Abstract
Epigallocatechin gallate was added to gelatin, and the changes in the gelatin were characterized to determine the effects of epigallocatechin gallate modification. The microstructural changes in the samples were analyzed using Fourier transform infrared spectroscopy, X-ray diffraction, differential scanning calorimetry, sodium dodecyl sulfate polyacrylamide gel electrophoresis, and scanning electron microscopy. The results indicated that the gel strength and thermal stability of gelatin can be improved by appropriate epigallocatechin gallate addition. The optimal final concentration of epigallocatechin gallate was 1.0 g l−1 in a gelatin solution (66.7 g l−1). The concentration was also verified using Fourier transform infrared spectroscopy and X-ray diffraction. Covalent bonds were not observed in the epigallocatechin gallate-gelatin samples. Hydrogen bonds were the main molecular interactions observed in the epigallocatechin gallate-gelatin samples. The color of the epigallocatechin gallate-gelatin hydrogels or xerogels was darker because of the epigallocatechin gallate oxidation.
INTRODUCTION
Gelatin, a fibrous protein extracted from collagen, is widely used in various industries such as in food products, material science, pharmaceutical, and photography. In particular, gelatin is used in food and pharmaceutical products because of its unique chemical and physical properties.[Citation1] However, this protein with high molecular weight possesses several disadvantages such as poor mechanical properties as well as low gelling and melting temperatures. These properties restrict the application of gelatin in numerous fields. Various approaches have been undertaken for better cross-linking and improvement of gel strength[Citation2] such as physical, chemical, or enzymatic modifications. These methods include the use of ultraviolet irradiation, transglutaminase (TGase),[Citation3] and aldehydes.[Citation4] Compared with physical and enzymatic modifications, optimal physical properties can be achieved by adding chemical-modifying agents. However, these agents, such as aldehyde, methyl aldehyde, and glutaraldehyde, are toxic to mammalian cells. In using modified gelatin in food, food-compatible reagents should be used to replace chemical-modifying agents.
Polyphenols comprise a structural class of natural organic chemicals characterized by the presence of large multiples of phenolic hydroxyl groups,[Citation5] which can modify protein by bonding peptide chains.[Citation6] The mechanism for the modification of polyphenols remain unclear. Polyphenols may be cross-linked with protein using hydrophobic bonds, hydrogen bonds, or esterification.[Citation7] In nature, plant polyphenols, which have antioxidant action,[Citation8,Citation9] are found in virtually all families of plants. However, various types of plant polyphenols, such as hydrolysable tannins, are toxic to humans.
A group of non-toxic and inexpensive flavonoids, known as tea polyphenols, is naturally found in tea.[Citation10] Tea polyphenols are water-soluble polyphenolic substances that include epicatechin-3-gallate (ECG), epigallocatechin, epigallocatechin-3-gallate (EGCG), and epicatechin (EC).[Citation11] Compared with EC and ECG, EGCG is the most significant active component that contains an additional phenolic hydroxyl group. The leaf buds and first leaves of tea are richest in EGCG. EGCG exhibits powerful anti-oxidant activities and plays an important role in preventing cancer,[Citation12] HIV,[Citation13] and cardiovascular diseases.[Citation14] EGCG may be used as a modifying agent because of its rich phenolic hydroxyl groups that can cross-link gelatin. The EGCG gelatin may possess some beneficial characters for health. This article aims to prepare modified gelatin using EGCG, characterize the changes in gelatin, and compare the modified gelatin with the untreated gelatin.
MATERIALS AND METHODS
Preparation of Modified Gelatin
Gelatin solutions (87 g l−1) were prepared by mixing dry gelatin (285 Bloom, originally from pig skin; Sinopharm Group, China) with chilled distilled water containing 0.1 g l−1 NaN3.[Citation15] The mixture was kept at 10°C for 60 min, transferred to a water bath, and then held at 50°C for 30 min.[Citation16] The EGCG (over 90% purity; Jiangxi Lukang Nature Products Co., Ltd., China) solutions (2.5, 5, 10, 15, and 20 g l−1) were obtained by dissolving EGCG in distilled water. Then, the EGCG solutions were mixed with gelatin solutions in a beaker to obtain the final concentrations of 0.5, 1, 2, 3, and 4 g l−1, respectively. A constant concentration of gelatin (66.7 g l−1) was also achieved. The gelatin–polyphenol solutions were exposed to oxygen at 50°C of water bath by bubbling oxygen through the solution for 1 h.[Citation15,Citation17] The mixture subsequently matured for 24 h at room temperature and was kept for 16 to 18 h at 7°C in a biochemical incubator (Shanghai Sanfa Scientific Instruments Co., Ltd., China). The untreated gelatin hydrogel (66.7 g l−1) was prepared using the same method as the modified gelatin hydrogels, except for the EGCG addition. A portion of the matured gelatin hydrogels was lyophilized for further testing.
Determination of Gel Strength
A total of 10 ml of gelatin–polyphenol solution, which was exposed to oxygen for 1 h, was added to a weighing bottle (27 mm in diameter), matured for 24 h at room temperature, and then kept for 16 to 18 h at 7°C. The gel strengths of the modified and untreated gelatin hydrogels were tested using a TAXT2 texture analyzer (Stable MicroSystems, UK) with a 5 kN load cell and equipped with a 5 mm diameter probe. The maximum force (g) was determined as the gel strength.
Thermal Properties
The thermal stability of the similar dry weight samples were determined via differential scanning calorimetry (DSC) using a Perkin-Elmer Pyris 1 DSC instrument. Then, 5 mg of each sample was hermetically encapsulated in aluminum pans and heated from 30 to 280°C with a heating rate of 10°C min−1. The denaturation temperatures were determined as the onset values of the resulting endothermic peak.
Fourier-Transform Infrared Spectroscopy (FTIR)
The freeze-dried, untreated, and modified gelatin xerogels were analyzed using attenuated total reflectance Fourier transform infrared spectroscopy (FTIR). The spectra were obtained using a Nicolet Nexus FTIR spectrometer (Thermo Electron Corporation) with an absorption mode at 4 cm−1 intervals and scanning frequency of 16 times in the wavenumber region of 500 to 4000 cm−1.[Citation18]
X-Ray Diffraction (XRD) Studies
The XRD patterns of xerogels were obtained using a D8 Advance X-ray diffractometer (Bruker, Germany) equipped with Cu Kα radiation (λ = 0.15406 nm), and operating at 40 kV and 40 mA. Data were recorded in the angular range of 2θ (3 to 90°), at a rate of 4°−min−1, and a step size of 0.02°. DS (the divergence slit)-SS (the scatter slit)-RS (the receiving slit) was set at 1, 1, and 0.1 mm, respectively. Each sample was measured once. MDI Jade 5.0 was used to analyze the diffractograms.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed using 50 g l−1 of resolving gel and 40 g l−1 of stacking gel. The samples (10 μl) were applied and subjected to electrophoresis. After electrophoresis, the gel was stained with Coomassie Brilliant blue R-250. Channel catfish skin collagen extracted according to the method of Liu et al.[Citation19] was applied as a molecular weight standard.
Scanning Electron Microscopy (SEM)
The samples were gold-coated using a sputter coater (BAL-TEC, SCD 005; Witten, Germany) prior to observation. SEM micrographs for each specimen were obtained using a scanning electron microscope (QUANTA-200, FEI).
Statistical Analysis
Statistical tests were performed using the SPSS computer program (SPSS Statistical Software Inc., Chicago, IL, USA). Differences between pairs of means were assessed based on the confidence intervals using the Duncan test. The level of significance was p < 0.01.
RESULTS AND DISCUSSION
Effects of EGCG on Gel Strength
The mechanism for polyphenol modification remains unclear. Haslam[Citation20] reported that polyphenols are multidentate ligands that can bind simultaneously via different phenolic groups at various sites on the protein molecule. Jobstl et al.[Citation21] explained the process of binding between polyphenols and protein. Polyphenol–protein precipitation occurs in three stages. Cross-linking initially occurs and then leads to aggregation. Protein forms a compact sphere at higher concentrations and at suitable reaction conditions.[Citation17] The polyphenols cross-link the different protein molecules as the polyphenol concentration rises, concurrently producing non-soluble materials. Finally, as more polypehnols are added, more protein molecules aggregate, resulting in large particles that precipitate out of the solution.[Citation21]
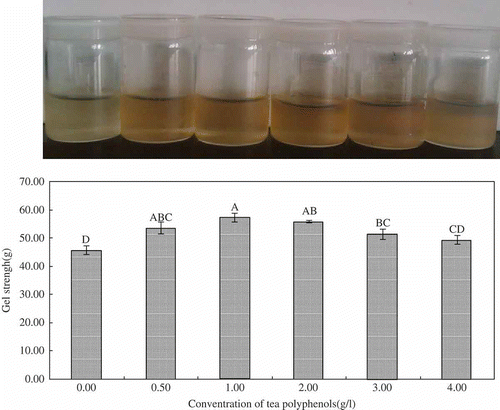
The appearance and morphology of samples were recorded using a Canon G12 camera. The EGCG solution became light brown after exposure to oxygen for 1 h. Consequently, the procedure made the color of the hydrogels darker. However, the color of the hydrogel became lighter when the EGCG concentration was increased to 4 g l−1. Jobstl et al.[Citation21] attributed the changes in the sample color to the precipitation that occurred when excess EGCG binds the gelatin in solution, thereby decreasing transparency.
As shown in , the gel strengths of the samples increased when the EGCG concentration increased. Gel strength reached the highest value when the EGCG concentration was 1.0 g l−1. The highest gel strength was 57.1 g, significantly higher than that of the untreated samples (45.7 g) (p < 0.01). Gel strength is a function of complex interactions and one of the most important functional properties of gelatin. The highest gel strength was obtained during the second stage of the cross-linking process.[Citation21] Kosaraju et al.[Citation17] reported that diphenol moiety is oxidized to an orthoquinone by molecular oxygen. Then, the quinone reacts with amino or sulfhydryl side chains of gelatin to form covalent bonds. Cross-linking occurs when a second protein molecule reacts with regenerated hydroquinone. Some researchers believe that only hydrogen bonding occurs between protein and polyphenols. In the present study, the cross-linking strengthened the network of gelatin hydrogels when EGCG concentration rose to 1.0 g l−1. However, as more EGCG were added, more gelatin precipitated. This phenomenon conforms to the theory of Jobstl et al.[Citation21] The results suggested that adding appropriate quantities of EGCG improved the gel strength of gelatin hydrogel.
Thermal Properties
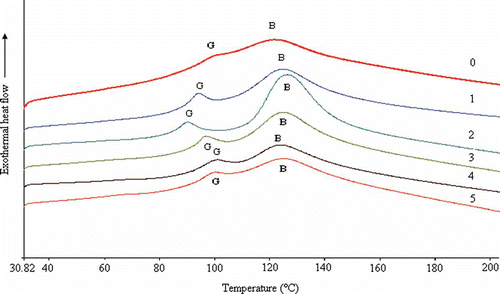
The thermal stability of xerogels was determined using DSC. As shown in , the DSC curve of porcine gelatin in the study is similar to that described by Rahman et al.[Citation22,Citation23] The DSC curves showed the melting transitions, B: an endothermic peak and a small endothermic peak at G. The melting temperatures of the EGCG samples that were attributed to a helix-coil transition of some fraction of the gelatin[Citation24] increased compared with that of the untreated gelatin. The EGCG cross-linking improved the thermal property of gelatin. The sample cross-linked with 1 g l−1 of EGCG had the highest melting temperature and biggest peak area (i.e., enthalpy of melting).
XRD
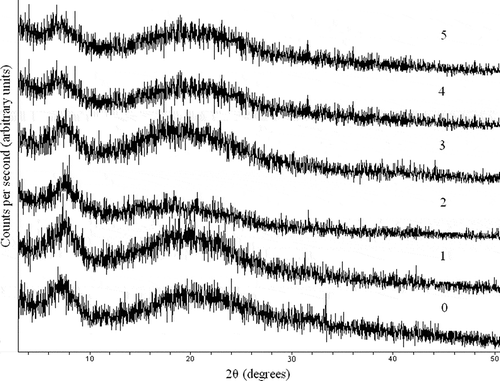
As shown in , drying gelatin exhibited two peaks at approximately 6.91 and 20.10°. The first peak (6.91°) indicated the distance between the molecular chains, and the second peak (20.10°) was attributed to diffused scattering.[Citation25] The results showed that all first diffraction peaks of EGCG-gelatin shifted toward higher angle values compared with those of the untreated gelatin (). Simultaneously, the corresponding d001 value that represented the basal spacing of the samples decreased considerably from 12.78 to 11.66 Å because of the EGCG added. The minimum value of d001 (11.66 Å) was obtained when the EGCG concentration was increased to 1 g l−1. The result indicated that the xerogel structure became more compact because of hydrogen bonding. EGCG could enter the spacing of polypeptide chains of gelatin to reinforce the intermolecular interaction. The results of XRD diffraction and FTIR were consistent, and both results may be confirmed.
Table 1 The d values of diffraction peaks in X-ray diffraction diagrams of gelatin xerogels treated with and without EGCG
Table 2 Fourier transform infrared absorption band assignment of gelatin hydrogels treated with and without EGCG
FTIR
FTIR was applied to analyze the difference between drying EGCG-gelatin samples and the control (). As shown in , the amide A band position was found in the untreated gelatin at 3294.79 cm−1, which was caused by the hydrogen-bonded hydroxyl groups.[Citation16] Various shifts of the amide A band to lower frequencies were observed in the modified samples. The shift implied that the hydrogen bonds in the EGCG-gelatins increased considerably. The most significant shift (24.59 cm−1) was obtained in the 1.0 g l−1 EGCG sample. The spectrum of the untreated gelatin dispersions also demonstrated the characteristic pattern reflecting the amide I, II, and III bands at 1629.55, 1543.74, and 1237.59 cm−1, respectively. The amide I band was the coil structure characteristic of gelatin.[Citation26] As shown in , no obvious shifts were observed in the modified gelatin compared with that in the untreated gelatin. To visualize the spectral changes of the amide I band region more clearly, the second derivative was applied. As shown in , almost all peak intensities of EGCG-gelatins decreased considerably compared with those of the untreated sample. The most significant decrease was obtained in the 1.0 g l−1 EGCG sample at 1628, 1656, 1661, and 1668 cm−l. Prystupa and Donald[Citation27] indicated that peaks near 1630, 1652, 1660 cm−l, and from 1660 to 1690 cm−l are related to the random coil regions, single α-helix, triple helix, and β-turn regions, respectively. Lazarev et al.[Citation28] reported that the collagen components at 1630, 1656, 1660, and 1670 cm−l were assigned to imide residues hydrogen bonded to the solvent, amino residues bonded to one another, and amino residues hydrogen-bonded to water. Consequently, the decrease in peak intensity in the second derivative showed that the amount of secondary structure of gelatin decreased, and it also suggested that the intermolecular interaction, especially hydrogen bond was enhanced.
Figure 4 Fourier transform infrared spectrum of gelatin xerogels treated with and without EGCG. (0) 0 g/l EGCG; (1) 0.5 g/l EGCG; (2) 1.0 g/l EGCG; (3) 2.0 g/l EGCG; (4) 3.0 g/l EGCG; (5) 4.0 g/l EGCG.
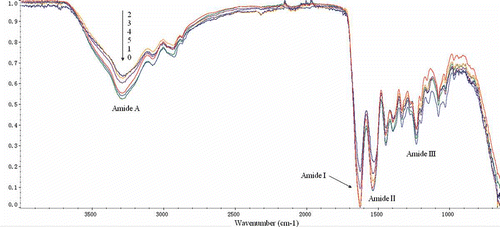
Figure 5 Second derivative of amide I band from FTIR spectra of gelatin xerogels treated with and without EGCG. (0) 0 g/l EGCG; (1) 0.5 g/l EGCG; (2) 1.0 g/l EGCG; (3) 2.0 g/l EGCG; (4) 3.0 g/l EGCG; (5) 4.0 g/l EGCG.
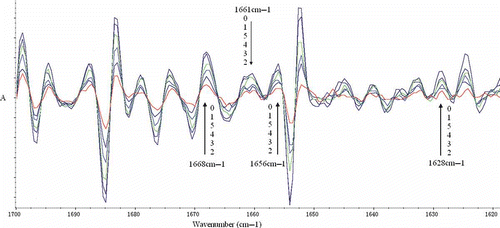
The wavenumbers of EGCG-gelatin at the amide II band were all lower than those of the untreated gelatin. The biggest shift (10.61cm−1) was obtained in the 1.0 g l−1 EGCG sample. This result indicated that N–H was more involved in bonding with the adjacent peptide chains.[Citation28] The amide III band demonstrated the existence of an α helical structure.[Citation16,Citation29] The small changes in the wavenumbers of EGCG-gelatin at amide III band (from 3.85 to 1.92 cm−1) suggested the helical structure of gelatin decreased as the EGCG addition.
Based on the results of the present work, the interaction between adjacent chains of gelatin was enhanced via hydrogen bonding after EGCG addition. In addition, N–H was employed in bonding with the adjacent gelatin peptides.[Citation30] Therefore, more intermolecular than intramolecular cross-linking occurred in the EGCG of the modified gelatins. Although the EGCG-gelatin solution was exposed to oxygen, the characteristic peaks of covalent bonds and function in polyphenol–protein cross-linking[Citation17] were not observed in the spectroscopy. This result suggested that hydrogen bonding was the main molecular interaction in the EGCG-gelatin samples, not covalent bonds. Similar cross-linking was also found between EGCG and amyloid β-protein.[Citation31]
SDS-PAGE
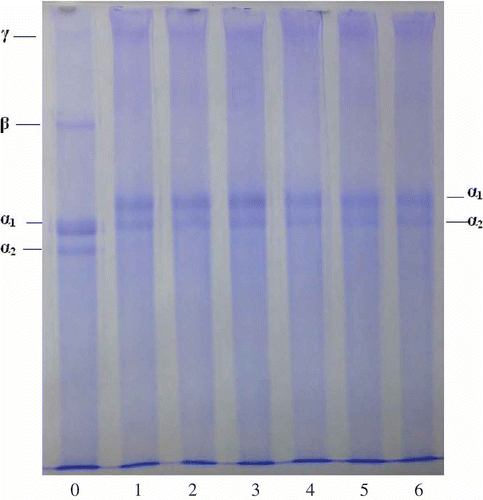
The untreated gelatin and EGCG-gelatins were analyzed using SDS-PAGE. As shown in , all EGCG-gelatin samples showed almost similar molecular weight distributions as that of the untreated gelatin. No production of macromolecule material with covalent bonds occurred. Given that the pretreatment of SDS-PAGE can completely break hydrogen bonds between peptide chains, it cannot disrupt the covalent bonds. Therefore, covalent bonding was not the main molecular interaction during the cross-linking between EGCG and gelatin. The FTIR result is similar to that from the SDS-PAGE.
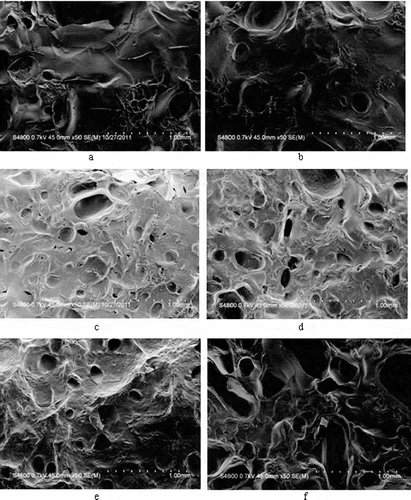
SEM
The surface morphology of untreated gelatin and EGCG-gelatins were analyzed using SEM. As shown in , no obvious difference was found between samples. However, with the increase in the final concentration of EGCG, pits formed on the surface, and pit depth increased. The EGCG strengthened the structure of gelatin. However, the pits became wide, and deep cracks developed when the final concentration of EGCG was increased to 4 g l−1. To some extent, excessive EGCG disrupted the network structure of gelatin.
CONCLUSION
Untreated gelatin and EGCG-gelatin were compared, in terms of macroscopic and microcosmic aspects. Results indicated that the physical properties of gelatin improved with the appropriate EGCG addition. The results were verified using gel strength, SDS-PAGE, DSC, FTIR, XRD, and SEM analyses. The results indicated that gelatin molecules became more compact in EGCG-gelatin because of the hydrogen bonds, not covalent bonds. Hydrogen bonds between EGCG and gelatin are easy to be broken under mild conditions. Therefore, EGCG-gelatin may be beneficial to human health as a kind of food material or biomaterial.
FUNDING
This study was supported by the Fundamental Research Funds for the Central Universities under Grant No. of JUSRP21010.
REFERENCES
- Jamilah, B.; Harvinder, K.G. Properties of gelatins from skins of fish-black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chemistry 2001, 77, 81–84.
- Prasertsung, I.; Mongkolnavin, R.; Damrongsakkul, S.; Wong, C.S. Surface modification of dehydrothermal crosslinked gelatin film using a 50 Hz oxygen glow discharge. Surface and Coatings Technology 2010, 205, S133–S138.
- Staroszczyk, H.; Pielichowska, J.; Sztuka, K.; Stangret, J.; Kołodziejska, I. Molecular and structural characteristics of cod gelatin films modified with EDC and T gase. Food Chemistry 2012, 130, 335–343.
- Boanini, E.; Rubini, K.; Panzavolta, S.; Bigi, A. Chemico-physical characterization of gelatin films modified with oxidized alginate. Acta Biomaterialia 2010, 6, 383–388.
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angewandte Chemie (International Ed. in English) 2011, 50, 586–621.
- Tang, H.R.; Covington, A.D.; Hancock, R.A. Use of DSC to detect the heterogeneity of hydrothermal stability in the polyphenol-treated collagen matrix. Journal of Agricultural and Food Chemistry 2003, 51, 6652–6656.
- Cao, N.; Fu, Y.H.; He, J.H. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocolloids 2007, 21, 575–584.
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16 (2), 1486–1507.
- Zujko, M.E.; Witkowska, A.M. Antioxidant potential and polyphenol content of selected food. International Journal of Food Properties 2011, 14 (2), 300–308.
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Haytowitz, D.; Holden, J.; Eldridge, A.L; Beecher, G.; Aladesanmi, J. Major flavonoids in dry tea. Journal of Food Composition and Analysis 2005, 18, 487–501.
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C. The chemistry of tea flavonoids. Critical Reviews in Food Science and Nutrition 1997, 37, 693–704.
- Das, A.; Banik, N.L; Ray, S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 2009, 116 (1), 164–176.
- Williamson, M.P.; McCormick, T.G.; Nance, C.L.; Shearer, W.T. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. The Journal of Allergy and Clinical Immunology 2006, 118 (6), 1369–1374.
- Wolfram, S. Effects of green tea and EGCG on cardiovascular and metabolic health. Journal of the American College of Nutrition 2007, 26 (4), 373S–388S.
- Strauss, G.; Gibson, S.M. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocolloids 2004, 18, 81–89.
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chemistry 2004, 86, 325–332.
- Kosaraju, S.L.; Puvanenthiran, A.; Lillford, P. Naturally cross-linked gelatin gels with modified material properties. Food Research International 2010, 43, 2385–2389.
- Guerrero, P.; Stefani, P.M.; Ruseckaite, R.A.; Caba, K. Functional properties of films based on soy protein isolate and gelatin processed by compression molding. Journal of Food Engineering 2011, 105, 65–72.
- Liu, H.Y.; Li, D.; Guo, S.D. Studies on collagen from the skin of channel catfish (Ictalurus punctaus). Food Chemistry 2007, 101, 621–625.
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited, Cambridge University Press: Cambridge, U.K., 1989.
- Jobstl, E.; O’Connell, J.; Fairclough, P.A.; Williamson, M.P. Molecular model for astringency produced by polyphenol/protein interactions. Biomacromolecules 2004, 5 (3), 942–949.
- Rahman, M.S.; Al-Saidi, G.S; Guizani, N. Thermal characterization of gelatine extracted from yellowfin tuna skin and commercial mammalian gelatine. Food Chemistry 2008, 108, 472–481.
- Rahman, M.S.; Al-Saidi, G. Thermal relaxation of gelatin and date flesh measured by isothermal condition in differential scanning calorimetry (DSC) and its relation to the structural and mechanical glass transition. International Journal of Food Properties 2010, 13 (4), 931–944.
- Sionkowska, A. Modification of collagen films by ultraviolet irradiation. Polymer Degradation and Stability 2000, 68, 147–151.
- Yan, M.Y.; Li, B.F.; Zhao, X.; Yi, J.B. Physicochemical properties of gelatin gels from walleye pollock (Theragra chalcogramma) skin cross-linked by gallic acid and rutin. Food Hydrocolloids 2010, 25, 907–914.
- Yakimets, I.; Wellner, N.; Smith, A.C.; Wilson, R.H.; Farhat, I.; Mitchell, J. Mechanical properties with respect to water content of gelatin films in glassy state. Polymer 2005, 46, 12577–12585.
- Prystupa, D.A.; Donald, A.M. Infrared study of gelatin conformations in the gel and sol states. Polymer Gels and Networks 1996, 4 (2), 87–110.
- Lazarev, Y.A.; Lazareva, A.V.; Shibnev, V.A.; Esipova, N.G. Infrared-spectra and structure of synthetic polytripeptides. Biopolymers 1978, 17, 1197–1214.
- Surewicz, W.K.; Mantsch, H.H. New insight into protein secondary structure from resolution enhanced infrared spectra. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology 1988, 952, 115–130.
- Bandekar, J. Amide modes and protein conformation. Biochimica et Biophysica Acta (BBA) Protein Structure and Molecular Enzymology 1992, 1120, 123–143.
- Wang, S.H.; Dong, X.Y.; Sun, Y. Thermodynamic analysis of the molecular interactions between amyloid β-protein fragments and (-)-epigallocatechin-3-gallate. Journal of Physical Chemistry B 2012, 116 (20), 5803–5809.