Abstract
Bitter bean (Parkia speciosa), also known as petai, is a popular non-timber forest product. Traditionally, its fruits are consumed as vegetables and herbal medicines in Malaysia. The present study aimed to evaluate the antioxidant activities of aqueous and ethanol extracts of P. speciosa empty pods using various antioxidant assays, as well as examining their polyphenolic constituent contents. Results showed that with the exception of superoxide radical scavenging activity, ethanol extracts possessed stronger DPPH and ABTS radical scavenging, anti-lipid peroxidation, metal chelating and reducing power activities than aqueous extracts. It was found to contain a higher level of total flavonoids and total phenols than aqueous extracts. The major polyphenolic constituents present in these extracts were gallic acid, catechin, ellagic acid, and quercetin. Although aqueous extracts contained a higher level in gallic acid, its catechin, ellagic acid, and quercetin contents were lower than ethanol extracts. Taken together, the higher amount of polyphenolic compounds present in ethanol extracts could have contributed to its stronger antioxidant activities than aqueous extracts; these results also provided the chemical basis for certain health benefits claimed of P. speciosa empty pods in folk medicine and as foods.
INTRODUCTION
Parkia speciosa or bitter bean, also known locally as petai, is a tropical leguminous tree of the family Fabaceae.[Citation1,Citation2] The plant grows to as height as 60 to 80 feet, and is found distributed in the Malaysian forests. It is a popular non-timber forest product. Its fruits are collected from the wild and consumed as vegetables and herbal medicines. The seeds are either eaten raw or cooked. The young leaves and the fleshy part of the flower stalk can also be eaten raw. In mature fruits, the empty pods account for more than 60% of the biomass (each pod contains 12 to 18 seeds).
Bitter beans have been used in folk medicine for their antibacterial effects on kidney, ureter, and urinary bladder; its effect is due to the presence of several cyclic polysulphides.[Citation3,Citation4] They are also believed to have diuretic and relaxing properties. Extracts prepared from P. speciosa seeds were reported to possess hypoglycemic,[Citation4,Citation5] anticancer,[Citation6] and antiangiogenic[Citation7] activities, and were able to reduce the blood glucose and blood pressure levels in hypertensive rats.[Citation8,Citation9] The pods with the seeds were shown to have anti diabetic activity.[Citation9] The P. speciosa empty pod extracts were rich in uronic acid and possessed good DPPH radical scavenging activity;[Citation10,Citation11] they were also reported to have a greater hypotensive action than the seeds.[Citation8]
Fruits, vegetables, and medicinal plants are well known to contain chemical compounds exhibiting good antioxidant properties.[Citation12−Citation14] Consumption of these antioxidants is believed to be beneficial in preventing oxidative stress related diseases, such as cancer, cardiovascular diseases, diabetes, etc.[Citation15−Citation17] Antioxidants also play an important role in lengthening the shelf life of food, reducing nutritional losses, and formation of harmful substances. In this study, the objectives were to have a detailed evaluation of antioxidant activities of aqueous (PH) and ethanol (PE) extracts of P. speciosa empty pods using various antioxidant assays, and to examine the polyphenolic contents and their constituents present in these extracts.
MATERIALS AND METHODS
Chemicals
Trolox, thiobarbituric acid (TBA), nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium (NBT), phenazine methosulphate (PMS), ferrozine, ethylenediamine-tetraacetic acid (EDTA), linoleic acid, and 2,2’-azino-bis[3-ethylbenthiazoline-6-sulfonic acid] (ABTS) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). 2,2-Diphenyl-1- picrylhydrazyl (DPPH) was obtained from MP Biomedicals Inc. (Eschwege, Germany). Dimethylsulphoxide (DMSO) and ferrous chloride were obtained from Wako Pure Chemical Industries (Osaka, Japan). All other chemicals used were of analytical grade.
Plant Materials
Fresh P. speciosa pods were purchased from the market in Bidor, Perak, Malaysia. After removing the seeds, the empty pods were immediately dried in an oven at 40°C for three days. The dried materials were ground using an electric grinding machine (Model RT-02B, Rong Tsong, Taipei, Taiwan) to produce the powder (≤ 20 mesh), which was collected and kept in an air-tight plastic bag until use.
Extract Preparation
To prepare the PH extract, 100 g of sample was extracted with 1 L of boiling water for 1 h. After filtering the extract with filter paper (Advantec No. 1; Toyo Roshi Kaisha Ltd., Tokyo, Japan), the residue was re-extracted under the same conditions twice. The filtrates obtained from three separate extractions were combined and concentrated using a rotary vacuum evaporator (EYELA, SB-651, Rikakikai Co. Ltd. Tokyo, Japan), followed by lyophilization for 72 h in a freeze-dryer (FD 5030/8530, Panchum Scientific Corp., Taipei, Taiwan). For the PE, 100 g of sample were soaked with 1 L of ethanol (95%) at room temperature for six days to improve the yield. After filtering the extract with filter paper (Advantec No. 1), the filtrate collected was concentrated with a rotary vacuum evaporator and then dried in a SpeedVac concentrator (Savant SPD121P, Thermo Scientific, MA, USA) under vacuum. The dried PH and PE were collected and stored at 4°C until use.
Anti-Lipid Peroxidation Assay
This assay was determined by the thiocyanate method as described by Ng et al.[Citation18] In brief, 2 mL of different concentrations of PH, PE or positive control (Trolox) were taken and well mixed with 3 mL of linoleic acid emulsion consisting of 2.5 g Tween-20, 2.5 g linoleic acid, and 0.04 mM of potassium phosphate buffer (pH 7.0), followed by incubating at 37°C. After reacting with FeCl2 and thiocyanate at several time intervals, the peroxide value was measured at wavelength 500 nm. The percentage inhibition of peroxidation (%) was calculated as 100−[(absorbance of the sample/absorbance of the control) × 100].
Superoxide Radical Scavenging Assay
This assay was conducted according to the method described previously.[Citation18] In brief, superoxide radicals were generated in 3 mL of phosphate buffer (0.1 M, pH 7.4) containing 1 mL of NBT (300 μM), 1 mL of PMS (120 μM), 1 mL of NADH (968 μM), and 1 mL of PH, PE or Trolox at various concentrations. The mixture was spectrophometically measured at 560 nm. The superoxide scavenging activity (%) of the extracts was calculated as [(absorbance of the control-absorbance of the sample)/absorbance of the control] × 100.
DPPH Radical Scavenging Activity
This assay was conducted according to the method as described by Wu and Ng.[Citation12] Briefly, 1 mL of 0.1 mM DPPH radical solution was mixed with 3 mL of various concentrations of PH or PE dissolving in methanol. Trolox was used as a positive control. The mixture was then vortexed vigorously and left to react in the dark for 30 min at 40°C. For the baseline control, 3 mL of methanol was used. The absorbance was measured at 517 nm. The DPPH scavenging activity (%) of the extracts was calculated as [(absorbance of the control-absorbance of the sample)/absorbance of the control] × 100.
ABTS Radical Scavenging Assay
The scavenging activity of ABTS + was measured according to the method described by Ng et al.[Citation18] Briefly, ABTS was dissolved in deionized water to 7 mM in concentration, which was then mixed with 2.45 mM potassium persulfate. The scavenging activity was determined by mixing with 180 μL of ABTS and 40 μL of extracts or positive control (Trolox), followed by measuring the absorbance at 734 nm. The ABTS scavenging activity (%) of the extracts was calculated as [(absorbance of the control-absorbance of the sample)/absorbance of the control] × 100.
Metal Chelating Activity
The chelating effect of ferrous ions by PH and PE was determined by the method described previously.[Citation12] In brief, 1 mL of sample at different concentrations was mixed with 3.7 mL of methanol and 0.1 mL of 2 mM FeCl2. The reaction was initiated by the addition of 0.2 mL of 5 mM ferrozine, followed by shaking vigorously and left to react at room temperature for 10 min. The absorbance was measured at 562 nm. EDTA, a strong metal chelator, was used as a positive control. The percentage inhibition of ferrozine-Fe2+ complex formation (%) was calculated as [(absorbance of the control-absorbance of the sample)/absorbance of the control] × 100.
Reducing Power
The reducing power of PH and PE was determined by the method of Wu and Ng.[Citation12] In brief, 2.5 mL of various concentrations of extracts or positive control (Trolox) was mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferric cyanide. After incubating the mixture at 50°C for 20 min, 2.5 mL of 10% trichloroacetic acid was added, followed by centrifuging at 3000 rpm for 10 min. Five milliliters of the upper layer solution were taken and mixed with 5 mL distilled water and 1 mL of 0.1% FeCl3. The mixture was then measured at wavelength 700 nm. The reducing power was estimated by the response of absorbance of the reaction mixture, with the increase in absorbance indicating an increase in reducing power and vice versa.
Analysis of Total Flavonoid and Total Phenolic Contents
The total flavonoid and total phenolic contents of PH and PE were determined by the colorimetric and Folin-Ciocalteu methods, respectively, as described by Wu and Ng.[Citation12] The total flavonoid content was expressed as grams quercetin equivalent per kg dry weight of sample, whereas the total phenolic content was expressed as grams gallic acid equivalent per kg dry weight of sample.
Chromatographic Analysis of Polyphenolic Constituents
The analysis of polyphenolic constituents of PH and PE was performed according to procedures as described by Suarez et al.[Citation19] using a HITACHI high performance liquid chromatographic (HPLC) system equipped with a pump (Model L-2130) and a UV/Vis Detector (Model L-2420) set at wavelength 280 nm. The separation was performed on an octadecyl column (Mightysil RP-C18; 4.6 mm × 250 mm, 5 μm) at room temperature. The analysis was carried out with 20 μL of sample injected into the column coupled with a gradient mobile phase consisting of solvent (A) 2% PH aqueous acetic acid and solvent (B) 100% methanol. The flow rate was set at 1.0 mL/min. The chromatographic gradient was as follows: 0–55 min solvent A from 100 to 55%, 55–75 min isocratic solvent A 55%, and 75–85 min solvent A from 55 to 100%. Quantification of individual polyphenolic compounds was carried out by external standard calibration curves.
Statistical Analysis
Results were presented as mean ± standard deviation (SD). Values were evaluated by one-way analysis of variance (ANOVA), followed by Duncan’s multiple range tests. Statistical analysis of two means was performed using Student’s t-test. Difference was considered significant when P-value was < 0.05.
RESULTS AND DISCUSSION
Antioxidant Activities
Fruits and vegetables are known to possess potent antioxidant activities[Citation12−Citation14] and beneficial effects in preventing chronic diseases.[Citation15−Citation17] Although the antioxidant capacities of different plant materials are influenced by many factors, they are known to associate with specific compounds or classes of compounds, such as polyphenols (flavonoids and phenolic acids), nitrogen compounds (alkaloids, chlorophyll derivatives, amino acids, and amines), carotenoids, lignans, and terpenes.[Citation20,Citation21] Studies have shown that P. speciosa extracts possessed good DPPH radical scavenging activity,[Citation11] and their high antioxidant activities were associated with high total phenolic and flavonoid contents.[Citation22,Citation23] In this study, results showed that both PE and PH exhibited a dose-dependent increase in antioxidant activities (–), their anti-lipid peroxidation and superoxide radical scavenging activities were more potent than Trolox as demonstrated by a lower IC50 value (). Although PE demonstrated a stronger DPPH radical scavenging activity than PH, its potency was weaker than Trolox. At 25 μg/mL, PH, PE and Trolox demonstrated an ABTS radical scavenging rate of 18.1, 62.9, and 41.5%, respectively (); the activity of PE (IC50: 19.57 μg/mL) was about four fold more potent than PH (IC50: 80.72 μg/mL), and was also better than Trolox (IC50: 29.03 μg/mL) (). In metal chelating assay, although both PE and PH showed a linearly decrease in the absorbance of Fe2+-ferrozine complex (), their activity was weaker than EDTA (). The reducing power of both PE and PH was moderate, and was much weaker than Trolox (, ). These results indicate that the antioxidant activities of P. speciosa extracts vary with the type of assays, and have suggested the different responses of bioactive compounds towards these assays.
Table 1 IC50 values of antioxidant activities of P. speciosa empty pods
Figure 1 Antioxidant activities of P. speciosa empty pods. PH and PE were the aqueous and ethanol extracts, respectively. (a) Anti-lipid peroxidation activity; (b) SOD: Superoxide radical scavenging activity; (c) DPPH: 2,2-Diphenyl-1-picrylhydrazyl radical scavenging activity; (d) ABTS: 2,2’-azino-bis[3-ethylbenthiazoline-6-sulfonic acid] radical scavenging activity; (e) Metal chelating activity; (f) Reducing power. Data are presented as mean ± SD (n = 3); values with the different superscript letters are significantly different at P < 0.05 as analyzed by Duncan’s multiple range tests.
![Figure 1 Antioxidant activities of P. speciosa empty pods. PH and PE were the aqueous and ethanol extracts, respectively. (a) Anti-lipid peroxidation activity; (b) SOD: Superoxide radical scavenging activity; (c) DPPH: 2,2-Diphenyl-1-picrylhydrazyl radical scavenging activity; (d) ABTS: 2,2’-azino-bis[3-ethylbenthiazoline-6-sulfonic acid] radical scavenging activity; (e) Metal chelating activity; (f) Reducing power. Data are presented as mean ± SD (n = 3); values with the different superscript letters are significantly different at P < 0.05 as analyzed by Duncan’s multiple range tests.](/cms/asset/a21f1ca5-f28f-4480-912e-cd1345f85959/ljfp_a_775152_f0001_b.gif)
Polyphenolic Contents and Individual Constituents
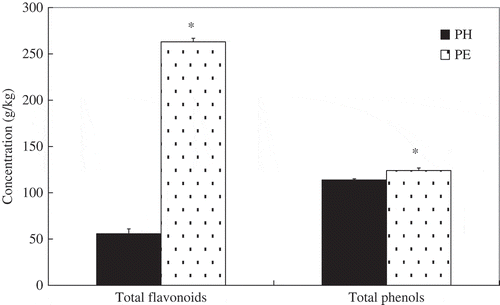
Compared with PE, PH appeared to have a lower total flavonoid and total phenolic contents (). Studies have shown that the free radical scavenging action of plant constituents were related to polyphenolic compounds and to caffeic acid derivatives.[Citation20,Citation24Citation−Citation26] Among them, flavonoids are considered to be the most important natural polyphenols, as they possess a broad spectrum of chemical and biological activities. As powerful free radical scavengers, flavonoids can effectively inhibit lipid peroxidation by neutralizing peroxyl radicals generated during the lipid oxidation. In this study, the level of total flavonoid level in PE was 4.7 times higher than PH; this would have contributed to stronger free radical scavenging and antioxidant activities of PE than PH. This finding was in agreement with reported results, indicating that flavonoids contribute significantly to the antioxidant activity and radical scavenging action of plant sources.[Citation24,Citation27]
Figure 2 Total flavonoid and total phenolic contents of P. speciosa empty pods. PH and PE were the aqueous and ethanol extracts, respectively. Data are presented as mean ± SD (n = 3). *Indicates a significant difference (P < 0.05) between PH and PE as analyzed by Student’s paired t-test.
The most abundant polyphenolic constituents present in both PH and PE were gallic acid, catechin, ellagic acid, and quercetin (). PH contained the highest level of gallic acid, of which the concentration was about 23.31 g/kg. In PE, gallic acid (6.58 g/kg), catechin (5.82 g/kg), ellagic acid (8.91 g/kg), and quercetin (4.86 g/kg) were the most abundant polyphenols. Among the 13 analyzed polyphenols, other constituents detected with significant amounts were chlorogenic acid, vanillic acid, caffeic acid, epicatechin, and kaempferol. These results indicate that gallic acid, catechin, ellagic acid, and quercetin could be the major polyphenolic constituents contributing to the potent antioxidant activities of P. speciosa empty pods.
Figure 3 The content of polyphenolic constituents in P. speciosa empty pods. PH and PE were the aqueous and ethanol extracts, respectively. Data are presented as mean ± SD (n = 3). *Indicates a significant difference (P < 0.05) between PH and PE as analyzed by Student’s paired t-test.
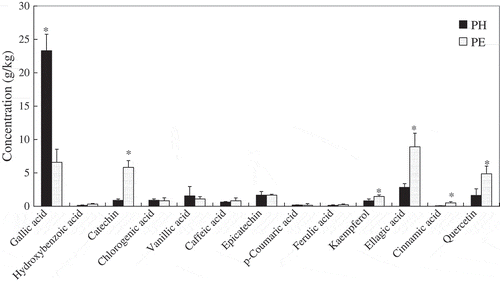
Previous phytochemical investigations of P. speciosa had led to the isolation of β-sitosterol and stigmasterol,[Citation4,Citation5] thiazolidine-4-carboxylic acid,[Citation5,Citation6] dichrostachinic acid, djenkolic acid, hexathionane, pentathiepane, pentathiocane, tetrathiane, tetrathiepane, and trithiolane.[Citation3,Citation5] In another study, using antioxidant activity guided fractionation, a large amount of gallic acid (4.9%) and quercetin (0.61%) was isolated from the ethylacetate fraction of P. speciosa empty pods (unpublished data). In this study, the HPLC analysis further confirmed that this component of P. speciosa contained a high level of gallic acid and quercetin.
Flavanoids and phenols, as secondary plant metabolites, are commonly found in fruits and vegetables, and have been shown to defend against oxidative stress from endogenous reactive oxygen species and free radicals.[Citation13,Citation24,Citation28] They are known to possess antioxidative, antiproliferative, anti-inflammatory, and antiallergic effects.[Citation12,Citation16,Citation17,Citation29] In addition, they can also be used as food additives to provide protection against oxidative degradation of foods.[Citation30] The high content of polyphenols in both PE and PH might have explained the potent antioxidant properties of P. speciosa empty pods, suggesting that their consumption is beneficial in protecting against oxidative damage in the body and hence decrease the occurrence of chronic diseases.
CONCLUSION
Regardless of the difference in solvents used for extraction, both PH and PE have demonstrated to possess potent antioxidant activities, of which appear to be largely influenced by the amounts of gallic acid, catechin, ellagic acid, and quercetin. With the exception of gallic acid, the polyphenolic compounds present in PE were higher than in PH, and that could have explained for having stronger antioxidant activities. The present study has provided the chemical basis for certain health claims of P. speciosa empty pods in folk medicine and as foods.
REFERENCES
- Burkill, I.H. A dictionary of the economic products of the Malay Peninsula. Ministry of Agriculture and Cooperatives, Kuala Lumpur, 1966, 1697–1700.
- Frérot, E.; Velluz, A.; Bagnoud, A.; Delort, E. Analysis of the volatile constituents of cooked petai beans (Parkia speciosa) using high-resolution GC/ToF–MS. Flavour and Fragrance Journal 2008, 23, 434–440.
- Gmelin, R.; Susilo, R.; Fenwick, G.R. Cyclic polysulphides from Parkia speciosa. Phytochemistry 1981, 20, 2521–2523.
- Fathaiya, J.; Mohamad, S.; Nordin, L.M. Hypoglycaemic effect of Parkia speciosa, seeds due to synergistic action of β-sitosterols and stigmasterol. Food Chemistry 1994, 49, 339–345.
- Susilo, R.; Gmelin, R. Precursor of cyclic polysulphides in seeds of Parkia speciosa. Zeitschrift für Naturforschung 1982, 37, 584–586.
- Suvachittanont, W.; Kurashima, Y.; Esumi, H.; Tsuda, M. Formation of thiazolidine-4-carboxylic acid (thioproline), an effective nitrile-trapping agent in human body, in Parkia speciosa seeds and other edible leguminous seeds in Thailand. Food Chemistry 1996, 55, 359–363.
- Aisha, A.F.A.; Abu-Salah, K.M.; Darvis, Y.; Abdul Majid, A.M.S. Screening of antiangiogenic activity of some tropical plants by rat aorta ring assay. International Journal of Pharmacology 2009, 5, 370–376.
- Bokhari, A.B. The pharmacological investigation of the hypotensive activity of extracts of “buah petai” (Parkia speciosa Hassk), BSc Thesis, School of Pharmaceutical Sciences, University Science Malaysia: Pulau Penang, Malaysia, 1980.
- Jamaluddin, F.; Mohamed, S.; Lajis, M.N. Hypoglycaemic effect of stigmast-4-en-3-one, from Parkia speciosa empty pods. Food Chemistry 1995, 54, 9–13.
- Gan, C.Y.; Abdul Manaf, N.Hj.; Latiff, A.A. Optimization of alcohol insoluble polysaccharides (AIPS) extraction from the Parkia speciosa pod using response surface methodology (RSM). Carbohydrate Polymers 2010, 79, 825–831.
- Aisha, A.F.A.; Abu-Salah, K.M.; Alrokayan, S.A.; Ismail, Z.; Abdul Majid, A.M.S. Evaluation of antiangiogenic and antioxidant properties of Parkia speciosa Hassk extracts. Pakistan Journal of Pharmaceutical Sciences 2012, 25, 7–14.
- Wu, S.J.; Ng, L.T. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. LWT-Food Science and Technology 2008, 41, 323–330.
- Vanzani, P.; Rossetto, M.; De Marco, V.; Sacchetti, L.E.; Paoletti, M.G.; Rigo, A. Wild Mediterranean plants as traditional food: A valuable source of antioxidants. Journal of Food Science 2011, 76, C46–C50.
- Wang, R.J.; Hu, M.L. Antioxidant capacities of fruit extracts of five mulberry genotypes with different assays and principle components analysis. International Journal of Food Properties 2011, 14, 1–8.
- Cook, N.C.; Samman, S. Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary sources. Journal of Nutritional Biochemistry 1996, 7, 66–76.
- Andarwulan, N.; Batari, R.; Sandrasari, D.A.; Bolling, B.; Wijaya, H. Flavonoid content and antioxidant activity of vegetables from Indonesia. Food Chemistry 2010, 121, 1231–1235.
- Araújo, J.R.; Gonçalves, P.; Martel, F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutrition Research 2011, 31, 77–87.
- Ng, L.T.; Ko, H.H.; Lu, T.M. Potential antioxidants and tyrosinase inhibitors from synthetic polyphenolic deoxybenzoins. Bioorganic and Medicinal Chemistry 2009, 17, 4360–4366.
- Suarez, B.; Palacios, N.; Fraga, N.; Rodriguez, R. Liquid chromatographic method for quantifying polyphenols in ciders by direct injection. Journal of Chromatography A 2005, 1066, 105–110.
- Hall, C.A.; Cuppett, S.L. Activities of natural antioxidants. In: Antioxidant Methodology in Vivo and in Vitro Concepts; Aruoma, O.L.; Cuppett, S.L.; Eds.; AOCS Press: Champaign, Illinois, 1997, 2–29.
- Banerjee, A.; De, B. Comparative study of antioxidant activity of the food flowers of west Bengal, India. International Journal of Food Properties 2013, 16, 193–204.
- Razab, R.; Abdul-Aziz, A. Antioxidants from tropical herbs. Natural Product Communications 2010, 5, 441–445.
- Gan, C.Y.; Latiff, A.A. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chemistry 2011, 124, 1277–1283.
- Popa, C.V.; Lungu, L.; Savoiu, M.; Bradu, C.; Dinoiu, V.; Danet, A.F. Total antioxidant activity and phenols and flavonoids content of several plant extracts. International Journal of Food Properties 2012, 15, 691–701.
- Kimura, Y.; Okuda, T.; Hatono, T.; Agata, I.; Arichi, S. Effects of extracts of leaves of Artemisia species and caffeic acid and chlorogenic acid on lipid metabolic injury in rats fed peroxidized oil. Chemical and Pharmaceutical Bulletin 1985, 33, 2028–2034.
- Hatono, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical and on DPPH radical. Chemical and Pharmaceutical Bulletin 1989, 37, 2016–2021.
- Pavagadhi, S.; Joseph, G.S.; Jena, B.S. Antioxidant principles in Peltophorum ferrugineum flower extracts. International Journal of Food Properties 2012, 15, 549–557.
- Shahidi, E.; Janitha, P.K.; Wanasundra, P.D. Phenolic antioxidants. Critical Reviews in Food Science and Nutrition 1992, 32, 67–103.
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. American Journal of Clinical Nutrition 2003, 78, 517S–520S.
- Madsen, H.L.; Bertelsen, G. Spices as antioxidants. Trends in Food Science and Technology 1995, 6, 271–277.
