Abstract
In this study, peptide fractions with strong antioxidant activity were obtained from Roselle seed protein hydrolysates. Pepsin followed by pancreatin were used to digest Roselle protein at different time in order to produce hydrolysate with antioxidant activity suitable for conversion to high-value products. The hydrolysates obtained after different hydrolysis times were analyzed for antioxidant activity using 1,1-diphenyl-2-picrylhydrazyl radical scavenging method. The 3 h hydrolysate showed the highest antioxidant activity therefore, it was separated into four fractions (I, II, III, and IV) by gel filtration on Sephadex G-15. The antioxidant efficacies of the 3 h Roselle seed protein hydrolysates and its fractions were investigated using different in vitro methods. All fractions were effective antioxidants, with fraction III showing the strongest antioxidant activity. The fractions were then analyzed for amino acid composition. The analysis revealed that fraction III contained higher amounts of Serine, Glycine, Arginine, Alanine, Tyrosine, Valine, Phenylalanine, and Proline compared to the other fractions. Most of these amino acids have been reported to show antioxidant activity. The results showed that the hydrolysate derived from Roselle seed protein, particularly fraction III, could be a natural antioxidant source suitable for use as a food additive.
INTRODUCTION
Hibiscus sabdariffa L. also known as Roselle, karkade belongs to the family of Malvaceae, is an herbal shrub plant reported as native to tropical Africa and are grown in tropical climate countries. The plant is generally considered as a medicinal plant. The calyces are extensively used to prepare herbal drinks, cold and warm beverages, as well as making jams and jellies.[Citation1,Citation2] The high nutrients content of the seeds have been demonstrated.[Citation3,Citation4] They have also been roasted and used as a substitute for coffee.[Citation5] In West Africa: Mali, Burkina, Niger, and northern Nigeria, Roselle seeds are used to prepare fermented food known as Mungza Ntusa,[Citation6] datou, and dawadawa.[Citation4] However, Roselle seeds are a by-product of calices production and are still under exploited. If the protein by-product is recovered and utilized, it can represent a significant economic and social benefit. Free radicals are constantly generated in the body tissues as a result of oxidative metabolism. There is much evidence that free radicals play a critical role in a variety of pathological conditions, including the processes of aging, cancer, multiple sclerosis, inflammation, coronary heart and cardiovascular diseases, senile dementia, arthritis, and atheroscelerosis.[Citation7,Citation8] Antioxidants are components that significantly delay or inhibit oxidation of a substrate when present at low concentrations compared to that of an oxidizable substrate.[Citation9,Citation10] In order to provide protection against serious diseases and to prevent foods from undergoing deterioration, many chemicals with strong antioxidant activity are used as additives, such as butylated hydroxyanisole, butylated hydroxytoluene, and n-propyl gallate. Moreover, their use in foodstuffs is restricted or prohibited in some countries due to their undesirable consequences on human health.[Citation11] Therefore, natural antioxidants have attracted more and more interest because of their safety and wide distribution.[Citation9,Citation12,Citation13] A vast number of studies have been conducted on the preparation of hydrolysates. Moreover, protein hydrolysates produced may possess some bioactivities not found in the original proteins, such as antioxidant activity. Roselle seed is recognized as a potential source of protein.[Citation4,Citation14] But until now, no data have been reported on the hydrolysis of Roselle seed protein (RSP) and on the investigation of the antioxidant activity of their hydrolysates.
The aim of this study was to produce hydrolysate with antioxidant activity (from RSP) suitable for conversion to high-value products. Therefore, RSPs have been hydrolyzed using pepsin followed by pancreatin to produce hydrolysate with good antioxidant properties and the hydrolysate was separated using sephadex G-15 into four fractions. Furthermore, the hydrolysate and its fractions have been investigated for antioxidant activity by measuring their free radicals scavenging ability and their ability to act as reducing agents. The amino acid composition of the fractions was also analyzed to evaluate its relationship with their antioxidant activity.
MATERIALS AND METHODS
Materials
Seeds of H. Sabdariffa were obtained from Koutiala, southern region of Republic of Mali and transported to Wuxi, China. All enzymes used were of food grade. Pepsin, pancreatin, Sephadex G-15, and DPPH were purchased from Sigma Chemical Co. (St. Louis, USA). All the other chemicals used in the experiments were from commercial source and of analytical grade.
Extraction of RSP
RSP was obtained from defatted flour as reported by El Tinay et al.[Citation15] with some modifications. The defatted flour was dispersed in distilled water at flour to water ratio of 1:10 (W/V); the pH was adjusted to 10 with 1 M NaOH and stirred for 3 h at room temperature. The extract was separated by centrifugation at 4000 rpm for 20 min. The residues were re-extracted twice as described above. The extracts were combined and protein was precipitated by adjusting the pH to 3.5 with 1 M HCL before centrifugation at 4000 rpm for 20 min. The RSP (precipitate) was washed twice with distilled water. It was then resuspended in distilled water and the pH was adjusted to 7.0 with 1 M NaOH prior to freeze-drying. The RSP powder was placed in Ziplock bags and stored in a desiccator at room temperature for subsequent use. The protein content was determined by Kjeldahl analysis according to the AOAC[Citation16] method.
Preparation of Protein Hydrolysates
To produce antioxidant peptide from RSP, enzymatic hydrolysis was performed using deux enzymes (pepsin followed by pancreatin) with their optimal conditions (). Whole Roselle seeds were ground and defatted with hexane, following a small-scale hexane extraction method described by Tzeng et al.[Citation17] The defatted Roselle meal samples were vacuum-packed and stored at –20°C prior to protein extraction as described above. The crude protein content of RSP was determined by Kjeldahl analysis according to the AOAC[Citation16] method in order to calculate the amount of sample required for the hydrolysis process, based on enzyme/protein ratio. The RSP samples were divided into three groups (each containing 20 g and 400 mL of water) and have been hydrolyzed in 500 mL reactor with temperature and pH control devices. Sample 1 was hydrolyzed by pepsin for 30 min followed by pancreatin for 1 h and sample 2 was hydrolyzed by pepsin for 1 h followed by pancreatin for 1 h. Finally, sample 3 was hydrolyzed, using pepsin for 1 h followed by pancreatin for 2 h. Conditions were constantly monitored and maintained throughout the process. Upon completion of the hydrolysis, the enzymes were deactivated by heating in a boiling water bath for 10 min. The reaction mixtures were then filtered and the hydrolysates were collected. The protein hydrolysates obtained were freeze-dried and stored at –20°C for subsequent analysis. The degree of hydrolysis (DH) was determined by measuring the nitrogen content soluble in 10% trichloroacetic acid as discussed by Kim et al.[Citation18]
Table 1 Hydrolysis conditions used for preparation of Roselle protein hydrolysates
Size Exclusion Chromatography
The freeze-dried Roselle Seed Protein Hydrolysate (RSPH) (1 mL) at a protein concentration of 100 mg/mL was dissolved in 50 mM sodium phosphate buffer (pH 7.0) and loaded onto a Sephadex G-15 gel filtration column (90 cm × 2 cm) which had previously been equilibrated with the same buffer. The column was then eluted with the same buffer. The elution peaks were monitored at 280 nm and the fractions eluted under same elution peak were pooled, lyophilized, and tested for antioxidant activities.
Scavenging Effect on DPPH Radical
The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical-scavenging activity of enzymatically prepared Roselle protein hydrolysates and the four fractions was determined, following the procedure described by Shahidi et al.[Citation19] with minor modifications. Freeze-dried hydrolysate samples were dissolved in 95% ethanol at a series of concentrations (0.5, 1, 1.5, 2, 2.5, 3, and 3.5 mg/mL for hydrolysates) and (0.5, 1, and 1.5 mg/mL for fractions). An aliquot (0.1 mL) of the sample solution was mixed with 1.9 mL of ethanolic DPPH solution (50 μM) and the mixtures were allowed to stand at room temperature for 30 min. The absorbance was then read at 517 nm using a spectrophotometer and the scavenging ability of DPPH by protein hydrolysates was calculated as the following equation:
Scavenging Effect on Superoxide Anion (O2−) Radical
The superoxide radical-scavenging capacity of the 3 h RPH and its fractions was determined by the nitroblue tetrazolium (NBT) reduction method.[Citation21] One milliliter of NBT solution (156 μM NBT in 100 mM phosphate buffer, pH 7.4), 1 mL of Nicotinamide adenine dinucleotide (NADH) solution (468 μM NADH in 100 mM phosphate buffer, pH 7.4), and 0.1 mL of sample solution (10 mg/mL) were mixed. The reaction was started by adding 100 μL of phenazine methosulphate (PMS) solution (60 μM PMS in 100 mM phosphate buffer, pH 7.4) to the mixture. The abilities to scavenge the superoxide radical were calculated using the following equation:
Scavenging Effect on Hydroxyl (HO·) Radical
The hydroxyl radical scavenging effects of the 3 h Roselle protein hydrolysate and its fractions were assayed using the method of Halliwell et al.[Citation22] The reagents were added to a test tube in the following order: 0.4 mL KH2PO4–KOH buffer (pH 7.4), 0.1 mL sample solution with concentrations (10 mg/mL), and 0.1 mL of 1 mM EDTA, 10 mM H2O2, 60 mM 2-deoxy-Dribose, 2 mM ascorbic acid, and 1 mM FeCl3 (0.1 mL distilled water was used as control instead of FeCl3). The reaction solution was incubated at 37°C for 1 h. Then, 1 mL of 20% Trichloroacetic Acid was added to stop the reaction. The color was developed by addition of 1 mL of 1% Tiobarbutiric Acid into the reaction tubes, which were placed in boiling water for 15 min. The tubes were cooled to room temperature and then the absorbance was read at 532 nm. For each concentration of the hydrolysates from one batch, samples were prepared in triplicate and the antioxidant activity of each was measured in duplicate. The scavenging effects were calculated according to Eq. 2.
Determination of Reducing Power
Reducing power of the 3 h RSPH and its fractions were measured according to the methods of Duh et al.[Citation23] with some modifications. Briefly, samples were dissolved in a 0.2 M phosphate buffer (pH 6.6) at concentrations of 1, 2, 4, and 5 mg/mL. An aliquot (2.5 mL) of sample solution was then added to 2.5 mL of a 10 mg/mL potassium ferricyanide solution and incubated at 50°C for 20 min. To the mixture, after incubation, deionized water (2.5 mL) and a ferric chloride solution (1.0 mg/mL, 0.5 mL) were added. The absorbance was then recorded immediately at 700 nm. The trichloroacetic acid step was omitted since it would precipitate out the protein whose antioxidant activity is being assessed. A control, devoid of any hydrolysates and a blank, containing only hydrolysate samples, were used because proteins also absorb at the same wavelength. Increased absorbance of the reaction mixture indicated increased reducing power.
Amino Acid Composition
The peptide fractions derived from the 3 h RSPH were digested with HCL (6 M) at 110°C for 24 h under nitrogen atmosphere. Reversed phase high performance liquid chromatography (RP-HPLC) analysis was carries out using Agilant 1100 (Agilent Technologies, Palo Alto, CA, USA) assembly system after precolumn derivatization with o-phthaldialdehyde (OPA). Each sample (1 μL) was injected on a Zorbax 80 A C18 column (i.d. 4.6 × 180 mm, Agilent Technologies, Palo Alto, CA, USA) at 40°C with detection at 338 nm. Mobile phase A was 7.35 mM/l sodium acetate/triethylamine/tetrahydrofuran (500:0.12:2.5, v/v/v), adjusted to pH 7.2 with acetic acid, while mobile phase B (pH 7.2) was 7.35 mM/l sodium acetate/methanol/acetonitrile (1:2:2, v/v/v). The amino acid composition was expressed as g of amino acid per 100 g of protein.
Statistical Analysis
Results were expressed as the mean values ± standard deviation (SD) of three separate determinations. The data were averages of triplicate observations and were subjected to a one way analysis of various (ANOVA), followed by Duncan’s multiple range test. The data subjected to correlation analysis, using SPSS software (version 191 16.0, the predictive Analytics Company, Chicago, USA).
RESULTS AND DISCUSSION
Enzymatic Hydrolysis
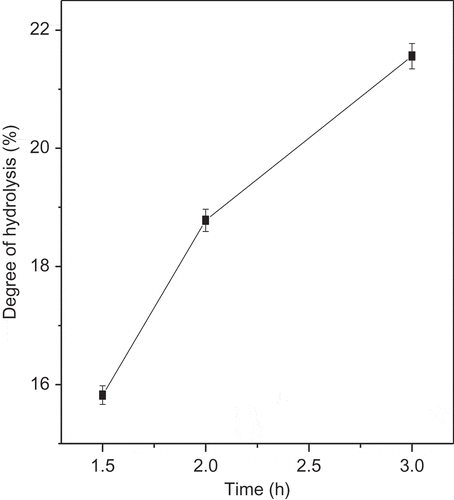
In the present study, RSP was hydrolyzed with pepsin followed by pancreatin at a different time in order to determine the more suitable hydrolysis time for production of antioxidant peptides. In quantitative work on protein hydrolysis it is necessary to have a measurement for the extent of hydrolytic degradation. It should be evident that the number of peptide bonds cleaved during the reaction is the parameter that most closely reflects the catalytic action of proteases.[Citation24] The DH is generally used as a parameter for monitoring proteolysis and is the most widely used indicator for comparison among different protein hydrolysates. From these results, the hydrolysis curves of RSP () showed that the DH of RSP for 1.5 and 2 h were 15.82 and 18.78%, respectively, which were lower than that observed at 3 h hydrolysis time (21.56%).
Fractionation of RSPH
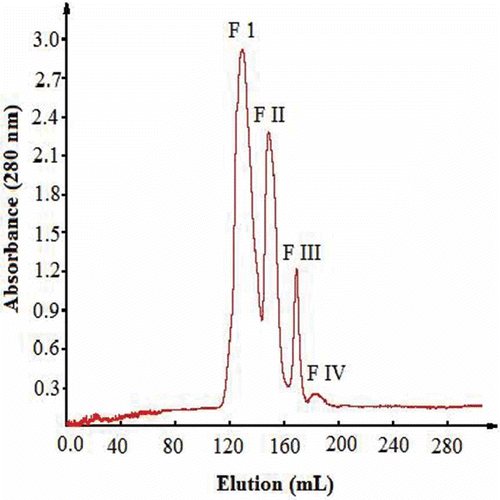
The 3 h RSPH sample was subjected to fractionation using gel filtration column chromatography on Sephadex G-15 (90 cm × 2 cm, American Pharmacie Biotech AB, Sweden). The hydrolysate was successfully separated into four fractions, I, II, III, IV (). The collected fractions were freeze-dried and stored at –20°C until antioxidant activity tests were done.
Scavenging Effect on DPPH Radical
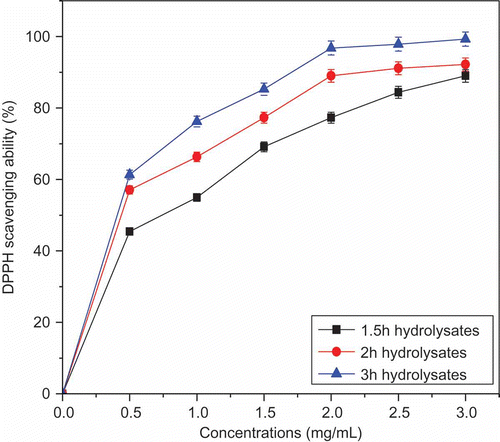
DPPH is a relatively stable organic radical characterized by a typical deep purple color and a maximum absorbance in the range at 515–520 nm. DPPH radical scavenging activity test system can be used for the primary characterization of the scavenging potential of compounds.[Citation25,Citation26] Therefore, the antioxidant activities of different hydrolysates were evaluated by the DPPH free radical scavenging activity test system. As shown in , all the hydrolysates resulting from different hydrolysis times were capable of scavenging DPPH radicals. However, the 3 h RSPH took the highest DPPH free radical scavenging activity at the concentration range of 0.5–3.5 mg/mL. From these results, the 3 h RSPH had the lower EC50 then had been chosen for gel separation. Four fractions were collected after gel separation then, the hydrolysate and its fractions on DPPH radical was assayed. The results in show that fraction III at 1.5 mg/mL exhibited excellent DPPH radical-scavenging activity (87.21%), which was higher than that of trypsin hydrolysate of the skin of both seela and ribbon fish (66 and 60% respectively).[Citation27] The other fractions also showed good DPPH radical-scavenging activity in the order of 3 h RSPH > fraction I > fraction II > fraction IV. From the results, it was concluded that the 3 h RSPH possibly contained some effective antioxidative peptides, which could convert DPPH free radical to more stable products and terminate the radical chain reaction. It has been reported that the DPPH radical-scavenging activity of food protein hydrolysates may depend on the size of their constituent peptides.[Citation28] Wang et al.[Citation29] studied the antioxidant properties of wheat gluten hydrolysate. Short peptides exhibiting antioxidant activity have also been isolated and characterized from soyabean protein,[Citation30] porcine myofibrillar proteins,[Citation31] hydrolyzed fermented mussel sauce,[Citation32] and Alaska pollack frame protein.[Citation33] However, to the authors knowledge, this is the first time that antioxidant activity of RSPH and its derived peptide fractions has been reported.
Table 2 The scavenging capacity of DPPH radicals (%) by various concentrations of samples
Scavenging Effect on Hydroxyl Radical
The radical system used for the antioxidant activity evaluation may influence the experimental results, hence two or more radical systems are required to investigate the radical-scavenging capacities of a selected antioxidant.[Citation34] Therefore, the superoxide radical (O2−) and hydroxyl radical (HO·) scavenging capacities of the 3 h RSPH and its fractions were also measured. Hydroxyl radicals are extremely reactive species and induce severe damage to adjacent biomolecules, resulting in lipid peroxidation in biological systems. Therefore removal of hydroxyl radicals is probably one of the most effective defense mechanisms through which living body defends its self against various diseases. indicates hydroxyl and the superoxide radical-scavenging effects of the 3 h RSPH and its fractions. Among all samples, fraction III exhibited the strongest hydroxyl radical-scavenging activity (62.30%). However, the 3 h RSPH, fractions I, II, and IV at the same concentration (10 mg/mL) exhibited 43.75, 56.25, 46.83, and 44.98% hydroxyl radical-scavenging activity respectively. These results revealed that all tested samples possessed hydroxyl radical scavenging activity. Admittedly, the hydroxyl radical possesses the strongest chemical activity among the active oxygen species, and easily reacts with biomolecules such as amino acids, proteins, and DNA.[Citation35] The antioxidant activity of hydrolysates from many kinds of food proteins has been studied in recent years. Peng et al.[Citation36] reported that whey protein hydrolysate and its peptide fractions showed antioxidant properties against hydroxyl radical similar to the results of this study.
Table 3 The hydroxyl and superoxide radical scavenging effects (%) by concentration (10 mg/mL) of samples
Scavenging Effect on Superoxide Radical
Superoxide radicals are generated by a number of biological reactions. Although they do not directly initiate lipid oxidation, superoxide radical anions are potential precursors of highly reactive species such as hydroxyl radicals and hydrogen peroxide.[Citation37] Not only superoxide anion radicals but also their derivatives are cell damaging, which can cause damage to the DNA and membrane of cells. Therefore it is of great importance to scavenge superoxide anion radicals. Pyrogallic acid can automatically oxidize under alkaline conditions to produce superoxide radicals directly, the constant rate of this autoxidation reaction being dependent on the pyrogallic acid concentration. shows the superoxide radical-scavenging effects of the 3 h RSPH and its fractions. Among all samples, fraction III possessed the highest superoxide radical-scavenging activity (70.87%). However, the 3 h RSPH, fraction I, fraction II, and fraction IV at the same concentration (10 mg/mL) exhibited 20.33, 25.43, 38.69, and 32.44% superoxide radical-scavenging ability, respectively. All tested samples possessed superoxide radical scavenging activity. Based on the results described above, RSPH and its fractions, especially fraction III, have good free radical-scavenging activities and could be a potential source of naturalantioxidants.
Reducing Power
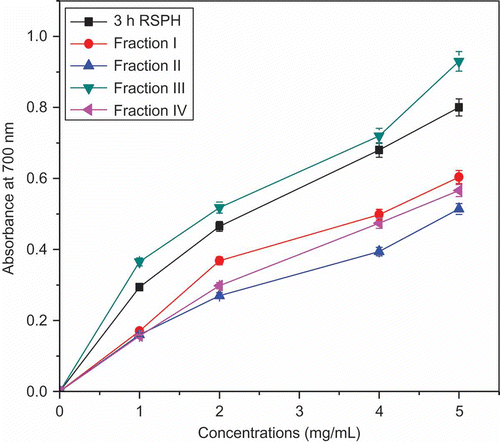
Reducing power determination is used to measure the potential antioxidant activities of bioactive compounds in different products, including peptides.[Citation26,Citation38] In this assay, the presence of antioxidants caused the reduction of the the Fe3+ ferricyanide complex to the ferrous form, and the yellow color of the test solution changed to various shades of green and blue depending on the reducing power of each compound. The Fe2+ was then monitored by measuring the formation of Perl’s Prussian blue at 700 nm.[Citation39] The 3h RSPH and its peptide fractions showed considerable amount of reducing power. showed that the reducing power of the RSPH and its peptide fractions increased with the increasing concentration. The data indicated that RSPH and its peptide fractions are capable of donating electrons, which can react with free radicals to convert them to a stable products and strongly inhibiting radical chain reaction. The results were in accordance with other investigators[Citation26,Citation40,Citation41] and correlated with the free radical scavenging tests.
Amino Acid Analysis
Chen et al.[Citation42] reported that several amino acids such as Glu, Asp, Tyr, Val, Leu, Phe, Lys, Ala, and Pro contribute to the scavenging of free radicals. For protein hydrolysates and peptides an increase in hydrophobicity will increase their solubility in lipid and therefore enhance their antioxidant activity.[Citation43] In particular, Histidine containing peptides exhibit strong antioxidant activity owing to the decomposition of the imidazole group of His.[Citation44] In the case of wheat gelatin peptides the abundance of amino acids such as His, Leu, Val, and Ala present in the sequence of hydrolysate peptides favors their radical scavenging properties.[Citation29] In order to further understand the antioxidant activity of the peptide fractions, the amino acid composition of the RSPH fractions was determined in our study and is shown in . The amino acid composition of these fractions revealed that fraction III contained high amount of Serine, Glycine, Arginine, Alanine, Tyrosine, Valine, Phenylalanine, and Proline compared with other fractions, most of which reportedly have relation to antioxidant activity. From these results, the study demonstrated that the antioxidant activity of the RSPH fractions was related to their amino acids composition and that the abundance of the amino Ser, Gly, Arg, Ala, Tyr, Val, Phe, and Pro in fraction III may correlate with its strong antioxidant activity.
Table 4 Amino acids composition of RSPH fractions (g/100 g)
CONCLUSION
Peptides with strong antioxidant activity were obtained by hydrolysis of RSP with gastro intestinal enzymes (pepsin followed by pancreatin). The RSPH sample was successfully separated into four fractions (I, II, III, and IV) by gel filtration column chromatography on Sephadex G-15. The results revealed that fraction III had the highest antioxidant and free radical-scavenging activities. The amino acid composition of the fractions was analysed and it was found that fraction III contained higher levels of Serine, Glycine, Arginine, Alanine, Valine, Tyrosine, Phenylalanine, and Proline. This may correlate with its strong antioxidant activity. The results suggested that the antioxidant peptide fractions from RSP might be useful as additive in food and pharmaceutical products. However, further study is undergoing to isolate the individual peptides responsible for the antioxidant activity of RSPH and to identify their amino acid sequences, which will allow a better understanding of the peptide structure–functionality relationship.
ACKNOWLEDGMENT
The authors wish to thank Mr. Coulibaly Oumar (Bamako, Mali) for providing Roselle seeds down to Wuxi, P.R. China.
FUNDING
This research was supported by the National Science Foundation of China (No. 30671525), the National High Technology Research and Development Program (“863” Program) of China (No. 2007.AA10Z325) and 111 project-B07029.
REFERENCES
- Rao, P.U. Nutrient composition and biological evaluation of mesta (Hibiscus sabdariffa) seeds. Plant Foods for Human Nutrition 1996, 49 (1), 27–34.
- Tsai, P.J.; Mc Intosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and antioxidant capacity in Roselle (Hibiscus Sabdariffa L.) extract. Food Research International 2002, 35 (4), 351–356.
- Abu-Tarboush, H.M.; Ahmed, A.A.; Kahtani, H.A. Some nutritional and functional properties of Karkade (H. sabdariffa) seed products. Cereal Chemistry 1997, 74 (3), 352–355.
- Tounkara, F.; Amadou, I.; Le, G.W.; Shi, Y.H. Effect of boiling on the physicochemical properties of Roselle seeds (Hibiscus sabdariffa L.) cultivated in Mali Africa. Journal of Biotechnology 2011, 10 (79), 18160–18166.
- Morton, J.F. Roselle. In: Fruits of Warm Climates; Morton, J.F.; Ed.; CF Dowling Media Inc.: Greensboro NCP: Miami, FL, 1987; 281–286.
- Balami, A. The effect of processing conditions, packaging, and storage on selected quality attributes of Mungza Ntusa. M.Sc. Thesis, University of Ibadan: Nigeria, 1998.
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, Oxford University Press: Oxford, 1999.
- Kehrer, J.P. Free radicals as mediators of tissue injury and disease. Critical Reviews in Toxicology 1993, 23 (1), 21–48.
- Lewis, N.G. Plant phenolics. In: Antioxidants in Higher Plants; Alscher, R.G.; Hess, J.L.; Eds.; CRC Press: Boca Raton, FL, 1993; 135–160.
- Halliwell, B.; Gutteridg, J.M.C. Free Radicals in Biology and Medicine, Oxford University Press: Oxford, 1989.
- Goli, A.; Barzegar, M.; Sahari, M. Antioxidant activity and total phenolic compounds of pistachio (pistachia vera) hull extracts. Food Chemistry 2005, 92 (2), 521–525.
- Hatate, H.; Nagata, Y.; Kochi, M. Antioxidative effect of bovine serum hydrolyzates and their synergistic effect with antioxidants. Yukagaku 1990, 39 (1), 42–46.
- Hettiarachchy, N.S.; Glenn, K.C.; Gnanasambandam, R.; Johnson, M.G. Natural antioxidant extract from fenugreek (Trigonella foenumgraecum) for ground beef patties. Journal of Food Science 1996, 61 (3), 516–519.
- El-Adawy, T.A.; Khalil, A.H. Characteristic of Roselle seeds as a new source of protein and lipid. Journal of Agricultural and Food Chemistry 1994, 42 (9), 1896–1900.
- El-Tinay, A.H.; Nour, A.M.; Abdel-Karim, S.H.; Mahgoub, S.O. Aqueous protein and gossypol extraction from glanded cottonseed flour: Factors affecting protein extraction. Food Chemistry 1988, 29, 57–63.
- AOAC. Official Method of Analysis of AOAC Intl, 17th Ed; Association of official Analytical communities: Arlington, VA, 2000.
- Tzeng, Y.M.; Diosady, L.L.; Rubin, L.J. Production of canola protein materials by alkaline extraction, precipitation, and membrane processing. Journal of Food Science 1990, 55 (4), 1147–1156.
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. Journal of Agricultural and Food Chemistry 2001, 49 (4), 1984–1989.
- Shahidi, F.; Liyana-Pathirana, C.M.; Wall, D.S. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chemistry 2006, 99 (3), 478–483.
- Zhang, H.; Chen, F.; Wang, X.; Yao, H.Y. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Research International 2006, 39 (8), 833–839.
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochemical and Biophysical Research Communications 1972, 46 (2), 849–854.
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The deoxyribose method-a simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals. Analytical Biochemestry 1987, 165 (1), 215–219.
- Duh, P.D.; Yen, C.G.; Yen, W.J.; Chang, L.W. Antioxidant effects of water extracts from barley Hordeum vulgare L. prepared under different roasting temperatures. Journal of Agricultural and Food Chemistry 2001, 49 (3), 1455–1463.
- Alder-Nissen, J. Enzymic Hydrolysis of Food Proteins, Elsevier Applied Science: New York, 1986; 122–124.
- Nagai, T.; Inoue, R.; Inoue, H.; Suzuki, N. Scavenging capacities of pollen extracts from cistus ladaniferus on autoxidation, superoxide radicals, hydroxyl radicals, and DPPH radicals. Nutrition Research 2002, 22 (4), 519–526.
- Zhu, K.X.; Zhou, H.M.; Qian, H.F. Antioxidant and free radical scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochemestry 2006, 41 (6), 1296–1302.
- Nazeer, R.A.; Deeptha, R. Antioxidant activity and amino acid profiling of protein hydrolysates from the skin of Sphyraena barracuda and Lepturacanthus savala. International Journal of Food Properties 2013, 16 (3), 500–511.
- Li, X.X.; Han, L.J.; Chen, L.J. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. Journal of the Science of Food and Agriculture 2008, 88 (9), 1660–1666.
- Wang, J.S.; Zhao, M.M.; Zhao, Q.Z.; Jiang, Y.M. Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Food Chemistry 2007, 101 (4), 1658–1663.
- Chen, H.M.; Muramoto, K.; Yamaguchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine containing peptides designed from peptide fragments found in the digests of a soybean protein. Journal of Agricultural and Food Chemistry 1998, 46 (1), 49–53.
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides from porcine myofibrillar proteins by protease treatment. Journal of Agricultural and Food Chemistry 2003, 51 (12), 3661–3667.
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Research International 2005, 38 (2), 175–182.
- Je, J.Y.; Park, P.J.; Kim, S.K. Antioxidant activity of peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Research International 2005, 38 (1), 45–50.
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free radical scavenging properties of wheat extracts. Journal of Agricultural and Food Chemistry 2002, 50 (6), 1619–1624.
- Cacciuttolo, M.A.; Trinh, L.; Lumpkin, J.A.; Rao, G. Hyperoxia induces DNA damage in mammalian cells. Free Radicals in Biology and Medicine 1993, 14 (3), 267–276.
- Peng, X.; Xiong, Y.L.; Kong, B. Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance. Food Chemistry 2009, 113 (1), 196–201.
- Chawla, S.P.; Chander, R.; Sharma, A. Antioxidant properties of Maillard reaction products obtained by gamma-irradiation of whey proteins. Food Chemistry 2009, 116 (1), 122–128.
- Mitsuta, H.; Yasumoto, K.; Iwami, K. Antioxidative action of indole compounds during the autoxidation of linoleic acid. Journal of Japanese Society of Nutrition and Food Science 1996, 19, 210–214.
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (Acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of Biochemistry and Biophysics 1994, 315 (1), 161–169.
- Kim, S.K.; Lee, H.C.; Byun, H.G.; Jeon, Y.J. Isolation and characterization of antioxidative peptides from enzymatic hydrolysates of Yellowfin sole skin gelatin. Journal of the Korean Fisheries Society 1996, 29 (2), 246–255.
- Amadou, I.; Le, G.W.; Shi, Y.H.; Jin, S. Reducing, radical scavenging, and chelation properties of fermented soy protein meal hydrolysate by Lactobacillus plantarum LP6. International Journal of Food Properties 2011, 14 (3), 654–665.
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from fragments found in the digests of a soybean protein. Journal of Agricultural and Food Chemistry 1998, 46 (1), 49–53.
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical mediated oxidative systems. Journal of Nutritional Biochemistry 2005, 16 (9), 562–569.
- Li, B.; Chen, F.; Wang, X.; Ji, B.; Wu, Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chemistry 2007, 102 (4), 1135–1143.