.B.; Upadhyay, R.G.; Singh, A. Proximate composition of jackfruit seeds. Journal of Food Science and Technology 1988, 25, 308–309.
- Kittipongpatana, O.N.; Sirithunyalug, J.; Laenger, R. Preparation and physicochemical properties of sodium carboxymethyl mungbean starches. Carbohydrate Polymers 2006, 63, 105–112.
- Rengsutthi, K.; Charoenrein, S. Physico-chemical properties of jackfruit seed starch (Artocarpus hetyerophyllus) and its application as a thickener and stabilizer in chili sauce. Journal of Food Science and Technology 2011, 44, 1309–1313.
- AOAC. Official Methods of Analysis, 16th Ed. Association of Official Analytical Chemists. 2000.
- Sowhagya, C.M.; Bhattacharya, K.R. Simplified determination of amylose in milled rice. Starch/Starke 1979, 31 (5), 159–163.
- Shanthy, A.P.; Sowbhagya, C.M.; Bhattacharya, K.R. Simplified determination of water-insoluble amylose content of rice. Starch/Starke 1980, 12, 409–411.
- Bertoft, E. On the nature of categories of chains in amylopectin and their connection to the super helix model. Carbohydrate Polymers 2004, 57, 211–224.
- Zobel, H.F.; Young, S.N.; Rocca, L.A. Starch gelatinization: An X-ray diffraction study. Cereal Chemistry 1988, 65, 443.
- Nara, S.; Noti, A.; Komiya, T. Study on relative crystallinity of moist potato starch. Starch/Starke 1987, 30 (4), 111–114.
- Khunae, P.; Tran, T.; Sirivongpaisal, P. Effect of heat moisture treatment on structure and thermal properties of rice starches differing in amylose content. Starch/Starke 2007, 59, 593–599.
- McCleary, B.V.; Monaghan, D.A. Measurement of resistant starch. Journal of AOAC International 2002, 85 (3), 665–675.
- Tulyathan, V.; Boondee, K.; Mahawanich, T. Characteristics of starch from water chestnut (Trapa Biipinosa Roxb.) Journal of Food Biochemistry 2005, 29, 337–348.
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of cereal starches. I. Effects of amylopectin, amylose, and lipids. Cereal Chemistry 1990, 67, 551–559.
- Tongdang, T. Some properties of starch extracted from three Thai aromatic fruit seeds. Starch/Starke 2008, 60, 199–207.
- Oates, C.G.; Powell, A.D. Bioavailability of carbohydrate material stored in tropical fruit seed. Carbohydrate Polymers 1998, 32, 301–312.
- Hanashiro, I.; Abe, J.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydrate Research 1996, 238, 151–159.
- Tulyathan, V.; Tananuwong, K.; Songjinda, P.; Jaiboon, N. Some physicochemical properties of jackfruit (Artocarpus heterophyllus Lam) seed flour and starch. Science Asia 2002, 28, 37–41.
- Kittipongpatana, O.S.; Sirithunyaluy, J.; Laerger, R. Preparation and physicochemical properties of sodium carboxymthyl mungbean starches. Carbohydrate Polymers 2006, 63, 105–112.
- Liu, W.; Shen, Q. Structure analysis of mung bean starch from sour liquid processing and centrifugation. Journal of Food Engineering 2007, 79, 1310–1314.
- Zobel, H.F. Molecules to granules: A comprehensive starch review. Starch/Starke 1988, 40, 44–50.
- Ahmad, F.B.; Williams, P.A.; Doublier, J.L.; Durand, S.; Buleon, A. Physico-chemical characterisation of sago starch. Carbohydrate Polymers 1999, 38, 361–370.
- Gunaratne, A.; Hoover, R. Effect of heat-moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydrate Polymers 2002, 49, 425–427.
- Mohan, B.H.; Gopal, A.; Malleshi, N.G.; Tharanathan, R.N. Characteristics of native and enzymatically hydrolyzed ragi (Eleusine coracana) and rice (Oryza sativa) starches. Carbohydrate Polymers 2005, 59, 43–50.
- Mustapha, B.; Karen, A.K.M.; Bruce, R.H. Rice amylopectin fine structure variability affects starch digestion properties. Journal of Agricultural and Food Chemistry 2007, 55, 1475–1479.
- Han, X.Z.; Hamaker, B.R. Amylopectin fine structure and rice starch paste breakdown. Journal of Cereal Sciences 2001, 34, 279–284.
- Charles, A.L. Some physical and chemical properties of starch isolates of cassava genotypes. Starch/Starke 2004, 56, 413–418.
- Jane, J.; Chen, Y.Y.; Lee, L.F.; McPherson, E.; Wong, K.S.; Radosavlijevic, M. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chemistry 1999, 76, 629–637.
- Lii, C.Y.; Shao, Y.Y.; Tseng, K.H. Gelation mechanism and rheological properties of rice starch. Cereal Chemistry 1995, 72 (4), 393–400.
- Tsai, M.; Li, C.; Lii, C. Effect of granular structures on the pasting behaviors of starches. Cereal Chemistry 1997, 74 (6), 750–757.